2020 年 67 巻 1 号 p. 81-89
2020 年 67 巻 1 号 p. 81-89
Although currently the primary strategy for the treatment of pheochromocytomas is surgery, it is associated with a high risk of intraoperative hemodynamic instability (IHD), even with adequate preoperative medical preparation, which may result in life-threatening situations. The aim of this study was to develop and validate a nomogram for preoperative prediction of IHD related to pheochromocytoma surgery. The development cohort consisted of 283 patients with pheochromocytoma who underwent unilateral laparoscopic or open adrenaletomy at our center between January 1, 2007 and December 31, 2016. The clinicopathological characteristics of each patient were recorded. The least absolute shrinkage and selection operator binary logistic regression model was used for data dimension reduction and feature selection, while multivariable logistic regression analysis was used to develop the prediction model. An independent cohort consisting of 119 consecutive patients from January 1, 2017 to December 31, 2018 was used for validation. The performance of the prediction model was assessed in regards to discrimination, calibration, and clinical usefulness. The predictors of this model included body mass index, coronary heart disease, tumor size, and preoperative use of crystal/colloid fluid. For the validation cohort, the model showed good discrimination with an area under the receiver operating characteristic of 0.767 (95% CI, 0.667–0.857) and good calibration (unreliability test, p = 0.852; Hosmer–Lemeshow test, p = 0.9309). Decision curve analysis demonstrated that the model was clinically useful. This nomogram to facilitate preoperative individualized prediction of IHD in patients with pheochromocytoma may help to improve the perioperative strategy and treatment outcome.
A PHEOCHROMOCYTOMA is a rare neuro-endocrine tumor, which arises from the chromaffin cells of the adrenal medulla with an incidence of 0.2–0.8 cases/100,000 persons/year, it affecting 0.1%–1% of patients with hypertension and approximately 5% of those with an adrenal incidentaloma [1, 2]. Pheochromocytoma presents with a series of clinical symptoms due to excessive catecholamine production, which include hypertension, headache, perspiration, palpitations, tremors, and facial pallor. These symptoms are often paroxysmal and can be spontaneous or induced by a variety of events, such as strenuous physical exertion, delivery, trauma, anesthesia induction, and surgery, among others [3].
Although resection is the mainstay treatment strategy for pheochromocytomas, surgery is associated with a high risk intraoperative hemodynamic instability (IHD) due to excessive release of catecholamines into the circulation, which may result in life-threatening situations [4, 5]. Mortality rate of pheochromocytomas has been reported can be as high as 50%; however, through widespread improvements in preoperative medical preparation [PMP], anesthesia strategy and surgical techniques, it has been significantly reduced to 0–2.9% [3]. Nonetheless, IHD is still common and difficult to manage. Previous studies have identified some independent risk factors that may be associated with IHD, which include tumor size, catecholamine levels, preoperative blood pressure, and surgical approach [6, 7]. However, the related risk factors remain unclear, as few studies addressed this issue and the conclusions have been inconsistent.
A nomogram derived from model is accepted as a reliable tool for predicting risk by incorporating and illustrating important predictors of significant clinical outcomes [8], which gives a numerical probability of the event. Therefore, the aim of the present study was to develop and validate the usefulness of nomogram for preoperative prediction of IHD in patients with pheochromocytoma.
The study protocol was approved by the Institutional Research and Ethics Committee of Sheng Jing Hospital Affiliated with China Medical University (Shengyang, China) on January 14, 2019 (approval no. 2019PS003K). Written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment at chictr.org.cn (ChiCTR1900020811, Principal investigator: Song Bai, Date of registration: June 20, 2019).
PatientsThe development cohort of this study consisted of 283 patients with pheochromocytoma who underwent unilateral adrenalectomy at our center between January 1, 2007 and December 31, 2016. The validation cohort comprised 119 consecutive patients from January 1, 2017 to December 31, 2018 who met the same inclusion and exclusion criteria. A detailed cohort flow chart is presented in Supplementary Fig. 1.
The diagnosis of pheochromocytoma was confirmed by pathologic examination and patients who underwent either unilateral laparoscopic or open adrenalectomy were included. The clinical stage was localized (apparently benign) disease with an American Society of Anesthesiologists (ASA) score of 1–3. All participants has normal renal function (confirmed by serum creatinine) and cardiac function (with more than 50% Ejection Fraction). Patients with a familial history of pheochromocytoma, were converted to laparotomy, or underwent bilateral adrenalectomy or surgery for ectopic pheochromocytoma were excluded.
Baseline characteristics and outcomesPatient demographics (sex, age, body mass index [BMI]), comorbidity (ASA score, diabetes mellitus [DM], coronary heart disease [CHD] hypertension, arrhythmia), disease characteristics (tumor side and tumor size, tumor necrosis, enhanced computed tomography difference), and extensive preoperative (use of alpha adrenoreceptor antagonists, use of crystal/colloid fluids, preoperative transfusion, 24-h urine metanephrines/upper normal limit value) and intraoperative (surgical approach, surgical duration, IHD, and estimated blood loss) characteristics were recorded.
IHD was defined as the presence of at least once instance of intraoperative systolic blood pressure >200 mmHg and a mean arterial pressure <60 mmHg, or the requirement for norepinephrine, phenylephrine or other vasoconstrictive agents or blood transfusion to maintain normal blood pressure intraoperatively [9]. Continuous vasoactive agents administration with invasive arterial blood pressure monitoring was applied to avoid IHD, such as sodium nitroprusside for controlling hypertension, norepinephrine or blood transfusion for hypotension.
Patients presenting with typical radiographic and biochemical findings of a pheochromocytoma were treated with prazosin, terazosin, or doxazosin for at least two weeks before surgery. A beta adrenergic blocker was added to control for tachycardia after the use of alpha blockader. Fluid intake was encouraged. Patients with high blood pressure or a larger tumor size were treated by intravenous crystalloid and colloid fluid (2,000 mL/day) or blood transfusion preoperatively. The criteria for preoperative medical preparation efficacy included blood pressure <130/80 mmHg, heart rate <90 beats/min, and hematocrit <0.45.
Statistical analysisData were analyzed using IBM SPSS Statistics for Windows, version 22.0. (IBM Corporation, Armonk, NY, USA USA), STATA 15.0. (Stata Corp., College Station, TX, USA) and R software (version 3.0.1; http://www.Rproject.org). The “rms” and “glmnet” packages in R were used in this study. Tests for statistical significance were all two-sided, with a probability (p) value of <0.05 considered statistically significant. The normality of continuous variables was determined by the Kolmogorov-Smirnov test. Normally distributed continuous variables are presented as the mean ± standard deviation and non-normally distributed continuous variables as the median (interquartile range). The means of two continuous normally distributed variables were compared using the independent samples Student’s t-test. The Mann–Whitney U test was used to compare two continuous non-normally distributed variables. Categorical variables are reported as the number (percentage). The chi-squared and Fisher’s exact tests were used for comparisons of categorical variables. In addition, patients with any missing data on the eligible variables were excluded from subsequent analysis.
The least absolute shrinkage and selection operator (LASSO) method, which is suitable for the reduction of high-dimensional data, was used to select the most useful predictive features from the primary data set in this study.
All the clinicopathologic variables were reduced to limited potential predictors on the basis of 283 patients in the development cohort using the LASSO binary logistic regression model. If the penalization coefficient lambda (λ) is large, there is no effect on the estimated regression parameters, but as the λ gets smaller, some coefficients may be shrunk towards zero. We then selected the optimal λ in the LASSO model by using 10-fold cross-validation via minimum criteria and one standard error of the minimum criteria (the 1-SE criterion). Finally, the model was re-fit by using all of the non-zero coefficients, which were selected by Lasso method (Fig. 1).
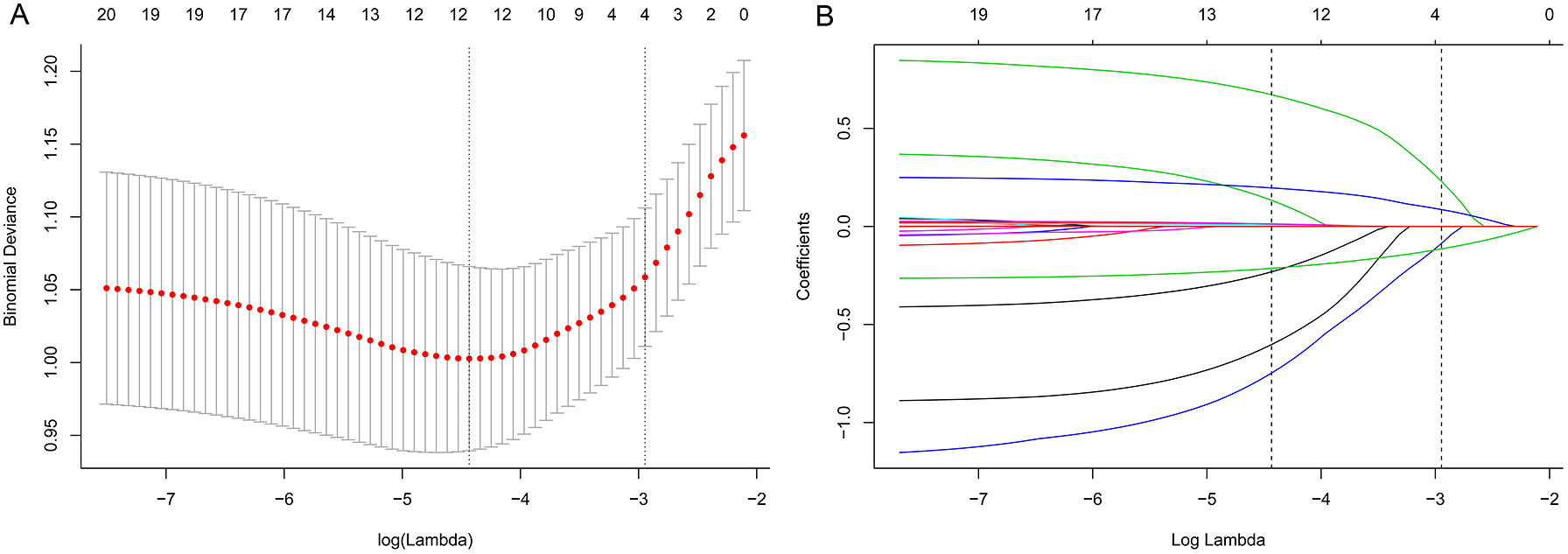
Texture feature selection using the LASSO binary logistic regression model.
A. Ten-fold cross-validation via minimum criteria was used to select the Tuning parameter (λ) in the LASSO model. Dotted vertical lines were drawn at the optimal values with the use of the minimum criteria and the one standard error of the minimum criteria (the 1-SE criteria).
B. A coefficient profile plot was produced against the log (λ) sequence. A vertical line was drawn at the value selected using 10-fold cross-validation.
The independent validation cohort was used to test the performance of this model. The logistic regression formula formed in the development cohort was applied to the validation cohort and the probability for each patient was calculated. To quantify the discrimination performance of the model, the area under the receiver operating characteristic (AUC) curve was measured, where an AUC of 0.5 indicates no discrimination and 1.0 indicates perfect discrimination. Calibration plots were used to assess the calibration of this model, accompanied with both the unreliability test and Hosmer–Lemeshow chi-square statistic (p > 0.05 supports the goodness of calibration). Perfect calibration is shown by the slope of the 45-degree line. Decision curve analysis was conducted to determine the clinical usefulness of this model by quantifying the net benefits at different threshold probabilities in the validation cohort.
Finally, 283 patients were included in the development cohort and 139 in the validation cohort after carefully screening with the same inclusion and exclusion criteria. Of these, 74 (26.1%) patients in the development cohort and 33 (27.7%) in the validation cohort had IHD, Supplement Fig. 1.
Univariate analysis of the development cohort showed that BMI, CHD, tumor size, and use of crystal/colloid fluid were significantly associated with IHD (in Table 1). Because the sample size in this study was inadequate to satisfy the recommended guide of events per variable [10], LASSO binary logistic regression was used to construct the model. The λ value was 0.015. Of all the relevant variables, 19 features were reduced to four potential predictors on the basis of the development cohort. The four variables with nonzero coefficients in the LASSO logistic regression model (i.e., BMI, CHD, tumor size, and use of crystal/colloid fluid) were used in the final model (in Fig. 1). Based on these results, a prediction model and a nomogram to predict IHD were generated (Table 2 and Fig. 2).
| Development Cohort (n = 283) | Validation Cohort (n = 119) | |||||
|---|---|---|---|---|---|---|
| Without IHD n = 209 (73.9) |
With IHD n = 74 (26.1) |
p-value | Without IHD n = 86 (72.3) |
With IHD n = 33 (27.7) |
p-value | |
| Demographic characteristics | ||||||
| Mean age (years) | 51.9 ± 12.3 | 54.0 ± 13.8 | 0.233 | 51.03 ± 13.00 | 55.52 ± 11.84 | 0.087 |
| Sex (male/female) | 110 (52.6)/99 (47.4) | 31 (41.9)/43 (58.1) | 0.112 | 47 (54.7)/39 (45.3) | 8 (24.2)/25 (75.8) | 0.003 |
| BMI (kg/m2) | 24.1 ± 3.5 | 21.9 ± 2.7 | <0.001 | 23.76 ± 3.57 | 22.15 ± 2.49 | 0.019 |
| ASA score 1/2/3 | 52 (24.9)/136 (65.1) /21 (10.0) |
14 (18.9)/53 (71.6) /7 (9.5) |
0.548 | 25 (29.1)/49 (57.0) /12 (14.0) |
7 (21.2)/23 (69.7) /3 (9.1) |
0.527§ |
| Comorbidity | ||||||
| Diabetes mellitus | 61 (29.2) | 23 (31.1) | 0.759 | 24 (27.9) | 8 (24.2) | 0.686 |
| Coronary heart disease | 66 (31.6) | 37 (50.0) | 0.005 | 32 (37.2) | 18 (54.5) | 0.086 |
| Hypertension Normal/Intermittent/Continuous | 82 (39.2)/47 (22.5) /80 (38.3) |
30 (40.5)/18 (24.3) /26 (35.1) |
0.883 | 39 (45.3)/18 (20.9) /29 (33.7) |
15 (45.5)/8 (24.2) /10 (30.3) |
0.903 |
| Arrhythmia | 12 (5.7) | 4 (5.4) | 0.914§ | 7 (8.1) | 3 (9.1) | 0.867§ |
| Preoperative data | ||||||
| Tumor side (left/right) | 103 (49.3)/106 (50.7) | 39 (52.7)/35 (47.3) | 0.613 | 41 (47.7)/45 (52.3) | 19 (57.6)/14 (42.4) | 0.333 |
| Radiographic tumor size (cm) | 5.2 ± 2.5 | 6.5 ± 3.1 | <0.001 | 5.42 ± 2.63 | 7.40 ± 3.46 | 0.005 |
| Tumor necrosis | 69 (33.0) | 33 (44.6) | 0.075 | 30 (34.0) | 19 (57.6) | 0.024 |
| Tumor enhanced CT difference (Hu) | 43.2 ± 20.6 | 45.6 ± 20.2 | 0.435 | 41.52 ± 17.34 | 44.36 ± 16.48 | 0.419 |
| Use of α adrenoreceptor antagonists | 115 (55.0) | 42 (56.8) | 0.797 | 50 (58.1) | 17 (51.5) | 0.514 |
| Use of crystal/colloid fluid | 118 (56.5) | 29 (39.2) | 0.011 | 50 (58.1) | 11 (33.3) | 0.015 |
| Use of blood transfusion | 54 (25.8) | 15 (20.3) | 0.338 | 20 (23.3) | 7 (21.2) | 0.812 |
| 24-hour urine metanephrines/normal upper limit | 1.4 (0.9–2.2) | 1.47 (0.9–2.7) | 0.153& | 1.51 (1.06–2.19) | 1.45 (1.10–2.51) | 0.350& |
| Intraoperative data | ||||||
| Laparoscopic vs. Open | 109 (52.2)/100 (47.8) | 42 (56.8)/32 (43.2) | 0.495 | 73 (84.9)/13 (15.1) | 26 (78.8)/7 (21.2) | 0.426 |
| Duration of surgery (minutes) | 151.9 ± 70.0 | 166.0 ± 63.6 | 0.269 | 157.73 ± 76.81 | 158.88 ± 65.55 | 0.940 |
| Estimated blood loss (mL) | 200 (100–400) | 200 (100–500) | 0.272& | 200 (100–400) | 200 (100–700) | 0.612& |
Continuous variables with normal distribution were reported as the mean ± standard deviation (SD), while non-normal continuous variables as the median (interquartile range) and categorical variables as numbers (percentages). The Student’s t-test was used to compare the mean values of two continuous normally distributed variables and the Mann–Whitney U test was used to determine mean values of two continuous non-normally distributed variables. The chi-squared or Fisher’s exact test was used for categorical variables.
& Mann-Whitney U test
§ Fisher’s exact test
BMI, body mass index; ASA, American Society of Anesthesiologists; CT, computed tomography; IHD intraoperative hemodynamic instability.
| Intercept and Variable | β | 95% CI | p |
|---|---|---|---|
| Intercept | 3.423 | 0.996, 5.850 | 0.006 |
| BMI (kg/m2) | –0.253 | –0.362, –0.145 | <0.001 |
| Coronary heart disease | 0.923 | 0.319, 1.525 | 0.003 |
| Use of crystal/colloid fluid | –0.695 | –1.288, –0.101 | 0.022 |
| Radiographic tumor size (cm) | 0.220 | 0.109, 0.3321 | <0.001 |
| Area under ROC curve | |||
| Development Dataset | 0.766 | 0.710, 0.823 | |
| Validation Dataset | 0.767 | 0.677, 0.857 | |
The β coefficient, odds ratio, and 95% confidence interval were measured through binary logistic regression.
OR, odds ratio; CI, confidence interval; BMI, body mass index; IHD, intraoperative hemodynamic instability.
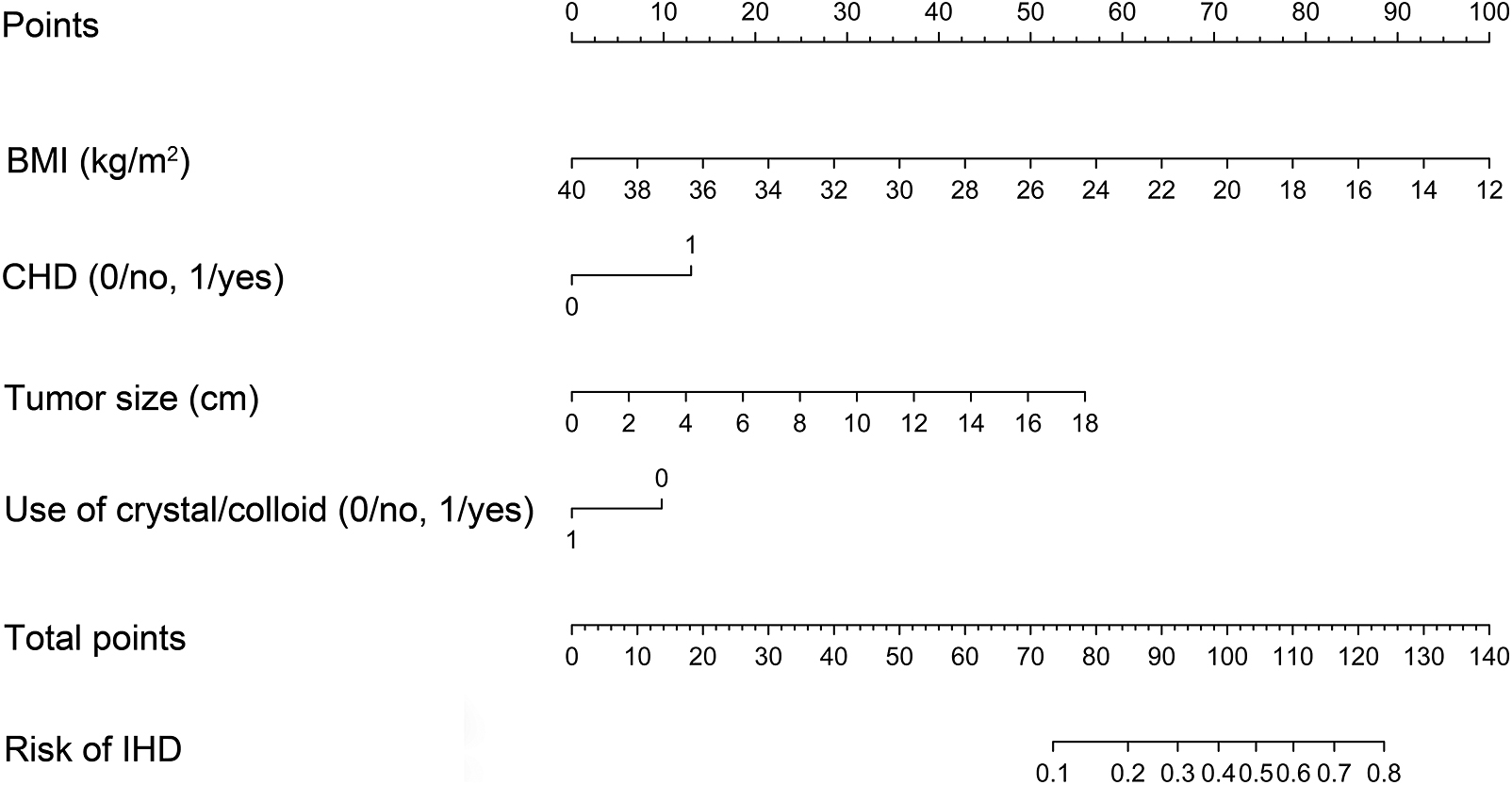
Nomogram to predict IHD in patients with pheochromocytomas
Each clinicopathological factor corresponds to a specific point by drawing a line straight upward to the Points axis. After sum of the points is located on the Total points axis, the sum represents the probability of IHD in patients with pheochromocytomas by drawing a line straight down to the risk axis.
Each clinicopathological feature corresponded to a specific point by drawing a line straight upward to the points axis. After the sum of the points is located on the Total Points axis, the sum represents the probability of IHD by drawing straight down to the risk axis. For example, for a patient with history of CHD (13 points), BMI of 20.0 kg/m2 (71 points), tumor size of 8 cm (25 points), and no use of crystal/colloid fluid (10 points) preoperatively, the total points were 119 and the suspected probability of IHD was approximately 73%. This calculated outcome could be used in decision making for treatment plans (Supplementary Fig. 2).
The AUC values of the development and validation cohorts were 0.766 and 0.767, respectively, and the cutoff value of risk probability in this model was 0.194, with a sensitivity of 89.19% and specificity of 53.59% (Table 2 and Fig. 3A and 3B). The unreliability test statistic of calibration for validation was –0.011 with a p value of 0.484 and Emax and Eavg values of 0.207 and 0.042, respectively (Fig. 3C). The Hosmer–Lemeshow chi-square statistic was 4.34 and the p value was 0.9309, demonstrating good calibration. The decision curve showed that if the threshold probability of a patient is in the range of 10% to 80%, the use of a nomogram to predict IHD in patients with pheochromocytoma is more beneficial than either the treat-all-patients or treat-none scheme. Within this range, net benefit was comparable (Fig. 3D).

Discrimination, calibration, and decision curve analysis for the model.
A. ROC of the development cohort representing the discrimination ability of the model, as measured by the C-index.
B. ROC of the validation cohort
C. A calibration plot showing the relationship between the predicted probabilities based on the nomogram and actual values of the validation cohort. A plot along the 45-degree line indicates perfect calibration of the model in which the predicted probabilities are identical to the actual outcomes.
D. Decision Curve Analysis: The y-axis measures the net benefit. The blue line represents the nomogram. The black line represents the assumption that all patients have IHD. The thin gray line represents the assumption that no patients have IHD.
Although the primary strategy for treatment of pheochromocytoma, surgery is associated with a high risk of IHD, even though there were greatly improved in PMP, anesthesia strategy, and surgical techniques have in recent years [6]. Exploring the risk predictors for IHD will lead to better treatment outcomes. Unfortunately, relatively few retrospective small-scale researches have addressed this issue in the literature, the risk factors remain unclear, especially there was no study focus on exploring the predictors of IHD. Nomogram has been accepted as reliable tools to predict risks by illustrating important predictors of clinical events. Therefore, the aim of this study was to develop and validate a nomogram for preoperative prediction of IHD in patients undergoing pheochromocytoma surgery.
There is no consensus on the criteria of IHD because of the low incidence of pheochromocytoma. In this study, the definition of IHD was at least one instance of systolic blood pressure ≥200 mmHg and mean arterial pressure ≤60 mmHg, or the requirement of vasoactive agents for balancing blood pressure intraoperatively, such as norepinephrine or transfusion to maintain normal blood pressure. As confirmed in previous studies, this criteria are closely related to severe morbidity, especially cardiovascular morbidity [9, 11]. In this study, 26.1% and 27.7% of patients in the development and validation cohorts, respectively, had IHD, which is consistent with a previous retrospective study that reported an incidence of IHD of 32.8% (44/134) in Chinese patients [4]. This nomogram incorporates four predictors based on extensive clinicopathological data, which were BMI, CHD, tumor size, and preoperative use of crystal/colloid fluid, for prediction of IHD.
This model was selected based on LASSO binary logistic regression instead of the minimal Akaike information criterion, as there were fewer IHD events, as compared to the number of variables, which was not in accordance with the events per variable principle. Validation of the nomogram is important to avoid overfitting and to determine generalizability. In this study, the AUC values of the development and validation cohorts demonstrated adequate discrimination (0.7660 and 0.7667, respectively), although the value of the validation cohort was a greater. Calibration plots also showed optimal agreement between the predicted and actual probability, as confirmed by both the Mann–Whitney test and Hosmer–Lemeshow chi-square statistic (p = 0.484 and 0.9309, respectively), which verified the repeatability and reliability of this model. Decision curve analysis was employed in this study to explore clinical usefulness, which showed that if the threshold probability of a patient is between 10% and 80%, the use of nomogram is more beneficial to predict the probability of IHD than either the treat-all-patients or the treat-none scheme. This range also covered the cutoff value of risk probability in this model (19.4%)
The risk regarding BMI, which was an independent risk factor for both severe and cardiovascular morbidity, has been previously reported [11, 12]. As a possible explanation, a lower BMI is associated with less effective circulatory volume, which could result in large fluctuations in blood pressure and a high incidence of IHD.
Usually, patients with pheochromocytoma have a higher incidence of heart disease than those with essential hypertension [13], such as CHD. The risk predictor of this model included patients with CHD becasue the myocardium and coronary arteries are exposed to abnormal elevated levels of catecholamines for prolonged periods, which would lead to collagen deposition and fibrosis formation in the myocardium [13]. In consistent with this, Zhang R et al. also found that acute left cardiac dysfunction due to chronically high level catecholamines exposure was the root cause of circulatory collapse and prolonged hypotension after pheochromocytoma surgery [14].
In the present study, tumor size was also an effective predictor for IHD, in accordance with the findings of previous studies [5, 15]. A large pheochromocytoma has a more prominent network of vessels and associated with greater intraoperative blood loss than smaller tumors [16, 17]. Meanwhile, large tumors secrete higher levels of catecholamines, which can easily to lead to greater fluctuations in blood pressure during the perioperative period [18]. Natkaniec et al. [19] reported that intraoperative blood loss was significantly greater in patients with tumor diameters ≥6 cm than <6 cm based on 530 patients who underwent laparoscopic adrenalectomy.
Another effective predictor for IHD involvement is the use of crystal or colloid for volume expansion before surgery. It was confirmed that preoperative medical preparation is important to decrease fluctuations in blood pressure during the perioperative period [20, 21]. All patients with pheochromocytoma should receive preoperative medical preparation and volume expansion to block the effects of released catecholamines [22]. However, the use of alpha-adrenergic receptor blockers and blood transfusion were not shown to be predictive. This may due to relative small sample size and events in this study, and may lead to underestimate its predictive effect. Nevertheless, both medical preparation and volume expansion are very important to achieve a good treatment outcome.
There were several limitations to this study that should be addressed. Firstly, our study was a retrospective study from one center. Secondly, peri-operative preparation strategies were varied and not standardized due to quite a long period of recruiting time; these differences may have influenced the final outcomes. Third, some variables related to IHD were not included, such as patient genomic background, radiomics and symptoms. Fourthly, this study is temporal external validation, which tested a model from older data on newer data in one center. It is superior to internal validation which used validation data sampled from a randomly splitting data set [23]. Therefore, a multicenter prospective cohort with standardization of PMP, anesthesia management, and the operative technique is required for validating this model. Lastly, validation using a Western cohort will also be needed prior to universal use of this model because of the significant differences in genetic characteristics between Eastern and Western cohorts should be considered [8]. Nonetheless, to the best of our knowledge, this is the first study to investigate the usefulness of a model to predict the risk of IHD. These findings should have a notable impact on preoperative preparation, treatment, and care options, such as the selection of patients who need additional preoperative preparation or intensive care. Both doctors and patients could perform an individualized prediction through this easy-to-use scoring system.
What are predictors of intraoperative hemodynamic instability (IHD) related to pheochromocytoma surgery and how to predict the probability of IHD individually?
FindingsThe predictors of IHD included body mass index, coronary heart disease, tumor size, and preoperative use of crystal/colloid fluid, by which facilitate individualized prediction of IHD in patients with pheochromocytoma.
MeaningThe least absolute shrinkage and selection operator binary logistic regression model was used for data dimension reduction and feature selection, while multivariable logistic regression analysis was used to develop the prediction model, which was assessed in regards to discrimination, calibration, and clinical usefulness in validated cohort.
Hongyan Wang certifies that all conflicts of interest, including specific financial interests, relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g., employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are none.
Song Bai helped acquire data, analyze and interpret of data, draft the manuscript and critically revise the manuscript for important intellectual content, and obtain funding and other (figures).
Bin Wu helped draft the manuscript and critically revise the manuscript for important intellectual content.
Zichuan Yao helped statistical analysis.
Xianqing Zhu helped statistical analysis.
Yunzhong Jiang helped statistical analysis.
Hongyan Wang helped study concept and design.
This study was financially supported (MC05) through the Shengjing Hospital Science and Technology Program. We give special thanks to all the teachers at the Department of Urology, Department of Graduate Medical Training, Department of Student Affairs Department of Shengjing Hospital for their help and support.
None.
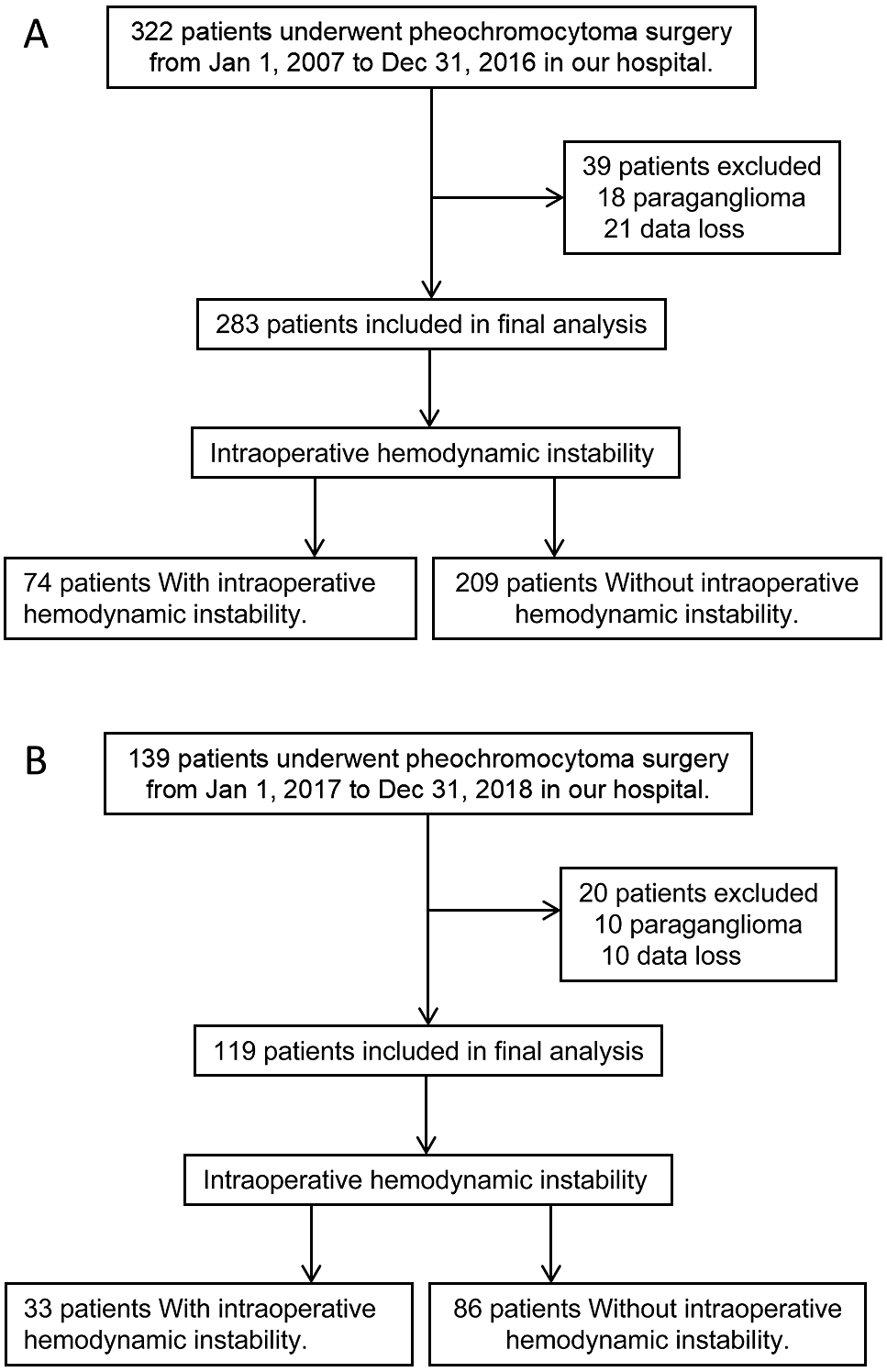
Inclusion and exclusion flow chart of development and validation cohorts
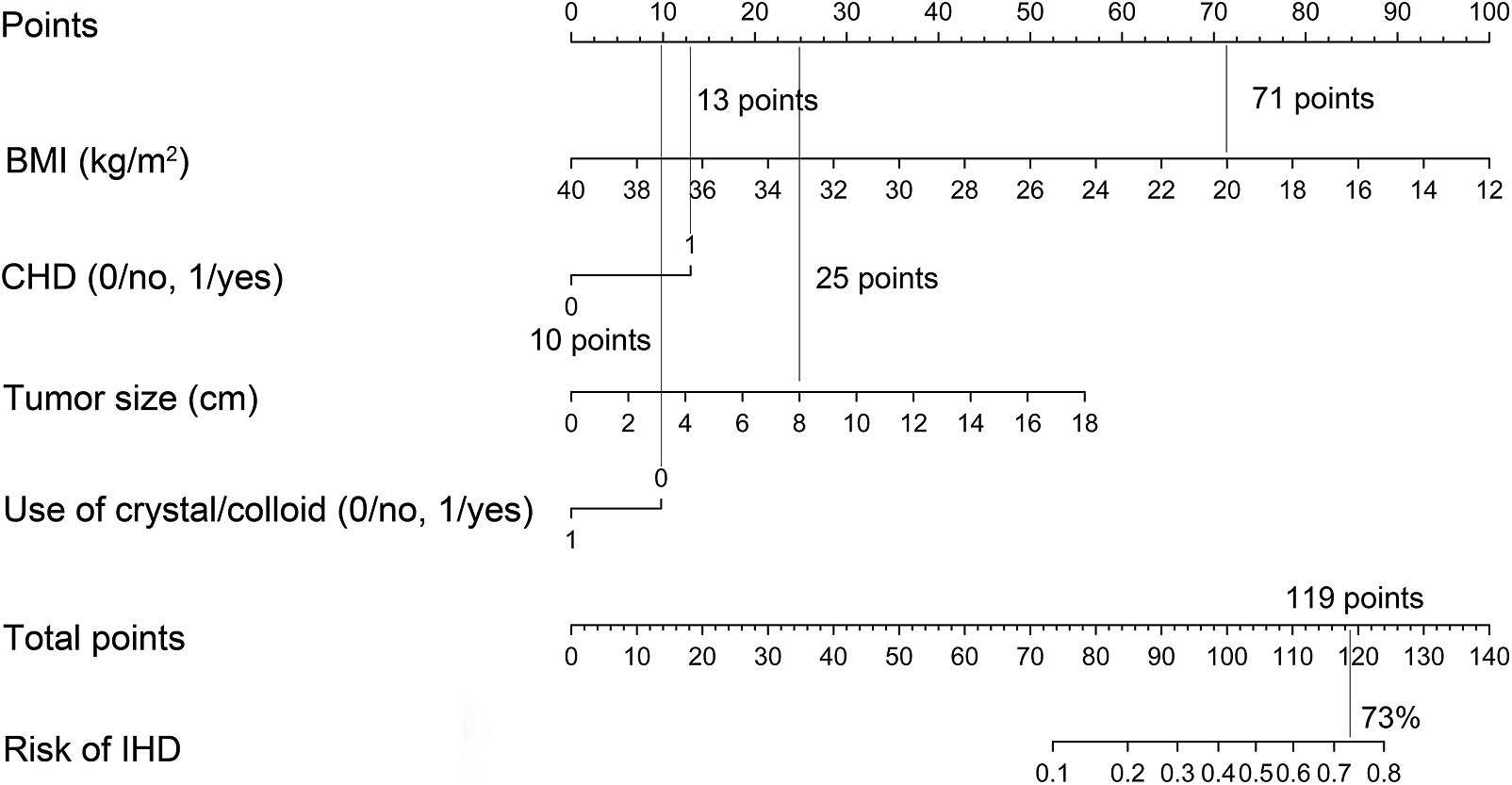
Nomogram to predict IHD after pheochromocytoma surgery for example