2021 年 68 巻 2 号 p. 195-200
2021 年 68 巻 2 号 p. 195-200
A silent pituitary adenoma (SPA) is characterized by the expression of pituitary hormones, detected by immunohistochemical staining, in the absence of clinical signs or symptoms of hormonal excess. Compared with functional pituitary adenomas, little is known regarding the involvement of SPAs in metabolic disorders. This study aimed to examine the correlations between SPAs and metabolic disorders, including obesity, abnormal glucose tolerance, hypertension and dyslipidemia. Seventy-four patients with nonfunctional pituitary adenomas who underwent a pituitary adenomectomy in Hokkaido University Hospital from 2008 to 2016 were retrospectively examined. Pituitary adenomas were immunohistochemically classified into pituitary hormone positive or negative groups. Twenty whole hormone-negative pituitary adenomas were excluded because we couldn’t identify pituitary transcription factors which is necessary for the diagnosis of a null cell adenoma. The preoperative rates of obesity, abnormal glucose tolerance, hypertension and dyslipidemia were compared between each group. Twenty-seven GH positive adenomas (50.0%), 32 gonadotroph positive adenomas (59.3%), 28 TSH positive adenomas (51.9%) and 21 ACTH positive adenomas (38.9%) were identified. Evaluation of the preoperative clinical data showed 25 cases of obesity (46.2%), 16 cases of abnormal glucose tolerance (29.6%), 29 cases of hypertension (53.7%) and 35 cases of dyslipidemia (64.8%). The rate of hypertension was significantly lower in the GH positive group (37.0%) than the GH negative group (70.4%) (p = 0.0140). In the GH negative group, postoperative systolic and diastolic blood pressure levels were significantly lower than preoperative values. GH positive SPAs may affect the homeostasis of blood pressure.
A SILENT PITUITARY ADENOMA (SPA) is defined as a pituitary adenoma that does not secrete enough anterior pituitary hormone products to cause an elevation of the serum concentration or result in clinical signs or symptoms of hormone hypersecretion, even though the expression of these hormones is detected by immunohistochemistry [1]. They are classified into silent somatotroph adenomas, silent gonadotroph adenomas, silent thyrotroph adenomas, silent corticotroph adenomas (SCAs) and silent lactotroph adenomas. In contrast, a pituitary adenoma with negative for pituitary hormones is termed a null cell adenoma. Regarding tumor characteristics, SCAs are generally macroadenomas and tend to be more aggressive with a higher chance of hemorrhage and invasion [2].
As for functional pituitary adenomas, the standardized mortality ratio is significantly worse for patients with persistent Cushing’s disease. In addition, hypertension, diabetes mellitus and hypercortisolism significantly contribute to mortality [3]. Several studies have investigated the correlation of GH or Insulin-like growth factor-1 (IGF-1) to comorbidities in patients with treated acromegaly, which revealed significant associations between increased GH burden and impaired glucose tolerance, diabetes mellitus and ischemic heart disease [4]. We previously reported that glucose tolerance was improved following an adenomectomy in patients with a silent somatotroph adenoma [5]. However, unlike functional pituitary adenomas, little is known about the involvement of SPAs in metabolic disorders, including obesity, abnormal glucose tolerance, hypertension and dyslipidemia. Furthermore, there is no definite standard care or surgical indications for SPAs. Thus, to explore the treatment strategy, this study analyzed the correlations of SPAs and markers of the metabolic disorders.
Patients with a clinically non-functioning pituitary adenoma who underwent an adenomectomy from 2008 to 2016 were retrospectively examined. All participants were admitted to Internal Medicine II at Hokkaido University Hospital for endocrinological examinations. The selection criteria were i) over 20 years old, ii) surgery performed to remove pituitary adenomas and iii) the surgical specimens immunostained. Patients who had overt functional pituitary adenomas or those with incomplete or inconsistent data excluded. The protocol used in this study and opt-out consent procedure were approved by the institutional review board of Hokkaido University Hospital (017-0120) and adhered to the provisions of the Declaration of Helsinki. The clinical examination of patients included documentation of their medical history, physical examinations, anthropometric measurements, blood tests and Magnetic resonance imaging scans. Blood samples and blood pressure measurements were collected from patients at rest and in the fasting state.
Immunohistochemical staining was performed using an automated staining machine (DAKO Autostainer Link 48, Santa Clara, USA). The following primary antibodies were used: anti-GH (polyclonal, 1:4000, DAKO), anti-PRL (monoclonal, 1:1000, Thermo, Waltham, USA), anti-ACTH (monoclonal, 1:1000, DAKO), anti-TSH (monoclonal, 1:400, Thermo), anti-FSH (monoclonal, 1:1000, Thermo), and anti-LH (monoclonal, 1:200, DAKO).
Clinical evaluationNon-functioning pituitary adenomas were confirmed by a medical examination and endocrinological evaluation based on the rapid ACTH loading test, growth hormone-releasing peptide-2 loading test, and CRH-TRH-LHRH loading test performed by several endocrinologists at Hokkaido University Hospital. All endocrinological tests were conducted when patients were at rest and in the fasting state. We classified pituitary adenomas into pituitary hormone positive and negative groups according to the results of the immunohistochemical staining. Whole hormone-negative pituitary adenomas were excluded because we couldn’t identify pituitary transcription factors which is necessary for the diagnosis of a null cell adenoma [6]. The preoperative rates of obesity, abnormal glucose tolerance, hypertension and dyslipidemia were compared between each group. These metabolic disorders were evaluated according to the patient’s medical records obtained during their preoperative hospitalization in our department and postoperative visits at an outpatient clinic. Each parameter was defined as follows: obesity = body mass index (BMI) ≥25 kg/m2 [7], abnormal glucose tolerance = either diabetes mellitus, impaired glucose tolerance, or impaired fasting glucose [8], hypertension = blood pressure ≥140/90 mmHg [7] or taking an antihypertensive drug, and dyslipidemia = either LDL cholesterol ≥140 mg/dL, HDL cholesterol <40 mg/dL, triglycerides level ≥150 mg/dL [7], or taking a lipid-lowering drug. Postoperative data were collected from a week to several months after surgery, and antihypertensive drugs were not added during the study period.
As we state later in the Results section, a significant difference was found in the preoperative rate of hypertension between the GH positive and negative groups, so we focused on these groups. We defined adrenal insufficiency as patients with a baseline cortisol level <4 μg/dL or who were taking hydrocortisone before surgery. We compared the rate of adrenal insufficiency between GH positive and GH negative groups.
Statistical analysisThe results were expressed as the mean ± S.D or median and interquartile range. A paired t-test was used for comparing pre- and post-operative values in the GH positive and negative groups. Significant differences between the means of the GH positive and negative groups were assessed with a Student’s t-test, or the Mann-Whitney U test when the data did not have a normal distribution. Expression data for each immunohistochemical marker were transformed into binary categorical variables (0 = negative and 1 = positive expression), and a chi-squared test was used to compare the percentage of each metabolic disorder between the hormone positive and negative groups. A multiple logistic regression model yielding odds ratios (ORs) and 95% confidence intervals (CIs) was used to identify the predictors of hypertension. The model included several independent variables, such as age, sex, BMI and the results of anterior pituitary hormone immunostaining. A p value <0.05 was considered statistically significant. We performed statistical analyses using JMP pro13 (SAS Institute Inc., Cary, NC, USA).
Seventy-four patients were recruited in this study, and 54 patients were identified as either pituitary hormone positive. The evaluation of preoperative clinical data showed 25 cases of obesity (46.2%), 16 cases of abnormal glucose tolerance (29.6%), 29 cases of hypertension (53.7%) and 35 cases of dyslipidemia (64.8%) (Table 1). In addition, 27 GH positive adenomas (50.0%), 32 gonadotroph positive adenomas (59.3%), 28 TSH positive adenomas (51.9%), 21 ACTH positive adenomas (38.9%), 9 PRL positive adenomas (16.7%) were identified (Table 2). The rate of hypertension was significantly lower in the GH positive group (37.0%) than the GH negative group (70.4%) (p = 0.0140) (Fig. 1). No significant differences were found in the rates of obesity, abnormal glucose tolerance, nor dyslipidemia between the pituitary hormone positive and negative groups. Furthermore, following multiple logistic regression analyses, GH positivity was identified as an independent factor for hypertension after adjusting for age, sex, BMI, and the results of pituitary hormone immunostaining (OR 0.18; 95% CI, 0.04–0.79; p = 0.0229) (Table 3). We also divided pituitary hormones into GH/PRL/TSH, LH/FSH and ACTH based on the transcription factors for pituitary cell lineages suggested in the 2017 World Health Organization guidelines [6]. However, no significant differences were found in the rates of obesity, abnormal glucose tolerance, hypertension or hyperlipidemia between positive and negative groups of the pituitary hormone lineages (Table 4). We next compared the baseline data between the GH positive and negative groups. Other than blood pressure, there were no significant differences (Table 5). The postoperative hypertension rate showed no significant difference between the GH positive (33.3%) and GH negative (46.2%) groups (p = 0.3401) (Table5).
| n | 54 |
| Age (years) | 59.4 ± 12.1 |
| Female (n(%)) | 28 (51.8) |
| BMI (kg/m2) | 24.8 (22.0–26.7) |
| Systolic blood pressure (mmHg) | 128.8 ± 16.8 |
| Diastolic blood pressure (mmHg) | 79.5 ± 11.1 |
| T-Chol (mg/dL) | 205.0 ± 51.2 |
| TG (mg/dL) | 149.0 (95.0–197.0) |
| LDL-Chol (mg/dL) | 118.8 ± 42.8 |
| HDL-Chol (mg/dL) | 54.5 (45.3–67.5) |
| FPG (mg/dL) | 101.0 (92.0–109.0) |
| HbA1c (%) | 5.7 (5.5–6.0) |
| Abnormal glucose tolerance (n(%)) | 16 (29.6) |
| Hypertension (n(%)) | 29 (53.7) |
| Dyslipidemia (n(%)) | 35 (64.8) |
| Obesity (n(%)) | 25 (46.2) |
| GH positive ((n(%)) | 27 (50.0) |
| LH positive ((n(%)) | 14 (25.9) |
| FSH positive ((n(%)) | 31 (57.4) |
| TSH positive ((n(%)) | 28 (51.9) |
| ACTH positive ((n(%)) | 21 (38.9) |
| PRL positive ((n(%)) | 9 (16.7) |
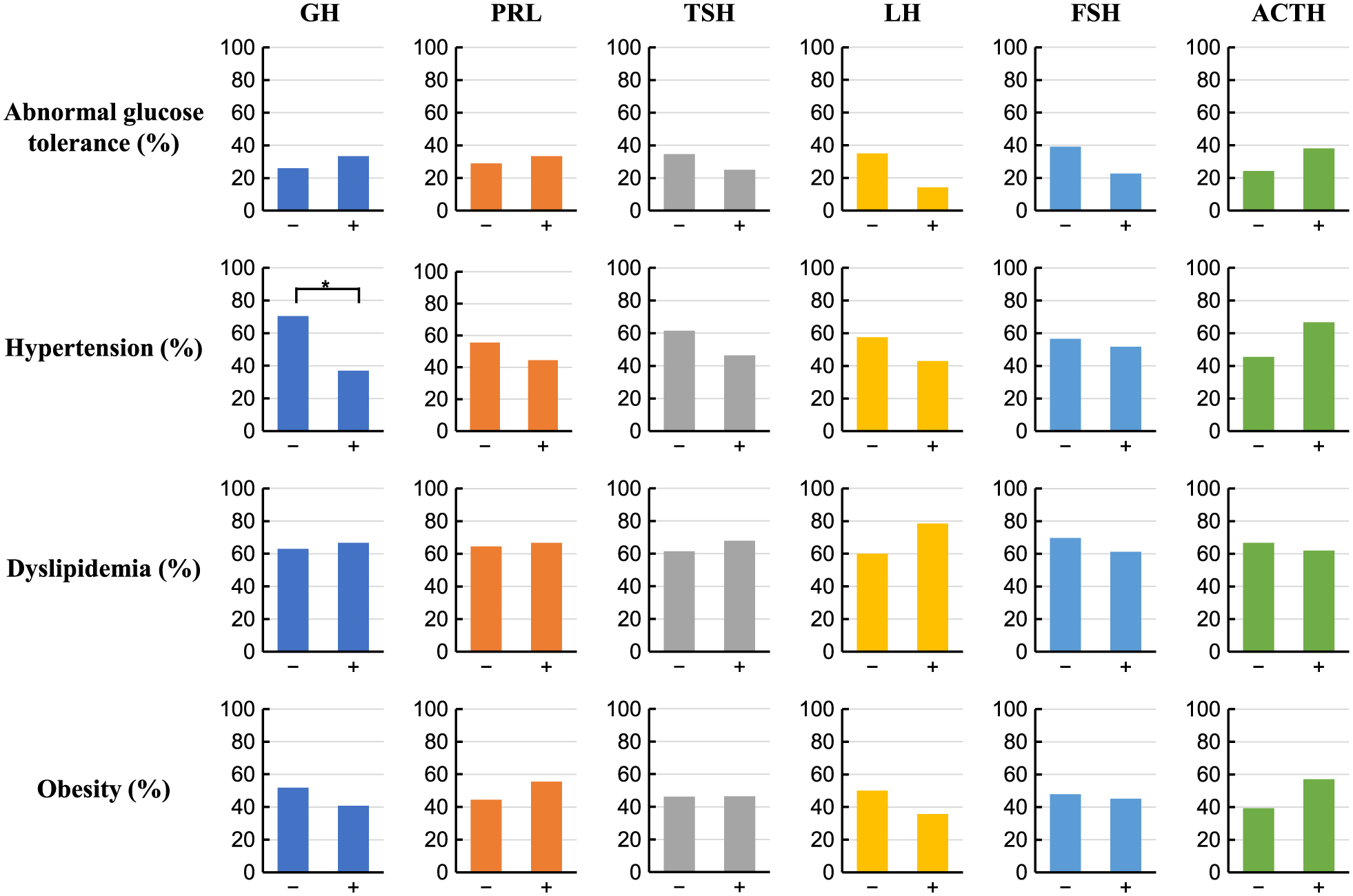
Comparison of the rates of abnormal glucose tolerance, hypertension, dyslipidemia and obesity between the pituitary hormone positive and negative groups. *: p < 0.05, chi-squared test.
| Odds ratio | 95% Confidence interval | p value | |
|---|---|---|---|
| GH positive | 0.18 | 0.04–0.79 | 0.0229 |
| GH/PRL/TSH negative | GH/PRL/TSH positive | p value | LH/FSH negative | LH/FSH positive | p value | ACTH negative | ACTH positive | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Abnormal glucose tolerance (%) | 26.7 | 30.8 | 0.7674 | 40.9 | 21.9 | 0.1323 | 24.2 | 38.1 | 0.2771 |
| Hypertension (%) | 73.3 | 46.2 | 0.0728 | 54.6 | 53.1 | 0.9181 | 45.5 | 66.7 | 0.1275 |
| Dyslipidemia (%) | 53.3 | 69.2 | 0.2732 | 68.2 | 62.5 | 0.6675 | 66.7 | 61.9 | 0.7209 |
| Obesity (%) | 66.7 | 38.5 | 0.0626 | 45.5 | 46.9 | 0.9181 | 39.4 | 57.1 | 0.2023 |
| GH negative | GH positive | p value | |
|---|---|---|---|
| n | 27 | 27 | |
| Age (years) | 60.1 ± 12.3 | 58.7 ± 12.2 | 0.6743 |
| Female sex (%) | 55.6 | 48.2 | 0.5860 |
| Body mass index (kg/m2) | 25.0 (21.7–26.9) | 24.7 (22.1–26.0) | 0.9112 |
| Systolic blood pressure (mmHg) | 133.4 ± 17.7 | 124.1 ± 14.9 | 0.0423 |
| Diastolic blood pressure (mmHg) | 79.8 ± 12.1 | 79.3 ± 10.3 | 0.8567 |
| Total Cholesterol (mg/dL) | 199.0 (179.0–221.0) | 205.0 (183.0–265.5) | 0.3014 |
| Triglyceride (mg/dL) | 138.5 (90.0–172.8) | 156.0 (96.0–266.0) | 0.1730 |
| LDL Cholesterol (mg/dL) | 121.6 ± 31.9 | 116.7 ± 50.2 | 0.6814 |
| HDL Cholesterol (mg/dL) | 60.0 (51.0–68.0) | 49.0 (42.0–68.0) | 0.9238 |
| Fasting plasma glucose (mg/dL) | 99.5 (92.3–107.0) | 103.0 (91.5–111.5) | 0.1923 |
| HbA1c (%) | 5.7 (5.4–6.0) | 5.8 (5.7–6.1) | 0.9289 |
| Adenoma size (mm) | 28.5 (22.8–37.5) | 30.0 (23.5–47.0) | 0.3313 |
| Adrenal insufficiency (%) | 37.0 | 25.9 | 0.3794 |
| Insulin-like growth factor-1-SD score | –0.9 ± 1.7 | –0.9 ± 1.9 | 0.8572 |
| GH (ng/mL) | 0.2 (0.1–0.3) | 0.2 (0.1–0.4) | 0.8871 |
| Preoperative Hypertension rate (%) | 70.4 | 37.0 | 0.0140 |
| Postoperative Hypertension rate (%) | 46.2 | 33.3 | 0.3401 |
In the GH negative group, the postoperative baseline level of cortisol (6.7 ± 4.7 μg/dL) was significantly lower than the preoperative value (8.9 ± 4.7 μg/dL) (p = 0.0155). Also, in the GH negative group, both postoperative systolic blood pressure (120.6 ± 14.3 mmHg) and diastolic blood pressure (73.4 ± 12.0 mmHg) were significantly lower than the preoperative measurements (p = 0.0003 and p = 0.0178, respectively) (Fig. 2).
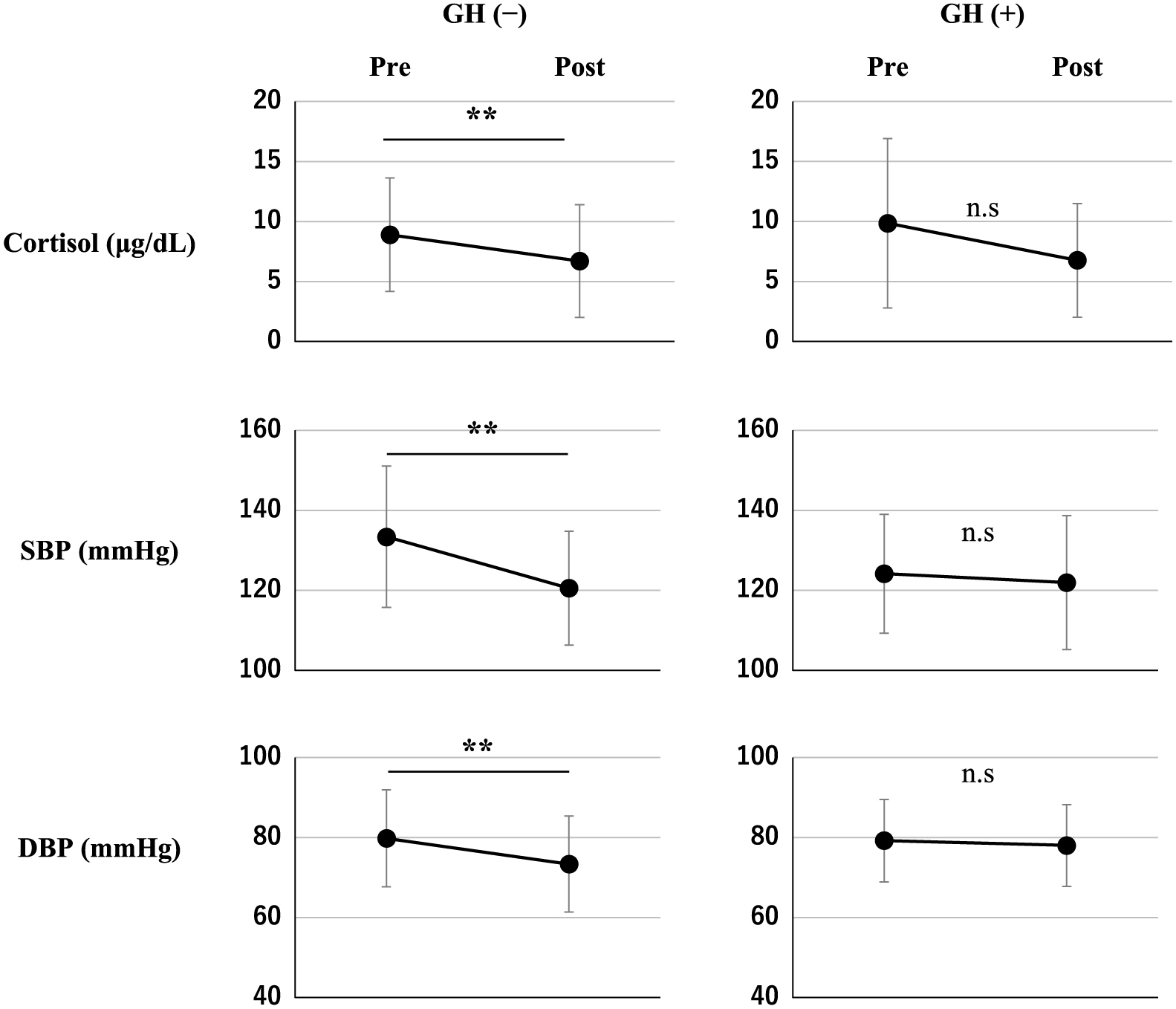
Baseline cortisol, systolic blood pressure (SBP), and diastolic blood pressure (DBP) at pre-and post-operation in the GH negative and positive groups. **: p < 0.01, n.s, not significant, paired t-test.
In this study, we showed that the rate of preoperative hypertension was significantly lower in the GH positive group than the GH negative group, but this difference was not sustained in the postoperative evaluation. In the GH negative group, the postoperative baseline cortisol and blood pressure levels were significantly lower compared with the preoperative measurements. Postoperative systolic blood pressure and diastolic blood pressure were significantly lower than those of pre-operation in the GH negative group even if we excluded nine patients with subclinical Cushing’s disease, suggesting the result did not be affected by improvement of subclinical Cushing’s disease.
In a previous report on silent acromegaly, three patients did not have substantially elevated serum GH levels and showed no clinical signs nor symptoms indicative of acromegaly, even though their tumor cells exhibited ultrastructural features of sparsely granulated somatotroph adenoma cells, secreted GH in vitro, and appropriately responded to stimulatory and inhibitory substances [9]. These tumors may produce more than one form of GH that is immunoreactive but lacks any biologic activity [9, 10]. These reports suggest that “silent” pituitary adenomas are not always be silent and may sometimes secrete a small amount of hormones.
The mechanism underlying the difference in blood pressure between the GH negative and positive groups is unclear. In our study, the postoperative cortisol level was less than the preoperative level in the GH negative group and unchanged in the GH positive group, suggesting that cortisol might play a role in the underlying mechanism. GH is an inhibitor of the glucocorticoid activation enzyme 11β-hydroxysteroid dehydrogenase type 1 [11]. Therefore, the rate of preoperative hypertension might be lower in the GH positive group because of a decrease in glucocorticoid activity. In the GH negative group, the baseline cortisol and blood pressure levels would decrease following a pituitary adenomectomy, whereas these would not change in the GH positive group because the preoperative effect of GH would disappear and the postoperative baseline of cortisol would be neutralized. Although blood pressure did not change significantly in the GH positive group during the observed period, the change in GH status after operation might change the homeostasis of blood pressure in the longer term. Therefore, clinicians need to monitor blood pressure carefully after removal of a SPA with positive GH immunostaining. However, this mechanism contradicts acromegaly, which involves the hypersecretion of GH and is often complicated by hypertension. The cause of the lower rate of hypertension in patients with GH positive SPAs remains to be elucidated, and thus further investigation is required.
This study had several limitations. First, the definition of positive immunohistochemical staining is broad and ranges from weakly positive to 80% positive in pituitary specimens. Second, we did not quantify cortisol levels by urine storage or methods other than collecting baseline data, which may be influenced by other factors, such as stress. Finally, the period from adenomectomy to postoperative endocrinological examinations varied.
In conclusion, the presence of a GH positive SPA may affect the homeostasis of blood pressure, providing the idea in one of the managing strategies in patients with SPA. Further large prospective studies will confirm our observation in future.
We thank Melissa Crawford, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript. We also thank Naoki Nishimoto, PhD, from Clinical Research and Medical Innovation Center of Hokkaido University Hospital for giving advices regarding statistical analysis.
None of the authors have any potential conflicts of interest associated with this research.