2020 Volume 67 Issue 2 Pages 231-240
2020 Volume 67 Issue 2 Pages 231-240
Exposure to ionized radiation in childhood has been recognized as a risk factor for the development of thyroid cancer and possibly for other thyroid disorders. However, the effects of neonatal radiation exposure on thyroid morphology and functions have never been explored despite its potential importance. One-week-old male Wistar rats were subjected to cervical X-irradiation at 6 and 12 Gy. Animals were examined at the ages of 2, 8 and 18 weeks old. For comparison, 8-week-old rats were cervically X-irradiated at the same doses. Thyroid histology was examined by computer-assisted microscopy to measure areas of colloid and epithelium of thyroid follicles as well as epithelial heights. In rats that received cervical X-irradiation at 1 week old, the colloid size of thyroid follicles decreased at the age of 8 weeks old in a radiation-dose dependent manner. This morphological change was persistently found at 18 weeks old. There were no significant differences in serum total T3 or T4 levels among the groups. Serum TSH levels increased significantly in 8-week-old rats neonatally X-irradiated. Thyroglobulin (Tg) mRNA and protein expressions were significantly decreased in the neonatally-irradiated group while thyroid peroxidase mRNA express increased at 18 weeks old. None of these changes were observed in the rats X-irradiated at 8 weeks old. In conclusion, our results clearly demonstrated that neonatal rat thyroid was sensitive to ionized radiation, developing specific morphological changes characterized by smaller thyroid follicles along with changes in serum TSH levels and Tg expressions in the thyroid tissue.
AN ASSOCIATION between childhood radiation and an increased risk of thyroid cancer has been well established. Early investigations of neoplasms in those who were treated with X-rays in infancy suggested that radiation exposure in childhood increases the risk of thyroid cancer [1]. A study in rats exposed to cervical X-irradiation showed that neonatal rats were more susceptible to developing thyroid cancer than adult animals [2]. Moreover, the rapid increase in childhood thyroid cancer after the Chernobyl nuclear plant accident clearly demonstrated the risk of radiation exposure in childhood and adolescence [3, 4]. A cohort study of Japanese atomic bomb survivors also showed an excess thyroid cancer risk associated with childhood radiation exposure [5]. It has been suggested that a high mitotic rate of thyroid follicular cells in infancy would be responsible for the high prevalence of thyroid cancer [6]. However, the mechanisms of thyroid carcinogenesis caused by childhood radiation exposure have been rarely investigated in laboratory. Recently, age-dependent sensitivity of the thyroid to X-irradiation was examined in rats regarding the short-term effects on DNA damage response, apoptosis, and autophagy, suggesting a possible involvement of autophagy in the development of thyroid cancer [7].
The effects of radiation on thyroid function are less clear. Some studies reported that an increase in serum TSH levels was related to childhood exposure to 131I in the Chernobyl accident [8, 9], while other studies did not find any changes [10, 11]. In experimental studies, an early work demonstrated that whole body or cervical X-irradiation could change serum thyroid hormone levels in adult rats [12]. More recently, 6-week-old rats were subjected to cervical X-irradiation and their serum TSH levels found to be significantly elevated in 6 weeks [13]. Although these animal experiments suggested that irradiation of the thyroid gland could cause short- and long-term effects on serum hormone levels, there have been no reports of the effects of neonatal radiation on thyroid morphology and status. In the present study, one-week-old male Wistar rats were subjected to cervical X-irradiation. Thyroid histology including measurements of colloid and epithelial areas and epithelial heights, serum hormone levels, and protein and gene expression in thyroid tissue were examined afterward in comparison with rats irradiated at 8 weeks old.
Three-month-old pregnant Wistar rats were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan). The rats were at 16–18 days of gestation, which allowed 3–5 days before delivery. They were maintained with free access to a basal diet (MF, Oriental Yeast Co., Tokyo, Japan) and tap water. Animal rooms were maintained at 23.0°C ± 2.0°C with 50.0% ± 10.0% relative humidity and a 12-h light cycle. After delivery, male pups were selected and divided into five groups. Three groups (10 rats per group) were exposed to sham, 6, or 12 Gy of cervical X-irradiation at 1 week old, designated as Control, NeoX-6Gy, and NeoX-12Gy, respectively. The other two groups (five rats per group) received 6 or 12 Gy of cervical X-irradiation at the age of 8 weeks, and were designated as 8wX-6Gy and 8wX-12Gy, respectively. At the ages of 8 and 18 weeks old, animals were killed by whole blood removal from an abdominal artery under anesthesia, which was induced by intraperitoneal injection of an anesthetic mixture: 0.3 mg/kg of medetomidine, 4.0 mg/kg of midazolam, and 5.0 mg/kg of butorphanol. A separate animal experiment was set up to examine the effects one week after the neonatal radiation exposure. After delivery, male pups were divided into three groups (five rats per group), and received cervical X-irradiation at 1 week old, before being killed at 2 weeks old. Necropsies were performed between 13:00 h and 15:00 h to reduce any potential circadian-dependent variations in gene expression. Left lobes of the thyroid tissues were stored in RNA Save solution (Biological Industries, Cromwell, CT, USA) for RNA extraction and the other lobes were fixed in 10% formalin for histological examination. For western blot analysis, three groups (six rats per group) were exposed to sham, 6, or 12 Gy of cervical X-irradiation at 1 week old and killed at 8 weeks old. The thyroid tissues were immediately frozen in liquid nitrogen and stored at –80°C. The animal experiment was approved by the Animal Experiment Committee of Hiroshima University (document # A19-69) and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals at Hiroshima University.
X-irradiationIrradiation was performed with an X-ray irradiator, MBR-1520R-3 (Hitachi Medical Co., Tokyo, Japan), set at 150 kV and 20 mA with 0.5 mm aluminum and 0.1 mm copper filters. The dose rate was approximately 0.9 Gy/min. During irradiation, rats were under anesthesia as described above and placed the ventral side up at which X-irradiation was given. Two plates of lead of 2 mm thickness were used to cover each animal, leaving a gap for the neck region. The widths of the gap were 3 mm for 1-week-old rats and 6 mm for 8-week-old rats. For ‘sham irradiation’, rats were covered completely with lead plates and irradiated.
To estimate the radiation doses in the pituitary area and the abdominal area under the shield of lead plates, 1 and 8 week-old rats (3 rats each) were killed and glass dosimeters, GM-552M (AGC Techno Glass Co., Shizuoka, Japan) were placed in the neck region, the pituitary area inside the cranial cavity and the abdominal area. Rat bodies were covered with lead plates and X-irradiated as described above. After X-irradiation at 3 Gy (the measurement limit of GM-553M), the irradiated doses were recorded with a glass dosimeter reader, FGD-1000 (AGC Techno Glass Co.). The ratios of doses in the pituitary and abdominal areas over those at the unshielded neck area were calculated.
Histological analysisThyroid tissues were fixed in 10% formalin and embedded in paraffin. The 4-μm-thick sections were prepared and stained with hematoxylin and eosin (HE). Images of HE staining of the thyroid were captured with an HS All-in-one microscope BZ-9000 (Keyence Co., Osaka, Japan). At a magnification of ×200, different areas were randomly selected from each specimen (n = 2–5 per group). The 5 largest follicles from each area were chosen to be measured. In each thyroid follicle of the captured images, the areas of follicles and colloids, as well as the epithelial height, were measured using image analysis software BZ-II analyzer (Keyence Co.). The areas of epithelium were obtained subtracting colloid areas from follicular areas [14]. The ‘peripheral zone’ was defined as the area within 2 layers of follicles from the outer layer of the gland, otherwise it was defined as ‘central zone’ [15].
Quantification of mRNA levels by quantitative RT-PCRTotal RNA was prepared using Isogen II (Nippon Gene Co., Tokyo, Japan) from a piece of thyroid tissue stored in RNA Save solution. First strand cDNA was synthesized by incubating 2 μg total RNA with 200 U M-MLV reverse transcriptase (Toyobo Co., Osaka, Japan) with a mixture of 5 pmol oligo-(dT)15 primer/25 pmol of random primer pdN6 (Takara Bio Inc., Shiga, Japan). A quantitative PCR instrument, StepOnePlus (Applied Biosystems/Life Technologies Co., Carlsbad, CA, USA), was employed for the measurement of cDNA with a KAPA SYBR Fast qPCR Kit (Kapa Biosystems, Inc., Woburn, MA, USA). Specific primer sets were designed for rat thyroid peroxidase (Tpo), 5'-ATCACAGTTCTCCACGGATGC and 5'-TCTGTTGTTGCACACCCCTG; rat thyroglobulin (Tg), 5'-TGCCTACCAGGGCTACTTCT, and 5'-TCAGCTGCTTTCTGTGAGAAC; rat TSH receptor (Tshr), 5'-TACATCGCCCTTGTTCTCCTG, and 5'-GATCAACACGGCCATCCTCT). GenBank Accession numbers were NM_019353, AB035201, and NM_012888, respectively. Prior to quantitative analysis, the PCR products were prepared separately and purified by gel electrophoresis. The DNA sequences were confirmed using a capillary DNA sequencer (310 Genetic Analyzer; Applied Biosystems/Life Technologies Co.). The extracted fragments were used as standards for quantification. PCR conditions were as follows: 30 s initial denaturation followed by 40 cycles of 5 s at 95°C and 35 s at 60°C. The measured mRNA levels were normalized with reference to the levels of Actb mRNA [16].
Western blotThyroid tissues were homogenized in RIPA lysis buffer containing phosphatase inhibitors (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Two μg of each lysate were applied to 10% SDS-PAGE and transfer to Hybond-P membranes (GE Healthcare Ltd, Buckinghamshire, UK). After blocking with skim milk, they were incubated with anti-Tpo (MoAb47, 1:250, Santa Cruz Biotechnology Inc.), anti-TG (2H11 + 6E1, 1:400, Novus Biologicals, Centennial, CO, USA), or anti-GAPDH (3H12, 1:1000, MBL Co., Nagoya, Japan). They were washed and incubated with peroxidase-conjugated anti-mouse IgG (1:5000, MBL Co.). The protein bands were visualized with Chemi-Lumi reagents (Nacalai Tesque Inc., Kyoto, Japan) and image-captured with a CCD camera system, ImageQuant LAS 4000mini (GE Healthcare Ltd). The band intensities were quantified using ImageJ analysis software (http://imagej.nih.gov).
Statistical analysisAll values are expressed as mean ± standard error of the mean (SEM). For multiple comparisons, one way ANOVA was applied after the equality of variances was checked by F-test. Then, Tukey HSD test was performed to compare each radiation-exposed group with the control group. The R’s package ‘multcomp’ was used for calculation (http://cran.r-project.org).
Increases in body weight were steady in all groups. However, the body weights in neonatally X-irradiated groups were lower than the control. In the NeoX-12Gy group, the reduction in body weight became significant at 2 weeks after the irradiation and continued through to the end of the experiment, while it became evident in 13 weeks after the irradiation in the NeoX-6Gy group (Fig. 1a). Conversely, X-irradiation at 8 weeks of age did not significantly affect the subsequent growth rate even in the 8wX-12Gy group (Fig. 1b).

Changes in body and thyroid weights. Male Wistar rats cervically X-irradiated at doses of 0, 6, and 12 Gy at 1 week old (Control, NeoX-6Gy, and NeoX-12Gy) (a). Male Wistar rats cervically X-irradiated at 8 weeks old (Control, 8wX-6Gy, and 8wX-12Gy) (b). Absolute and relative thyroid weights at 8 and 18 weeks old in Control, NeoX-6Gy, and NeoX-12Gy groups (c, left) and at 18 weeks old in Control, 8wX-6Gy, and 8wX-12Gy groups (c, right) ; *p < 0.05, ** p < 0.01.
Absolute and relative thyroid weights were illustrated in Fig. 1c. Neonatal X-irradiation did not affect either absolute or relative thyroid weights at 8 weeks old. At 18 weeks old, the absolute thyroid weights decreased along with reduced body weights, but the relative thyroid weights were not significantly affected. X-irradiation at 8 weeks old did not cause any changes in thyroid weights at 18 weeks old.
Thyroid follicles in X-irradiated ratsAt 2 weeks old, the thyroid follicle structure was similar among groups (Fig. 2a). At 8 weeks old, however, the thyroid follicle sizes were evidently reduced with thickening and fusing of epithelial cells in the NeoX-12Gy group, while it was less prominent in the NeoX-6Gy group (Fig. 2b). The smaller thyroid follicles in the NeoX-6Gy and NeoX-12Gy groups remained similar at 18 weeks old (Fig. 2c). In addition, the variation in nucleus size seemed to increase in the NeoX-12Gy group (Fig. 2b, 2c).
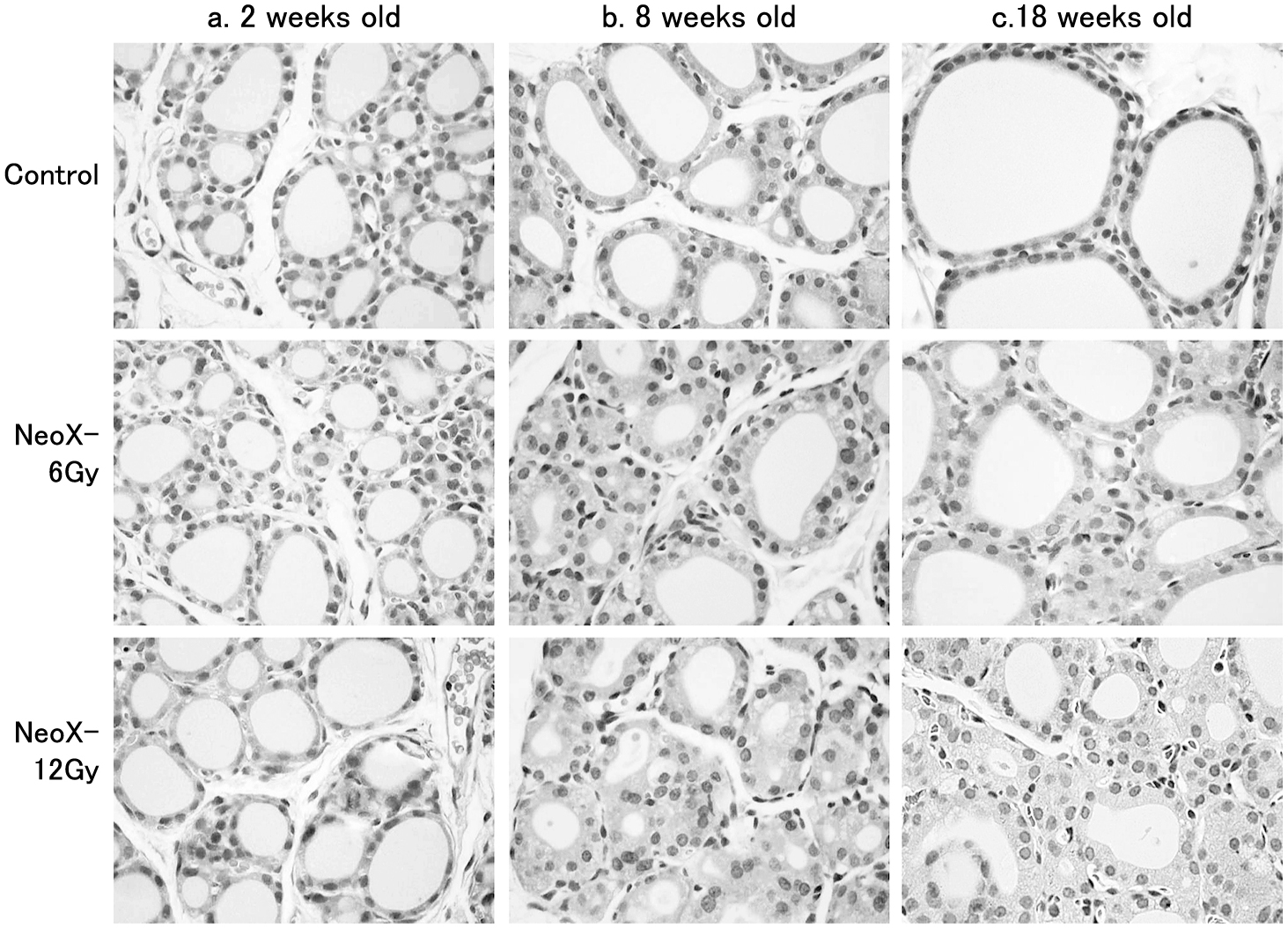
HE staining of the thyroid in rats cervically X-irradiated at 0, 6, and 12 Gy at 1 week old (Control, NeoX-6Gy, and NeoX-12Gy). Thyroid follicles at 2 weeks old (a), 8 weeks old (b) and 18 weeks old (c). Thyroid follicle size was evidently reduced with thickening of the epithelium in NeoX groups. The variation in nuclear size of the follicles was increased in NeoX groups.
The changes in follicular structure were further analyzed by measuring areas of thyroid colloids and epithelium with computer-assisted microscopy (Fig. 3a). Ratios of the epithelium to colloid areas were also calculated (Fig. 3b). The measurements were performed separately in the peripheral zone and the central zone of each thyroid gland. At 2 weeks old, there were no differences in areas in colloids or epithelium among the three groups. At 8 weeks old, the average peripheral colloid areas were significantly decreased to 47% of the control value in NeoX-12Gy, while colloid areas in the central zone did not decrease significantly. At 18 weeks old, both peripheral and central colloid areas reduced significantly to 51% and 52% of the control values, respectively, in NeoX-12Gy. Epithelium-colloid ratios significantly increased at 8 and 18 weeks old in NeoX-12Gy (Fig. 3b). Epithelial heights were also affected by neonatal cervically X-irradiation, which increased significantly at 8 weeks old in the NeoX-12Gy group (Fig. 4). It is noteworthy that colloid sizes increased with maturation; control peripheral colloid areas were 1.8 ± 0.1, 12 ± 1.7 and 22 ± 1.6 × 103 μm2 at 2, 8, and 18 weeks old, respectively, while central colloid areas were 1.4 ± 0.1, 4.5 ± 0.7 and 5.8 ± 0.4 × 103 μm2.

Colloid and epithelial areas and the ratios of thyroid follicles in rats cervically X-irradiated at 1 week old. Areas of colloid and epithelium of the follicles were measured in the peripheral zone (top) and the central zone (bottom) of the gland at 2, 8 and 18 weeks old (a). The ratios of epithelial areas to colloid areas were calculated (b); * p < 0.05, ** p < 0.01.

Epithelial heights of thyroid follicles in rats cervically X-irradiated at 1 week old. Epithelial heights were measured in the peripheral zone (top) and the central zone (bottom) of the gland at 2, 8 and 18 weeks old.
When rats were cervically X-irradiated at 8 weeks old, no changes in thyroid morphology, colloid and epithelial areas, epithelium-colloid ratios or epithelial heights were noted at 18 weeks old, 10 weeks after the irradiation (Fig. 5).

HE staining of the thyroid and histomorphological parameters of thyroid follicles in 18-week-old rats cervically X-irradiated at 8 weeks old. No morphological changes were observed in the thyroid (a). Areas of colloid and epithelium (b), the ratios (c) and epithelial heights (d) of the thyroid follicles were measured in the peripheral zone (top) and the central zone (bottom) of the gland at 18 weeks old.
Serum hormone levels are summarized in Table 1. At 2 weeks old, there were no significant changes in serum TT3, TT4, or TSH levels among the NeoX-6Gy, NeoX-12Gy, and control groups. At 8 weeks old, serum TSH levels were significantly increased over the control in the NeoX-12Gy group. At 18 weeks old, however, no differences in serum TT3, TT4, or TSH levels were noted among the groups. X-irradiation at 8 weeks old did not affect the serum hormone levels at 18 weeks old either (8wX-6Gy or 8wX-12G).
| Groups | Total T3 (ng/mL) | Total T4 (ug/dL) | TSH (ng/mL) |
|---|---|---|---|
| 2 weeks old | |||
| Control | 3.7 ± 0.10 | 9.0 ± 0.20 | 2.80 ± 0.54 |
| NeoX-6Gy | 3.9 ± 0.30 | 7.9 ± 0.63 | 3.40 ± 0.28 |
| NeoX-12-Gy | 4.3 ± 0.16 | 8.2 ± 0.62 | 3.80 ± 0.40 |
| 8 weeks old | |||
| Control | 4.0 ± 0.71 | 3.8 ± 0.42 | 0.80 ± 0.06 |
| NeoX-6Gy | 3.8 ± 0.45 | 3.4 ± 0.27 | 0.87 ± 0.04 |
| NeoX-12Gy | 3.1 ± 0.22 | 3.1 ± 0.19 | 1.06 ± 0.08* |
| 18 weeks old | |||
| Control | 3.2 ± 0.33 | 3.2 ± 0.28 | 1.09 ± 0.11 |
| NeoX-6Gy | 3.0 ± 0.39 | 3.4 ± 0.15 | 1.09 ± 0.05 |
| NeoX-12Gy | 3.0 ± 0.15 | 3.3 ± 0.18 | 1.26 ± 0.16 |
| 8wX-6Gy | 3.3 ± 0.30 | 3.7 ± 0.47 | 1.11 ± 0.07 |
| 8wX-12Gy | 3.4 ± 0.13 | 3.6 ± 0.38 | 1.28 ± 0.09 |
a) Each value shows mean ± SEM (n = 5)
b) * indicate significantly different from each contol level by p < .05 (*).
Table 2 summarizes mRNA levels of Tpo, Tg, and Tshr in the thyroid tissue. Tpo mRNA levels in NeoX groups were similar to the control at 2 weeks old, increased at 8 weeks old, and became significantly higher than the control at 18 weeks old. Tg mRNA expression was similar among the groups in 2-week-old animals but significantly decreased in the NeoX-6Gy and NeoX-12Gy groups at 8 weeks old. Tg mRNA levels were still low at 18 weeks old in the NeoX groups. There were no changes in Tpo or Tg mRNA levels in the 8wX-6Gy or 8wX-12Gy groups. No changes in thyroidal Tshr mRNA expression were noted among the groups in NeoX-6Gy and NeoX-12Gy groups.
| Groups | Tpo | Tg | Tshr |
|---|---|---|---|
| 2 weeks old | |||
| Control | 3.1 ± 0.24 | 13.5 ± 1.78 | 0.75 ± 0.05 |
| NeoX-6Gy | 3.2 ± 0.64 | 12.9 ± 0.11 | 0.76 ± 0.10 |
| NeoX-12Gy | 2.9 ± 0.19 | 12.3 ± 0.92 | 0.61 ± 0.06 |
| 8 weeks old | |||
| Control | 3.9 ± 0.69 | 34.6 ± 1.28 | 0.66 ± 0.07 |
| NeoX-6Gy | 5.7 ± 0.85 | 26.0 ± 3.0* | 0.75 ± 0.04 |
| NeoX-12Gy | 5.0 ± 0.21 | 25.4 ± 1.69** | 0.76 ± 0.07 |
| 18 weeks old | |||
| Control | 3.9 ± 0.27 | 35.9 ± 2.71 | 0.82 ± 0.13 |
| NeoX-6Gy | 5.1 ± 1.07 | 32.2 ± 5.51 | 1.12 ± 0.20 |
| NeoX-12Gy | 8.1 ± 0.60** | 22.3 ± 1.01** | 1.03 ± 0.03 |
| 8wX-6Gy | 4.4 ± 1.10 | 33.4 ± 2.64 | 0.83 ± 0.15 |
| 8wX-12Gy | 3.4 ± 0.62 | 38.0 ± 5.09 | 0.82 ± 0.06 |
a) Each value shows mRNA levels in fg/fg β-actin, mean ± SEM (n = 5)
b) * and ** indicate significantly different from each contol level by p < .05 (*) and p < .01 (**).
Fig. 6 showed the Tpo and Tg protein expressions in the thyroid at 8 weeks old in the NeoX-6Gy and NeoX-12Gy groups. Tpo protein expression was similar between groups, while Tg protein expression significantly decreased in both NeoX-6Gy and NeoX-12Gy.

Tpo protein and Tg protein expressions in the thyroid in 8 week-old rats cervically X-irradiated at 1 week old. Lysates extracted from the thyroid were examined by western blot ; *p < 0.05.
In 1-week-old rat bodies, the received dose ratios in the pituitary area and the abdominal area were 4.8 ± 0.4% and 2.8 ± 0.3%, respectively. In 8-week-old rats, they were 8.0 ± 0.4 and 2.7 ± 0.2%, respectively (n = 3, each group). Then, the estimated pituitary doses were 0.58 and 0.96 Gy in NeoX-12Gy and 8wX-12Gy groups, respectively.
The present study clearly demonstrated that the thyroid glands of one-week-old rats were more sensitive to X-irradiation than that of 8-week-old rats in terms of the effects on thyroid morphology, serum TSH levels and expression of thyroid function-related genes and proteins. These neonatal effects may be persistent for a long period of time since the changes found at 8 weeks old still remained at 18 weeks old.
It has been well established that exposure to radiation during infancy and childhood increases the risk of thyroid carcinoma in later life. Early investigations of neoplasms in persons treated with X-rays in infancy suggested an increased risk of thyroid cancer [1]. A study in a Marshall island population exposed to radioactive fallout from a nuclear test in 1954 also reported a high incidence of thyroid nodules among the young people who were then under 10 years old [17]. After the accident at the Chernobyl nuclear power plant, a rapid increase in the number of pediatric thyroid cancer cases was reported. An association between 131I exposure during childhood and thyroid cancer risk has been proven in Ukraine, Belarus, and other nearby regions [3, 4, 18]. Radiation-induced thyroid cancer was extensively studied in rodent models from the 1950s and through the 1970s [19, 20]. A study of rats with neck region X-irradiation demonstrated that neonatal rats (10 days old) have a higher susceptibility to developing radiation-induced thyroid tumors than adult rats [2]. In rodent models, the development of thyroid tumors is strongly associated with an increased level of serum TSH [21, 22]. When rats were treated with anti-thyroid drugs that increase serum TSH levels, they eventually develop thyroid follicular adenoma and carcinoma. Studies in rats fed an iodine-deficient diet in combination with a chemical carcinogen showed that TSH played a role mainly as a promoter for carcinogenesis [23]. However, in humans, the relationship between serum TSH levels and the development of thyroid carcinoma seems to be more complex, although serum TSH may have a role in the progression of differentiated thyroid cancer [24]. Contrasting with the evident correlation between childhood radiation exposure and an increase in thyroid cancer incidence, the effects of radiation on thyroid status are less clear. Belarusian and Ukrainian cohort studies suggested that an increase in serum TSH levels was related to childhood exposure to 131I [8, 9], while other studies did not suggest any relationship [10, 11]. In rodent models, an early study demonstrated that whole body or cervical X-irradiation could change serum thyroid hormone levels in adult rats [12]. More recently, in a study of 6-week-old Wistar rats exposed to X-rays, serum TSH levels were found to increase after the exposure [13]. Since epidemiological data regarding the radiation effects on thyroid function at younger ages are controversial, we investigated the effect of cervical radiation in neonatal rats compared with adult rats at 8 weeks old. Our results suggested the neonatal thyroid gland was indeed sensitive to radiation in terms of the effects on thyroid morphology, serum TSH levels, and expression of essential thyroid proteins, although relatively high dosages of X-irradiation were required. This is consistent with the epidemiological data of the Ukrainian cohort in which the positive relationship between childhood radiation and hypothyroidism was found only in those who received more than 5 Gy (ranging from 5 to 27 Gy) as the thyroid dose [9], which accounts only 0.9% of the total number of registered specimen. Since a previous study in rats showed that neonatal cervical irradiation at 3 Gy induced thyroid cancer in 15 months after the exposure [2], our current model probably develops thyroid cancer in the long-term. It would be worth investigating how the changes in thyroid morphology and function found in the present study can be related to the development of thyroid cancer in a future study.
The histological changes in the thyroid gland after neonatal X-irradiation are characterized by smaller-sized but still round-shaped follicles. These follicles are packed densely and sometimes fused each other, but clearly different from the diffused follicular hyperplasia typically induced by anti-thyroid chemicals [25]. In fact, there were no increases in thyroid weights in the Neo-X groups. In the thyroid gland, follicle size is not uniform and depends on the location in the gland: peripheral follicles are larger than central follicles [15, 26]. Then we performed the histomorphological analysis both in the peripheral zone and the central zone separately. In control group, the average area of peripheral colloids is about 2.8 × 103 μm2 at 2 weeks old, then increases to 12 × 103 and 22 × 103 μm2 at 8 and 18 weeks old, respectively, while central follicle areas were smaller as 1.4 × 103 μm2 at 2 weeks old and increased to 5.8 × 103 μm2 at 18 weeks old. Colloid areas in the NeoX groups were significantly smaller than the control at 8 and 18 weeks old. It appears that neonatal X-irradiation reduced the ‘growth’ of follicles or follicular colloids during the maturation of the thyroid gland from 1 to 18 weeks old, although it is unknown how the follicular colloid size is regulated in the thyroid. The effects of neonatal X-irradiation on the follicles were overall similar between peripheral and central zones. Histomorphological parameters in thyroid gland have been known as sensitive indicators for toxicological and pathological effects on the thyroid hormone homeostasis. When rats were exposed to thyroid toxicants to induce hypothyroidism, epithelial heights increase probably responding to stimulation of TSH [14]. In the present study, the epithelial heights were significantly increased only at 8 weeks old in NeoX-12Gy group with higher serum TSH, which may suggest that the epithelial height is a reversible parameter being able to respond to serum TSH.
Although rats were cervical X-irradiated, the head area also received considerable doses of radiation. The estimated doses in the pituitary area were 0.58 and 0.96 Gy in NeoX-12Gy and 8wX-12Gy, respectively. These levels of radiation, however, do not affect the pituitary, since the studies examining the effect of brain radiation in newborn rats demonstrated that even the irradiation at 5 Gy did not cause any changes in body weight or histopathology of the brain, while the newborn rats at ages of 2 days or younger were more sensitive [27, 28]. A recent study examining the effect of radiation on the pituitary function found that more than 20 Gy of X-irradiation was required to change the hormonal contents in the pituitary [29]. Then the functional change in the thyroid, as well as the decrease in body weight gain found in the present study, were probably caused by the effect of X-irradiation primarily on the thyroid and then through the pituitary-thyroid axis, although the mechanisms are yet to be analyzed.
It has been demonstrated that mRNA and protein expressions of Tg and Tpo in the thyroid gland increased in rats treated with a thyroid toxicant [30]. We found decreases in mRNA expression of Tg at 8 and 18 weeks old in the NeoX-12Gy group, while Tpo mRNA expression increased at 18 weeks old, which may indicate the change in the thyroid function. Although protein levels were only determined at 8 weeks old, we confirmed Tg protein expression was indeed decreased together with the mRNA level. Expression of Tshr mRNA may reflect the endocrine and molecular status of the thyroid [31], but no significant changes were found. Neonatal X-irradiation may directly act on the thyroid tissue to induce these morphological and functional changes, but it may affect the development of the pituitary–thyroid axis during the neonatal period. In rats, it has been reported that disruption of thyroid hormone status during the neonatal period can cause permanent impairment of thyroid hormone homeostasis [32, 33]. For instance, a single administration of a high dose of T4 in rats during the first week of life leads to a decrease in both body weight and serum T4 levels throughout life [34]. These alterations occurred probably because the development of the pituitary–thyroid axis was disrupted. Similar to these neonatal thyroid hormone disruptions, increases in body weight were significantly suppressed by neonatal X-irradiation. Lately there have been many concerns regarding the possible adverse effects of neonatal exposure to endocrine-disrupting chemicals such as tetrachlorodibenzo-p-dioxin and polybrominated diphenyl ethers [35, 36]. In a similar way, neonatal X-irradiation may disrupt neonatal thyroid function and affect the development of the thyroid axis including the hypothalamus, the pituitary, and the liver. Further studies are needed to understand the mechanisms of the higher sensitivity of the neonatal thyroid to radiation.
We thank Mr. Shingo Sasatani for his excellent technical help measuring radiation doses with glass dosimeters, and H. Nikki March, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This research was partially supported by a Joint Research Grant from the network-type joint Usage/Research Center for Radiation Disaster Medical Science of Hiroshima University, Nagasaki University, and Fukushima Medical University.
None of the authors have any potential conflicts of interest associated with this research.