2020 年 67 巻 1 号 p. 59-71
2020 年 67 巻 1 号 p. 59-71
Little is known about type 1 diabetes mellitus (T1DM) impact on the male sexual and reproductive functions. We aim to evaluate the influence of T1DM on male sexual function, quality of sexual life, and sex hormone levels. A total of 57 male patients aged 18 to 50 years (mean = 33) with T1DM (duration mean = 15 years) had a medical examination and completed a set of questionnaires - International Index of Erectile Function-5 (IIEF-5), Beck Depression Inventory (BDI) and Sexual quality of life questionnaire male (SQoL-M). The prevalence of erectile dysfunction was 28.1% (IIEF-5 ≤21). Patients without diabetic nephropathy had better erectile function (p = 0.008). Subjects with better glycemic control (HbA1c <65 mmol/mol) had also better erectile function (p = 0.041). At least 8.8% patients had retrograde ejaculation. Blood serum levels of sex hormones were determined and compared to laboratory reference values of healthy men. Total testosterone level was not significantly changed, sex hormone binding globulin was higher (p < 0.001) and its level correlated with daily insulin dose adjusted to body weight (p = 0.008). Free androgen index and calculated free testosterone were lower (p = 0.013; p < 0.001), estradiol was not significantly changed, LH was higher (p < 0.001), FSH was unchanged, and prolactin was higher (p < 0.001). Prostate-specific antigen (PSA) negatively correlated with HbA1c (p < 0.001). To conclude, we found significant changes in sexual functions and sex hormone blood concentrations that indicate impairment of sexual and reproductive functions in T1DM males.
DIABETES MELLITUS is a major public health problem. Globally, an estimated 422 million adults were living with diabetes in 2014 compared to 108 million in 1980. This rise is related to an increase in obesity and other risk factors of type 2 diabetes mellitus (T2DM) [1]. Type 1 diabetes mellitus (T1DM) incidence is also rising by 3% every year in high income countries according to The World Health Organization report [1]. T2DM is among the well documented risk factors of erectile dysfunction and other male sexual dysfunctions [2]. There are numerous studies showing an association of T2DM and insulin resistance with androgen deficiency and with disruption in the hypothalamic-pituitary-gonadal axis [3-7]. T1DM is known to have similar complications as T2DM but there are many differences between these two diseases. T1DM onsets mostly in childhood or young adulthood and subsequently affects men prior to and during their reproductive years. It has been demonstrated by Sjöberg et al. [8] that childhood-onset T1DM patients have a lower number of offspring than healthy controls. A number of studies reported higher prevalence of erectile dysfunction in T1DM [9-11]. There is evidence of testicular tissue and cell damage in T1DM animal models [12, 13]. Previous studies showed contradictory results regarding changes in conventional sperm parameters [14, 15]. In recent years, there has been a raising interest in sperm nuclear and mtDNA damage, which is believed to be increased in diabetes [14, 16]. Although previous studies indicated hormonal changes in T1DM males [17, 18], the impact of T1DM on sexual hormone levels in male patients has been mostly overlooked. The effect of endocrine disruptions on diabetic male reproduction is poorly understood. Here, for the first time, we provide a comprehensive evaluation of the effects of T1DM on male sexual and reproductive health, including the assessment of sex hormone profiles, sexual functions, sexual life quality, and fertility.
The data were collected as a part of ongoing study on the impact of diabetes mellitus on male reproduction. Written informed consent was obtained from all participants, and the study was approved by the local ethics committee. Recruitment of the patients was conducted between March 2016 and March 2018 in The Department of Internal Medicine of Motol University Hospital in Prague. Only consenting adult (18–50 years of age) male patients with T1DM were included. T1DM was diagnosed according to clinical criteria recommended by the World Health Organization [19]. Patients with known cancer or disease of reproductive system unrelated to diabetes were excluded as well as patients with heart, lungs, liver or kidney failure.
Questionnaires and examinationsAll study-consented patients initially underwent an interview and clinical examination to collect demographic and anthropometric data. Subsequently, they were asked to complete a personal questionnaire designed by our group to establish their education level, marital status, number of offspring, stability of a long-term relationships, number of sexual partners in lifetime, and frequency of sexual activities. The questionnaire included inquiry about the patient’s perception of T1DM influence on their sexual life initiation (if they already had the disease at the time of initiation), impact of the disease on their current sexual life and initiation of new sexual relationships. Subjects treated with an insulin pump were questioned about their partner’s attitude to the insulin pump during sexual activities, and how often, if ever, they disconnect the pump during sexual practices. There was a section for patient’s comments on their sexual problems in personal questionnaire. Furthermore, patients were screened for erectile dysfunction using a simplified version of the International Index of Erectile Function Questionnaire (IIEF-5) [20]. Erectile dysfunction was defined as IIEF-5 score of less than 22 points. Sexual life quality was assessed by the Sexual Quality of Life Questionnaire for male (SQoL-M) [21]. To evaluate depression, the 21-item Beck Depression Inventory was applied and cut off for depression was established as a score higher than nine points [22]. All the questionnaires were used in the Czech language translations.
An interview, personal questionnaires, clinical examination, hospital’s information database including previous laboratory results, and patient’s medical history were all reviewed by the same physician to determine the general status of the patient’s health and to evaluate diabetic complications, including diabetic retinopathy, diabetic neuropathy, diabetic nephropathy, diabetic foot syndrome, macroangiopathic complications (myocardial ischemia, chronic lower limb ischemia, cerebral stroke), and other diabetic comorbidities, such as autoimmune thyroiditis, arterial hypertension, and hyperlipemia. If present at the time of the interview, complications were noted regardless of their severity. Diabetic retinopathy was assessed from the recent screening examination by ophthalmologist, diabetic retinopathy was marked if any, mild or severe form of diabetic retinopathy were diagnosed. Diabetic nephropathy was recorded in a presence of microalbuminuria, macroalbuminuria or a history of diabetic nephropathy. Diabetic neuropathy was screened for and if need be additional tests were conducted during clinical examination. Smoking habit (if applicable) was recorded as daily cigarette consumption. The Body Mass Index (BMI) was calculated using the following formula: weight (kg)/height2 (m). Mean daily doses of basal and bolus insulin were recorded and total daily dose adjusted to body weight was calculated (IU/kg).
Laboratory measurementsBlood and semen samples were collected from every consenting subject on one single occasion not later than 30 days from the day of clinical examination. The blood samples were all analyzed in the hospital’s laboratory using routine methods. Plasmatic glucose level was determined by enzymatic hexokinase assay and blood glycated hemoglobin A1c (HbA1c) was measured by capillary electrophoresis. Hormone profiles consisting of thyroid-stimulating hormone (TSH), free thyroxine (fT4), luteinizing hormone (LH) [23], follicle-stimulating hormone (FSH) [23], prolactin [23], total testosterone [24] and sex hormone binding globulin (SHBG) [25] were all determined from the blood serum using chemiluminescent immunoassay (CLIA). Prostate-specific antigen (PSA) and 17β‐estradiol (estradiol) [26] serum levels were measured by electrochemiluminescence immunoassay (ECLIA). Free androgen index (FAI) was calculated by dividing total testosterone level by the sex hormone binding globulin level, and then multiplying by 100 [24]. Free testosterone (CFT) was calculated from total testosterone and SHBG by using the equation proposed by Ly and Handelsman [27]. We opted to use reference values provided by the manufacturer for each assay because a) using reference values is the routine laboratory procedure and b) the reference values represent the optimal selection of healthy male controls. As reference values for hormone results and FAI, we used the reference measurements of healthy males provided by assays’ manufacturers. Reference limits for CFT were adopted from Ho et al. [28]. If the sample value was lower or higher then reference range published by the assay’s manufacturer, the sample was considered as abnormal. Semen samples were collected from consenting patients who were able to ejaculate on the day of blood sample collection.
Statistical analysisThe data distributions were tested using the Shapiro-Wilk test of normality, Q-Q plots and histograms. The patients’ characteristics were expressed as the mean and standard deviation (SD) in case of normal data distribution or as median and interquartile range (IQR) in data without normal distribution. The Independent-Samples T Test was used to test for differences between the two groups of continuous normally distributed variables. The nonparametric Mann-Whitney U test was used to test for differences in continuous variables without normal data distribution. To compare hormone levels of T1DM patients with median level in healthy males acquired from the assay’s manufacturer, we used the Wilcoxon signed-rank test. Associations were tested using the Pearson’s chi-square test in categorical variables. All correlations were analyzed using the Spearman rank order correlation test. All statistical tests were two-tailed. A p value <0.05 was considered to be statistically significant. Statistics were performed using SPSS software, version 23 (IBM Corp., Armonk, N.Y., USA).
A total of 64 adult male patients with T1DM were approached. Seven patients refused to participate in the study. Eleven agreed to participate without blood or sperm testing. Forty-six consented to participate in a full extend; however, seven patients were later not able to produce sperm sample due to sexual dysfunctions. In total, we examined 57 consenting T1DM patients. The mean age of the participants was 33 years with an average of 15 years for the duration of diabetes (see Table 1). Demographic characteristics of the subjects are shown in Table 2. Percentages of diabetic complications and other comorbidities are displayed in Table 3. Prevalence of diabetic complications was included regardless of their severity. All patients were appropriately treated by intensive insulin therapy from the day of T1DM diagnosis [29]. Sixty percent of patients were applying multiple daily injections (MDI), and 40% were on continuous subcutaneous infusion of insulin (CSII) by an insulin pump. In addition to insulin, six patients (10.5%) were taking metformin for insulin resistance. Complications and comorbidities mentioned above were accordingly treated with antihypertensives, lipid-lowering agents, thyroid hormone replacement therapy and other drugs.
| Variables | Median (IQR) |
|---|---|
| Age (years)a | 33.0 (7.4) |
| BMI (kg/m2) | 24.3 (4.1) |
| Diabetes duration (years) | 16.0 (14.0) |
| Daily insulin dose (IU/kg) | 0.62 (0.4) |
| HbA1c (mmol/mol) | 65.0 (19.0) |
a Expressed as Mean; Standard Deviation (SD)
IQR, Interquartile Range; BMI, Body mass index
Total number of responders: 55
| Variables | n | % |
|---|---|---|
| Education, N = 57 | ||
| Primary | 6 | 10.5 |
| Secondary | 38 | 66.7 |
| Higher | 13 | 22.8 |
| Occupation, N = 56 | ||
| Full-time | 44 | 78.6 |
| Part-time | 5 | 8.9 |
| Unemployed | 7 | 12.5 |
| Marital status, N = 56 | ||
| Single | 34 | 60.7 |
| Married | 19 | 33.9 |
| Divorced | 3 | 5.4 |
| Widowed | 0 | 0 |
| Children, N = 55 | ||
| Yes | 19 | 34.5 |
| No | 36 | 65.5 |
N = total number of responders
| Variables | n | % |
|---|---|---|
| Microangiopathic complications | ||
| Retinopathy | 22 | 39 |
| Neuropathy | 13 | 23 |
| Nephropathy | 13 | 23 |
| Macroangiopathic complicationsa | 3 | 5 |
| Diabetic foot syndrom | 1 | 2 |
| Autoimmune thyroiditis | 11 | 19 |
| Arterial hypertension | 17 | 30 |
| Hyperlipemia | 20 | 35 |
| Smoking | 24 | 43 |
| Cigarette per dayb | 7.8 (17.8) | |
a Myocardial ischemia, chronic lower limb ischemia or cerebral stroke
b Expressed as Median and Interquartile Range (IQR), smokers only
Total number of responders: 57, in case of smoking, only 56 responded
The majority of the study participants completed a personal questionnaire and the results are summarized in Table 4. Over 70% of the surveyed T1DM patients had a steady relationship. Over half of the respondents reported having sex at least twice a week but almost every fourth responder did not have a sex partner at the time. More than third of the patients felt affected by T1DM in their current sexual life. In the comment section, they mostly complained about erectile dysfunction, fatigue and hypoglycemia during sex. Surprisingly, over half of the participants never discussed the influence of diabetes on their sexual life with any doctor. The majority of the evaluated patients, who use the insulin pump, reported that they have disconnected it during sexual activities.
| n | % | ||
|---|---|---|---|
| Number of sexual partners to datea | 7.0 (7) | ||
| Steady relationship | 39 | 72.2 | |
| How often do you have sex with your mate? | |||
| I don’t have any mate. | 12 | 23.1 | |
| More than twice a week | 13 | 25.0 | |
| About twice a week | 15 | 28.8 | |
| Less than twice a week but more often than once a month | 8 | 15.4 | |
| About once a month | 3 | 5.8 | |
| A few times a year but less than once a month | 1 | 1.9 | |
| Has diabetes affected your sexual life initiation negatively? | Yes | 5 | 12.5 |
| No | 35 | 87.5 | |
| Does diabetes influence negatively your current sexual life? | Yes | 17 | 33.3 |
| No | 34 | 66.7 | |
| Does diabetes disturb your relationship starting? | Yes | 5 | 10 |
| No | 45 | 90 | |
| Do you think your sexual partner is disturbed by your insulin pump? | Yes | 1 | 5.0 |
| No | 15 | 95.0 | |
| Do you disconnect insulin pump during sexual activities? | |||
| Yes, always | 18 | 85.7 | |
| Sometimes | 2 | 9.5 | |
| No, never | 1 | 4.8 | |
| Have you been satisfied with the information on T1DM influence on sexual function provided by your doctors ? | |||
| Yes | 17 | 36.2 | |
| No | 3 | 6.4 | |
| I have never discussed this topic with any doctor. | 27 | 57.4 | |
a Expressed as Median and Interquartile Range (IQR)
The remaining participants provided no response or did not have T1DM at the time of sexual life initiation
The results of the Beck depression inventory (BDI) indicate the prevalence of an undiagnosed depression was 14.8% (8 out of 54). Three additional patients (5.6%) had depression previously diagnosed and were already treated. Thus, every fifth T1DM patient in our study was suffering from depression. We found a strong negative correlation between the depression, expressed as BDI score, and sexual quality of life, represented by SQoL(M) score, (rs = –0.783, N = 52, p = 0.001, summarized in Fig. 1A). Diabetic patients with depression had lower sexual life quality than diabetic patients without depression (Mann-Whitney U = 303.5, N = 52, p = 0.001; Fig. 1B). We verified no associations between depression and diabetes duration, HbA1c or diabetic complications.
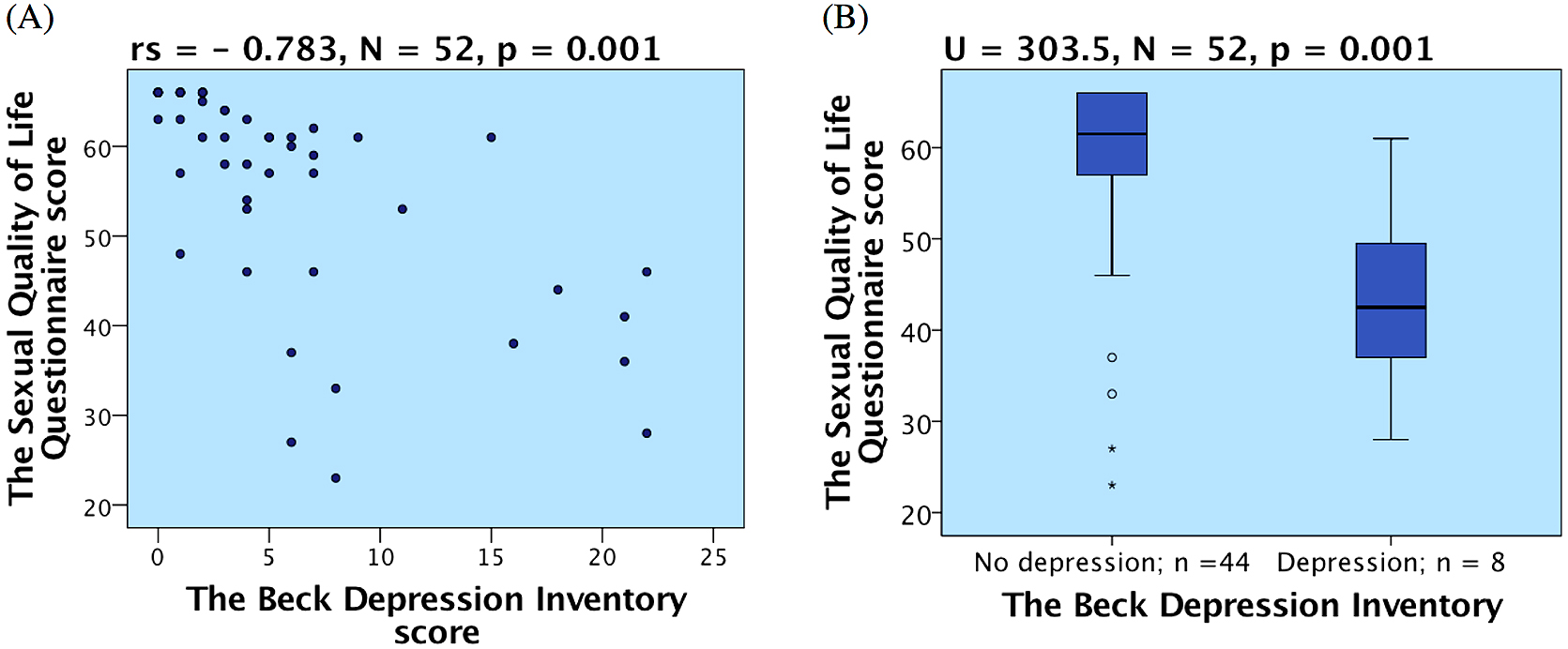
(A) Correlation of sexual quality of life and depression; (B) Difference in sexual quality of life in patients with and without depression
The IIEF-5 questionnaire results pointed to a high prevalence of erectile dysfunction in our group of T1DM patients. Almost every third patient (28.1%, N = 54) had self-reported erectile dysfunction expressed as the IIEF-5 score of 21 and lower. Erectile function expressed as the IIEF-5 score positively correlated with SQoL(M) (rs = 0.489, N = 52, p < 0.001). The men with erectile dysfunction had lower sexual life quality (Mann-Whitney U = 477.0, N = 52, p < 0.001; Fig. 2A). Patients, who reported disturbed sexual life by diabetes had a significantly lower erectile function score (Mann-Whitney U = 446.0, N = 50, p < 0.001; Fig. 2B). Patients with better glycemic control (expressed as HbA1c <65 mmol/mol) had higher IIEF-5 score and therefore, better erectile function (Mann-Whitney U = 235.0, N = 53, p = 0.041; Fig. 2C). We compared erectile function (expressed as the IIEF-5 score) in groups of patients with and without specific diabetic complications and comorbidities. Patients suffering from diabetic nephropathy had lower IIEF-5 than patients without this complication (Mann-Whitney U = 378.5, N = 54, p = 0.008; Fig. 2D). For the retinopathy, neuropathy, hypertension, hyperlipemia, and smoking, the differences were not statistically significant. No significant correlations were found between erectile dysfunction and diabetes duration or age. Additionally, we diagnosed at least five cases of retrograde ejaculation in the study group of 57 patients (8.8%). This problem was indicated during the interview by the fact that patients complained of frequent unejaculation or small amount of ejaculate. Urine samples were analyzed only when retrograde ejaculation was suspected and the diagnosis was confirmed in all suspected cases. Two more patients reported orgasmic dysfunction and some others complained about lack of sexual libido in the comment section of the personal questionnaire.

(A) Difference in sexual quality of life in patients with and without erectile dysfunction according to IIEF-5 score; (B) Difference in IIEF-5 score in patients who feel affected by diabetes in their sexual life compared to those who do not feel affected. (C) Difference in IIEF-5 score in patients with lower and higher glycated hemoglobin concentrations (HbA1c); (D) Difference in IIEF-5 score in patients with and without diabetic nephropathy
We compared levels of sex hormones in 46 consenting T1DM male patients to the median levels of healthy men, as discussed in the Methods section. The summary of the results is displayed in Table 5. We found that the levels of several sex hormones were significantly affected in our group of T1DM patients compared to healthy males.
| Healthy men | Diabetic group | Stand. Test Statistic | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | Reference range | N | Lowa | Higha | Median | IQR | |||
| Testosterone (nmol/L) | 122 | 14.12 | 5.72–26.14 | 40 | 1 (2.5) | 2 (5.0) | 15.78 | 6.74 | 1.116 | 0.265 |
| SHBG (nmol/L) | 122 | 34.97 | 14.55–94.64 | 40 | 0 | 1 (2.5) | 47.12 | 22.95 | 3.979 | <0.001*** |
| FAI (%) | 122 | 40.05 | 14.53–80.29 | 39 | 1 (2.6) | 0 | 31.91 | 14.12 | –2.477 | 0.013** |
| CFT (pmol/L) | 126 | 333 | 174–589 | 39 | 13 (33.3) | 0 | 206 | 107 | –5.289 | <0.001*** |
| Estradiol (pmol/L) | 150 | 90.9 | 41.4–159.0 | 41 | 8 (19.5) | 1 (2.4) | 67.5 | 51.0 | –1.899 | 0.058 |
| LH (IU/L) | 59 | 2.8 | 1.5–9.3 | 41 | 0 | 1 (2.4) | 4.1 | 2.5 | 4.510 | <0.001*** |
| FSH (IU/L) | 117 | 4.5 | 1.4–18.1 | 40 | 0 | 2 (5.0) | 4.3 | 2.6 | 1.042 | 0.298 |
| Prolactin (μg/L) | 139 | 7.0 | 2.1–17.7 | 41 | 0 | 2 (4.9) | 8.5 | 5.0 | 3.307 | 0.001** |
| PSA (μg/L) | 0–1.30 | 41 | 0 | 4 (9.8) | 0.65 | 0.50 | ||||
a Expressed as number of cases (percentage of total)—compared to reference range
Testosteron, total testosterone; SHBG, sex hormone binding globulin; FAI, free androgen index; CFT, calculated free testosterone; Estradiol, 17β‐estradiol; LH, luteinizing hormone; FSH, follicle-stimulating hormone; PSA, prostate-specific antigen
Testosterone. Total testosterone levels were not significantly changed in our T1DM patient group compared to healthy men. We found a moderate but significant correlation of total testosterone level with age (rs = –0.315, n = 40, p = 0.048). Patients who were overweight or obese (BMI >25 kg/m²) manifested lower testosterone levels than the patients with BMI ≤25 kg/m², as shown in Fig. 3A. As expected, total testosterone levels correlated with SHBG (rs = 0.492, N = 39, p = 0.001; Fig. 3B). We identified no significant associations of total testosterone with the duration of diabetes, HbA1c, diabetic complications, erectile dysfunction or sexual quality of life.
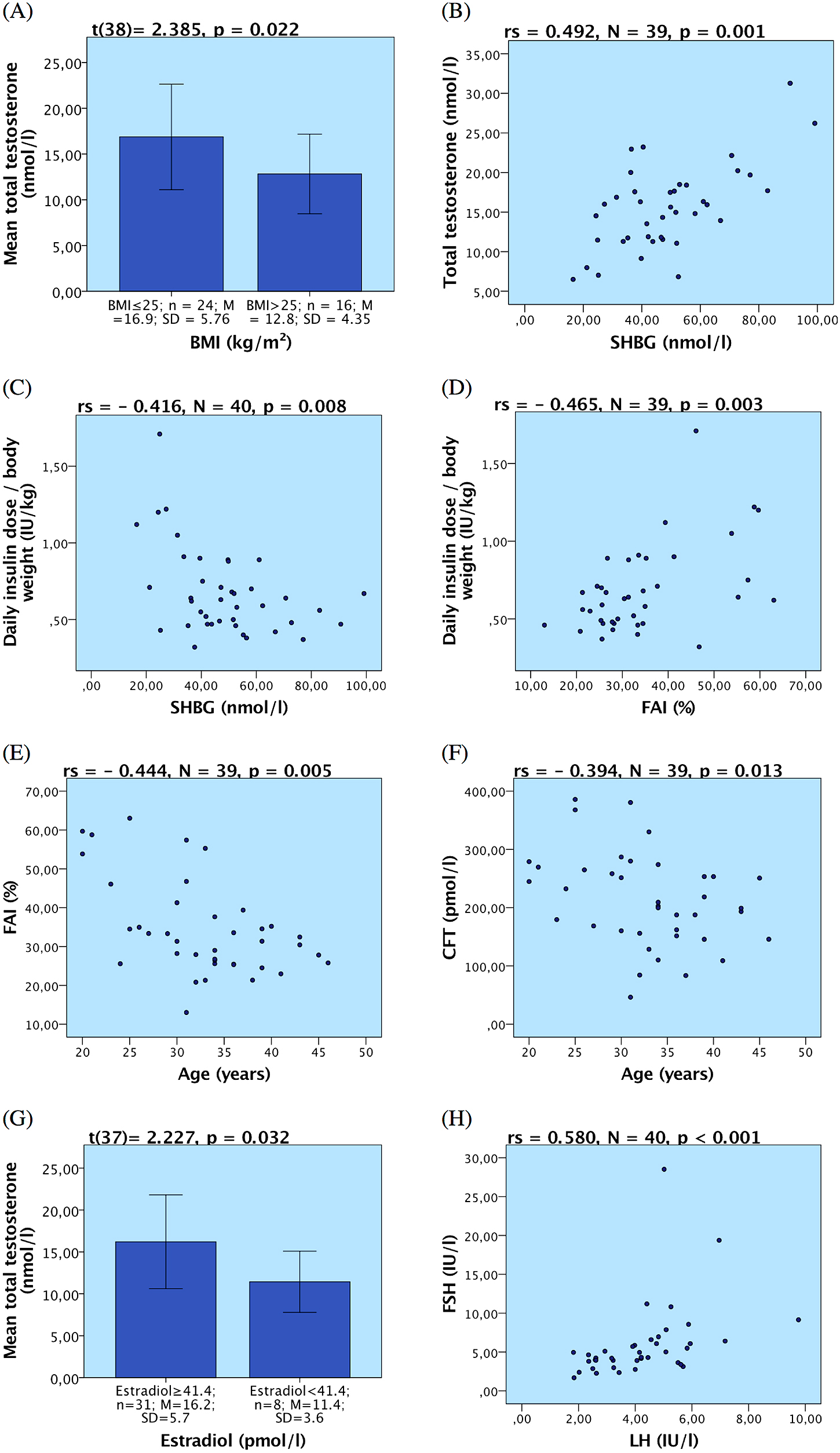
(A) Difference in mean total testosterone plasma concentration in two different BMI groups; (B) Correlation of total testosterone plasma concentration and plasma sex hormone binding globulin (SHBG); (C) Correlation of total daily insulin requirement adjusted to body weight with concentration of plasma SHBG; (D) Correlation of total daily insulin requirement adjusted to body weight with free androgen index (FAI); (E) Correlation of FAI with age; (F) Correlation of Calculated free testosterone (CFT) with age; (G) Difference in mean total testosterone in two groups with normal and subnormal plasma 17β-estradiol level (H) Correlation of luteinizing hormone (LH) and follicle-stimulating hormone (FSH)
Sex hormone binding globulin (SHBG). Although we found only one case (2.5%, N = 40) of SHBG higher than the reference range, further analyses showed overall significantly higher SHBG levels in the T1DM patients compared to healthy men (Table 5). Interestingly, SHBG negatively correlated with the mean daily insulin dose adjusted to body weight (rs = –0.416, N = 40, p = 0.008; Fig. 3C). Patients who required higher daily insulin doses had lower SHBG levels and vice versa. However, we did not find any associations between SHBG increase and diabetes duration, HbA1c, diabetic complications, erectile dysfunction or sexual life quality.
Free androgen index (FAI) was lower in our T1DM subjects compared to the reference values in healthy men. FAI significantly negatively correlated with the daily insulin dose adjusted to the body weight (rs = –0.465, n = 39, p = 0.003; Fig. 3D). We found negative correlation between FAI and age (rs = –0.444, n = 39, p = 0.005; Fig. 3E). We did not detect any associations between FAI and HbA1c, diabetes duration, diabetic complications, erectile dysfunction, sexual quality of life or depression.
Calculated free testosterone (CFT) was also lower (see Table 5) in our group of T1DM patients compared to the reference limits proposed by Ho et al. [28]. CFT significantly negatively correlated with age (rs = –0.394, n = 39, p = 0.013; Fig. 3F). No associations between CFT and HbA1c, diabetes duration, diabetic complications, erectile dysfunction, sexual quality of life or depression were found.
Estradiol. Eight patients (19.5%, N = 41) had estradiol levels below the reference range but overall the concentration of estradiol was not significantly changed compared to healthy men (Table 5). We detected a positive correlation between estradiol concentration and prolactin levels (rs = 0.338, N = 41, p = 0.031). We found no association between estradiol concentration and the duration of diabetes, HbA1c, diabetic complication, BMI or age. Correlation of estradiol with testosterone was weak and not statistically significant. However, when we compared 8 cases with abnormally low estradiol to the rest of the patients, we found they had significantly lower total testosterone (Fig. 3G).
Luteinizing hormone (LH), follicle-stimulating hormone (FSH). When measuring LH, only one case with abnormally high value was diagnosed, yet, we found significantly higher concentrations of LH compared to healthy men reference value, as displayed in Table 5. On the other hand, our measurements showed that FSH concentrations were not significantly different in T1DM males compared to healthy men. Levels of LH and FSH positively correlated (rs = 0.580, N = 40, p < 0.001; Fig. 3H). Our study demonstrated no association of gonadotropin concentration with diabetes duration or complications. When we compared patients with better glycemic control (HbA1c <65 mmol/mol) and poorly controlled (HbA1c ≥65 mmol/mol), we found no significant difference in gonadotropin concentrations.
Prolactin. Two patients (4.9%, N = 41) manifested hyperprolactinemia defined as serum prolactin concentration above the reference range. Further tests confirmed the median prolactinemia in our T1DM study group was significantly higher compared to reference median in healthy men (Table 5). Prolactin concentrations were not associated with age, HbA1c, diabetes duration or other comorbidities, including hyperlipemia. However, a positive correlation between estradiol concentration and prolactin levels was detected.
Prostate-specific antigen (PSA) and glycated hemoglobin A1c (HbA1c). We observed a strong negative correlation between HbA1c and PSA (rs = –0.590, N = 41, p < 0.001; Fig. 4A). Patients with poor metabolic compensation (HbA1c 65 mmol/mol or higher) showed lower PSA levels (Mann-Whitney U = 109.0, N = 41, p = 0.012; Fig. 4B). We found no statistically significant associations between PSA and BMI, age, diabetes duration, diabetic complications or hormone levels.
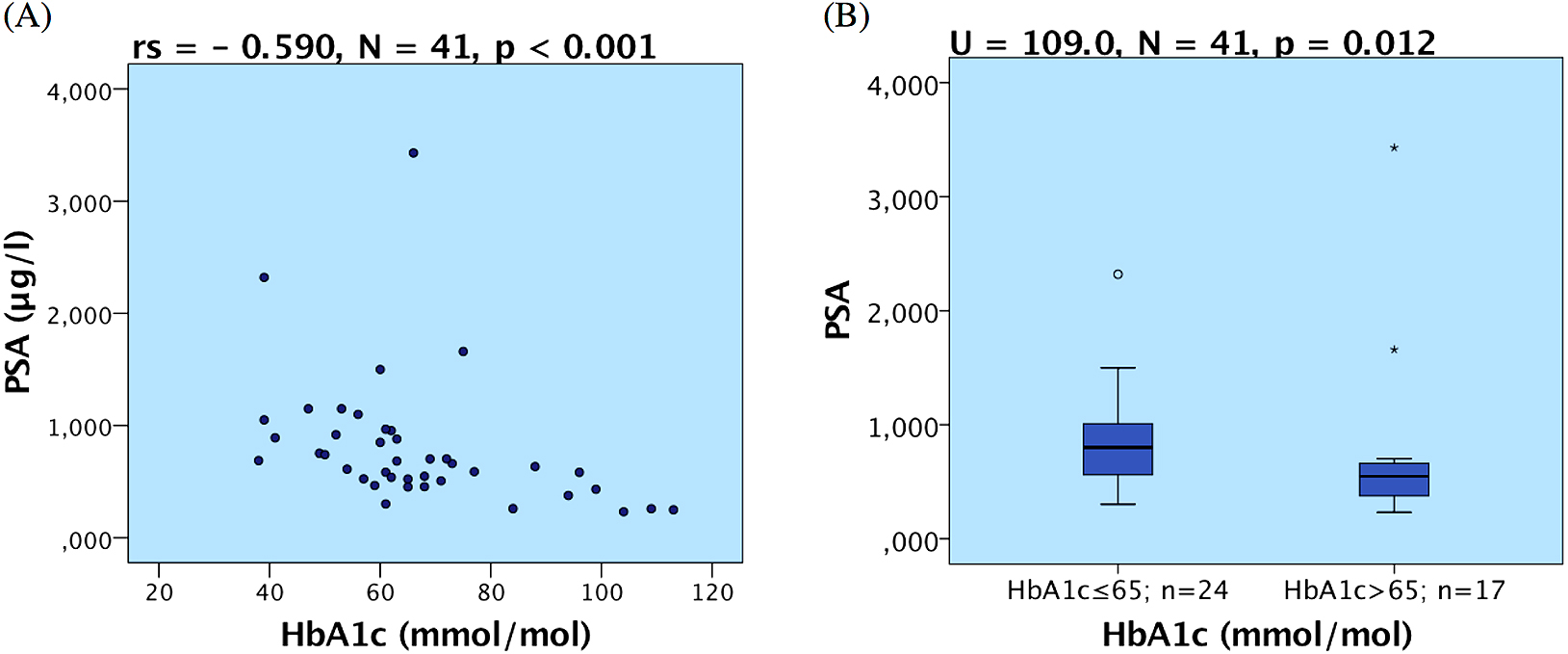
(A) Correlation of Prostate-specific antigen level (PSA) and glycated hemoglobin A1c (HbA1c); (B) Box plots representing different PSA level in groups of patients with low and high HbA1c
In the present study, we investigated the influence of T1DM on male sexual and reproductive functions. Our results show that T1DM negatively affects the quality of sexual life and sexual function, and alters levels of sex hormones. The limitations of this study should be noted. The number of participants is relatively small and therefore results should be interpreted with caution. Due to the cross-sectional design of the study, causality cannot be determined and requires further investigation.
Depression and sexual life qualityOver the past decade, an increasing number of studies reported a higher prevalence of depression in diabetic patients [30] and the negative influence of depression on glycemic control [31, 32]. Fisher et al. [33] proposed that a major part of depression cases emerging from T1DM depression screening may be attributed to the distress associated with having chronic disease and other life stressors. However, far too little attention has been paid to sexual life quality in diabetes apart from erectile dysfunctions. Pinhas-Hamiel et al. [34] in their study of 53 young adult male and female patients with T1DM (mean age 27.9 years) found out 35% of their patients fear of hypoglycemia during sex and 40% reported occasional mild hypoglycemic events during sexual practice. Every third participant in our study reported a negative influence of diabetes on his current sexual life. Patients who used free space to write text in our questionnaires often mentioned fear of hypoglycemia. The majority of our patients never discussed sexual problems with their physician. These findings confirm caregivers should actively inquire about fear of hypoglycemia and other sex-related difficulties in diabetic patients. This is the first study reporting a strong association between depression symptoms and decreased sexual quality of life in T1DM males. Further investigation is needed to estimate a cause-effect relationship. Greater emphasis should be placed on sexual quality assessment and early detection of depression in T1DM male patients.
Sexual dysfunctionsA sexual dysfunction is another aspect influencing sexual life quality and possibly reproduction. A number of researchers reported higher prevalence of erectile dysfunction in T1DM [9-11]. Our study contributed to this growing area of research by exploring the prevalence of erectile dysfunction in young (mean 33 years of age) Central European T1DM patients. In our study, the patients, who reported disturbed sexual life by diabetes in the personal questionnaire, had significantly lower erectile function score. These results provide further support for the hypothesis that erectile dysfunction has profound negative effect on T1DM patients’ quality of life. Lower erectile function in patients with diabetic nephropathy is difficult to explain given the cross-sectional design of the study. One possible explanation is microangiopathy, which is known to cause both erectile dysfunction and nephropathy. But we did not find any association between erectile dysfunction and the other microangiopathic complications. Another possible explanation is alteration in sex hormone levels in diabetic nephropathy [18], which may cause erectile dysfunction. To determine the cause-effect relationship between erectile dysfunction, nephropathy, and sex hormone alterations, additional prospective studies with a higher number of patients with diabetic nephropathy are needed.
We diagnosed at least 5 cases of retrograde ejaculation in the study group of 57 patients. Post-ejaculatory urine sample was not routinely examined in the study, so it may be reasonably assumed, prevalence of retrograde ejaculation was higher than documented. Consistent with this conclusion, a study by Fedder et al. [35] found that every third T1DM patient in their study group suffered from retrograde ejaculation, although none of their diabetic patients complained of anejaculation. Thus, this indicates that retrograde ejaculation is a more common and overlooked problem in young T1DM patients.
Sex hormonesWe opted to use the reference values provided by the manufacturers for each assay because this is a common laboratory procedure. These reference values were obtained from the large groups of healthy males and in case of the hormones affected by an age, we chose a subgroup of the similar age to our participants. The limitation of this approach is that we don’t know the exact anthropometric characteristics (i.e. BMI) of the healthy individuals in the manufacturer’s test group but we can reasonably assume these characteristics were similar to our patients as they were randomly selected from a healthy population.
Testosterone. The total testosterone level was not significantly changed in our group compared to healthy men but we found an association of testosterone and body mass index. These results substantiate previously published findings by Tomar et al. [17], who observed normal total testosterone in a group of 50 T1DM males with the average age of 42.8 years. Biswas et al. [6], investigated a group of 93 T1DM patients with the age mean of 45.5 years and 121 healthy controls (age mean 40.3). They found no significant change in the testosterone level between T1DM and controls and no correlation of the testosterone level with erectile function. Van Dam et al. [36] studied 52 T1DM patients (mean age 36.6) without microvascular complications and 53 controls matched in the age and BMI. They also reported the same total testosterone in T1DM patients as in the healthy controls. Our results confirm and extend the previous findings in the younger T1DM patients. Correspondingly to previous studies, we found no significant correlation of testosterone level and quality of sexual life or sexual dysfunction in T1DM patients. By contrast, Grossmann et al. [3] reported lower total testosterone levels in a cohort of 68 T1DM males (mean age of 45 years). This discrepancy could be attributed to higher BMI (mean 27 kg/m²) in their group. The association between testosterone and BMI is well documented in T2DM [4, 37] and it was also observed in T1DM in other studies [6, 17], including our own. Low total testosterone levels in T2DM and the patients with metabolic syndrome have been reported by a number of studies as reviewed in [38]. It has been hypothesized that low total testosterone in T2DM patients could be due to aromatase activity of adipose tissue, which would change testosterone to estradiol and subsequently suppress hypothalamo-hypophyseal-gonadal axis. However, this hypothesis was not supported by Dhindsa et al. [5], who found decreased instead of increased estradiol concentrations in T2DM patients with subnormal free testosterone concentrations. Thus, the pathogenesis of subnormal testosterone concentrations in T2DM is not clear. A comparison of different testosterone status in T1DM and T2DM suggests that testosterone level is not likely to be influenced by hyperglycemia, which is common for both diabetes types. High prevalence of erectile dysfunction and normal levels of testosterone in our subjects suggest that hypogonadism was not the dominant cause for presented erectile dysfunction.
SHBG is a transport protein, secreted in the liver that binds testosterone and affects its circulating level. SHBG concentrations in T2DM have been widely investigated. Meta-analysis conducted by Brand et al. [38] documented SHBG is lower in T2DM and metabolic syndrome patients, and SHBG levels negatively correlated with insulin resistance. In contrast, our study showed that SHBG levels in T1DM patients were significantly higher compared to healthy male controls. This is consistent with other T1DM studies [17, 36, 39]. Additionally, our study demonstrated, for the first time, the negative correlation between the mean daily insulin dose adjusted to body weight and SHBG. Previously, insulin was shown to have inhibitory effect on SHBG secretion by Pasquali et al. [40]. Recently, Boering et al. [41] postulated an influence of portal insulin concentrations on the SHBG production in the liver. In T1DM patients, portal insulin concentration is diminished, hence, it could conceivably be hypothesized that in absence of insulin, the liver production of SHBG is not inhibited and therefore, its blood levels increase. We did not identify any associations between SHBG increase and glycemic control, diabetes duration, or diabetic complications, therefore, it is unlikely SHBG increase could be mediated by hyperglycemia. Clinical consequences of increased SHBG in T1DM males remain to be investigated. Our results did not prove any association between SHBG levels and erectile function or sexual life quality.
FAI and CFT. Circulating testosterone is mostly bound to albumin, SHBG and other proteins, only a small portion remains free. It is assumed that unbound testosterone mediates the majority of its biological effects. Direct measurement of free testosterone is often impractical, and therefore, different calculations to estimate free testosterone have been adopted. Our calculations showed that FAI was significantly lower in our T1DM subjects compared to the reference values in healthy men. CFT was lower as well in our group of T1DM patients compared to reference limits proposed by Ho et al. [28]. Compared to our study, Ho et al. [28] used different method to determine SHBG levels in their group of healthy men. Similar results of decreased CFT in T1DM were also reported by the other authors [3, 36]. Our data indicate that T1DM has significant effect on the level of free testosterone. To determine cause and effect of low free testosterone in T1DM further investigation is needed.
Estradiol. Historically, estrogens have been associated with female reproductive functions, but over the past two decades it has been established that estrogens play an important role in male reproduction as well [42]. Lower estradiol concentration has been reported in T2DM males [5]. Serum estradiol concentration was inversely associated with carotid atherosclerosis in T2DM males, as determined by ultrasonographically evaluated intima-media thickness [43]. Despite these interesting findings, a very few studies have investigated estradiol levels in T1DM male patients. Maric et al. [18] investigated estradiol level in 101 T1DM males with normal renal function and 96 healthy controls. They found reduced levels of estradiol. We found a similar trend of lower estradiol concentration in T1DM males but this result was slightly above significance threshold (p = 0.058). Van Dam et al. [36] reported no significant change in estradiol level in T1DM patients. Further studies with higher number of T1DM male patients are needed to investigate estradiol level. Our finding of low estradiol in patients with low total testosterone might be attributed to decreased synthesis of estradiol from testosterone by aromatase activity.
LH and FSH. Pituitary gonadotropins are important for the initiation and maintaining of spermatogenesis. LH affects receptors on Leydig cells and enhances testosterone production. FSH interacts with Sertoli cells and, besides other effects, it mediates synthesis of the aromatase enzyme that converts testosterone to estradiol. LH secretion is inhibited by gonadal steroids [44]. Study of Van Dam et al. [36] reported higher LH in their group of 52 T1DM patients but this result did not reach statistical significance (p = 0.178). We found significantly higher concentrations of LH in T1DM patients. Higher levels of LH should be accompanied by low sex steroids in peripheral hypogonadism. Accordingly, we detected a significantly lower CFT, and free androgen index but no changes in total testosterone in our T1DM patients. These results may indicate LH secretion is stimulated by low free testosterone rather than low total testosterone. Our results confirm previous hypothesis of a tendency towards hypogonadism in T1DM male patients. FSH secretion is mainly regulated by estradiol in healthy men [44]. Our measurements showed that FSH concentrations were not significantly changed in T1DM males compared to healthy men. Correspondingly to unchanged FSH levels, our T1DM patients have normal levels of estradiol. Van Dam et al. [36] found slightly higher levels of FSH in a group of adult men with fairly controlled T1DM without complications. Ballester et al. [45] detected a decrease in FSH levels in streptozotocin-induced diabetic rats, and this decrease was followed by LH decline, reduced testosterone and impaired spermatogenesis. Lopez-Alvarenga et al. [46] concluded that the hypothalamic-pituitary axis was compromised in young men with very poorly controlled T1DM, leading to the disruption in LH secretion, central hypogonadism, and normal total testosterone concentrations. In contrast to our study, both previous reports analyzed the effects of poorly controlled glycemia on the hypothalamic-pituitary axis. Our study with T1DM patients with overall better glycemic control, on the other hand, demonstrated no association of LH concentration with glycemic control, diabetes duration or complications. These diverse data indicate that glycemic control of T1DM is probably an important factor in the production of pituitary gonadotropins and the regulation of the hypothalamic-pituitary axis.
Prolactin. The physiological function of prolactin in male reproduction is not clearly understood. Higher prolactin is known to cause hypogonadotropic hypogonadism in men, which is manifested by erectile dysfunction, impaired fertility, gynecomastia, and rarely galactorrhea [47]. Despite the profound negative influence of prolactin on the male reproduction, a larger study evaluating prolactin levels in T1DM male patients has not been performed. Our study is the first study involving a larger cohort of T1DM male patients, and clearly indicating negative effects of T1DM on prolactin levels. We detected significantly higher prolactin levels in our T1DM patients. In contrast, one report showed lower 24-hour prolactin release in 11 T1DM males [48]. However, compared to our study, the reported study involved a low number of patients with worse glycemic control. Thus, our data provide the first more comprehensive analysis of negative T1DM effects on prolactin levels.
PSA and HbA1c. PSA serum concentration reflects the amount of prostate glandular epithelium in men without prostate cancer. PSA blood concentration is often elevated in men with prostate cancer and it is used for screening prostate cancer. We detected a strong negative correlation between HbA1c and PSA. This finding is in accordance with previous reports in T1DM patients [49] and also in T2DM patients [50]. These results indicate that a cause of decrease in PSA levels is hyperglycemia, which is common for both T1DM and T2DM. Although mechanisms linking HbA1c to PSA level remain to be investigated, there are several possible implications of presented data. This phenomenon may affect prostate cancer screening in patients with T1DM, producing false negative results in early stages of prostate cancer. A similar possible mechanism was described in T2DM, where higher risk of positive prostate biopsy and higher odds of more aggressive disease were reported [51, 52]. Thus, these findings highlight the necessity to consider that diabetes may affect PSA levels and negatively influence prostate cancer screening in diabetic patients.
Our results indicate alterations in reproductive function of T1DM males on several levels. Firstly, we demonstrated impaired sexual life quality and high prevalence of sexual dysfunction. Secondly, we found significant alterations in the levels of calculated free testosterone, SHBG, LH and prolactin. And finally, we observed the correlation of PSA serum concentration with HbA1c. Overall, these psychological, physiological, and hormonal changes may lead to reproductive problems in T1DM patients. Our future work will focus on the impact of T1DM on the male reproduction, particularly on sperm quality.
The authors want to acknowledge the other physicians of The Department of Internal Medicine in Motol University Hospital in Prague who helped with patient recruitment and examination. This study was supported by Czech health research council (grant number: 15-30880A) and by The Department of Internal Medicine of Second Faculty of Medicine, Charles University in Prague and Motol University Hospital.
None of the authors have any potential conflicts of interest associated with this research.