2021 年 68 巻 7 号 p. 791-805
2021 年 68 巻 7 号 p. 791-805
Somatostatin analogs are recommended for pharmacotherapy of TSH-secreting pituitary adenoma (TSHoma). A multicenter clinical trial was conducted to evaluate the efficacy and safety of lanreotide autogel treatment for TSHoma. A total of 13 Japanese patients with TSHoma were enrolled from February to December 2018 and treated with lanreotide autogel 90 mg every 4 weeks, with dose adjustments to 60 mg or 120 mg. Analysis was performed on data from patients receiving preoperative treatment (n = 6) up to 24 weeks and from those receiving primary or postoperative treatment (n = 7) up to 52 weeks. The primary efficacy endpoints were serum concentrations of TSH, free triiodothyronine (FT3), and free thyroxine (FT4). The secondary efficacy endpoints were pituitary tumor size and clinical symptoms. The serum concentrations of TSH, FT3, and FT4 decreased with treatment, and euthyroid status was maintained until final assessment. FT4 at final assessment was within reference ranges in 10/13 patients. The median (interquartile range) percent change in pituitary tumor size from baseline at final assessment was –23.8% (–38.1, –19.8). The clinical symptoms were also improved. The patients receiving preoperative treatment did not develop perioperative thyroid storm. Regarding safety, adverse events were observed in 12/13 patients, but none discontinued treatment. The common adverse events were gastrointestinal disorders (12/13 patients) and administration site reactions (5/13 patients). Lanreotide autogel may be effective for controlling thyroid function and reducing the pituitary tumor size, and is tolerable in patients with TSHoma (Japic Clinical Trials Information; JapicCTI-173772).
TSH-SECRETING PITUITARY ADENOMA (TSHoma) has an incidence of 0.26 per million population/year [1], accounting for 1–2% of all pituitary adenomas [2], and is a rare cause of hyperthyroidism. Excessive secretion of TSH from the tumor causes the syndrome of inappropriate secretion of TSH (SITSH), which is a condition of normal or elevated levels of TSH with high levels of thyroid hormones: free triiodothyronine (FT3) and free thyroxine (FT4). The clinical symptoms associated with TSHoma include palpitations, tachycardia, hyperhidrosis, weight loss, and diffuse goiter, and the pituitary tumor may cause headache and visual impairment [3].
The first-line treatment of TSHoma is pituitary adenomectomy, and medical therapy is indicated depending on the patient’s clinical conditions [3, 4]. Patients with hyperthyroidism have an increased risk of developing thyroid storm due to stress such as surgery [5], and patients with TSHoma are also at risk [6, 7]. To avoid the risk of thyroid storm, preoperative medical therapy is necessary to control thyroid function [6, 7]. Medical therapy is also selected for patients with TSHoma who cannot undergo surgery or who did not achieve remission after surgery. The aim of pharmacotherapy for TSHoma is to control both thyroid function and pituitary tumor size, and administration of long-acting somatostatin analogs (SSAs), such as lanreotide and octreotide, is generally recommended [4]. However, a limited number of clinical trials investigating these drugs for TSHoma have been conducted [8-10]. In Japan, observational studies on octreotide were reported [11-13]. Pharmacotherapies for TSHoma had been eagerly expected in clinical practice [14].
Lanreotide exerts its effects on TSHoma by suppressing hormone secretion and tumor proliferation via binding to somatostatin receptors (SSTRs), especially the SSTR2 subtype [15]. In most TSHomas, the tumor cells are strongly positive for SSTR2 [16, 17]. Two formulations of lanreotide have been used to treat TSHoma [4]: lanreotide prolonged release (LAN-PR, Somatuline® LA) for intramuscular injection every 7 to 14 days [18], and lanreotide autogel (LAN-ATG, Somatuline® Autogel®), which is a sustained-release, supersaturated formulation of the lanreotide and is supplied in ready-to-use, pre-filled syringes, for deep subcutaneous injection every 4 weeks [19, 20]. The use of LAN-PR for TSHoma has been approved in 17 countries [18] based on a clinical trial conducted in Europe [8]. Although no clinical trials have evaluated LAN-ATG for TSHoma, it has been approved only in the Netherlands [21]. In Japan, LAN-ATG (Somatuline®) has been approved for the treatment of acromegaly, pituitary gigantism, and gastroenteropancreatic neuroendocrine tumors [22]; however, to date there have been no clinical trials to investigate this drug for the treatment of TSHoma. Therefore, this clinical trial aimed to evaluate the efficacy and safety of LAN-ATG in patients with TSHoma.
This was a multicenter, single-arm, dose-adjustment, preoperative short- or long-term phase 3 study conducted in Japan (clinical trial registry: Japic Clinical Trials Information; JapicCTI-173772). The study was conducted in accordance with the ethical principles of the Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the institutional review board at each study site. Written informed consent was obtained from each patient before enrollment.
Patients 1) aged 20 years or older, 2) presenting with SITSH and having a pituitary tumor on diagnostic imaging [23], and 3) requiring pharmacotherapy for TSHoma (unable to undergo surgery, having a postoperative residual tumor, or requiring control of thyroid function before surgery) were included in the study. Patients who received octreotide subcutaneous injection within 1 week, had sustained release-SSA injection within 8 weeks, or underwent surgery (within 8 weeks) or radiation therapy (within 52 weeks) for pituitary tumor were excluded.
Study treatmentPatients received a preoperative treatment (hereafter, preoperative LAN-ATG) or a primary or postoperative long-term treatment (hereafter, long-term LAN-ATG); for both treatments, lanreotide ATG 60 mg, 90 mg, or 120 mg was administered by deep subcutaneous injection in the buttocks every 4 weeks (Fig. 1). The investigators adjusted the dose of LAN-ATG based on the following criteria: 1) starting dose was 90 mg, which is the same dose for the treatment of acromegaly [22], 2) the dose was decreased by 30 mg to the dose of 60 mg if a clinically relevant adverse event (AE) occurred, and 3) the dose could be increased by 30 mg to the dose of 120 mg after Week 16 if the effect was insufficient (serum FT4 > the upper limit of the reference range or symptoms of thyrotoxicosis).

Study design
The treatment period of preoperative LAN-ATG was 3 weeks (single injection) to 24 weeks (7 injections) prior to surgery. For long-term LAN-ATG, data from the treatment period of 52 weeks were analyzed.
LAN-ATG, lanreotide autogel.
Study treatment was discontinued if a pituitary adenoma progressed without any beneficial effects of LAN-ATG, an unacceptable AE, or patient consent withdrawal occurred. Preoperative LAN-ATG was administered at a minimum of a single injection (3 weeks prior to surgery) and a maximum of 6 injections (24 weeks) until the time when surgery could be performed. Long-term LAN-ATG was continued until approval of LAN-ATG as treatment for TSHoma in Japan; since the study was ongoing at the time of analysis, the data from the treatment period of 52 weeks were reported here.
During the study, therapy with other SSAs and cyclosporine drugs (which were classed as prohibited concomitant drugs) and radiation therapy were prohibited. Changes in the dosage and administration of antithyroid drugs, iodine preparations, corticosteroids, dopamine agonists, and GH receptor antagonists (which were classed as restricted concomitant drugs) were restricted until Week 24 or study treatment completion, whichever came first.
AssessmentsFor efficacy evaluation, the primary endpoints were serum concentrations of TSH, FT3, and FT4. Measurements were performed every 4 weeks at the time of drug administration up to Week 24 (or study treatment completion before Week 24) for all participants, on the preoperative visit for the preoperative LAN-ATG, and every 12 weeks after Week 24 for long-term LAN-ATG. The final assessment was set as the “assessment of efficacy at Week 48” for patients who participated in this study until Week 52 and as the “assessment of efficacy at discontinuation” for patients who discontinued this study. Blood sampling was performed before LAN-ATG administration. Serum concentrations were measured at a central laboratory in the United Kingdom using electrochemiluminescence immunoassay (ECLIA, cobas® 8000 e801, Roche Diagnostics, Switzerland).
The secondary endpoints were the size of pituitary tumor and clinical symptoms associated with TSHoma. The size of pituitary tumor (volume) was measured by central radiology review performed by two independent expert radiologists using an image assessment system (mint LesionTM, Mint Medical GmbH, Germany) for magnetic resonance imaging (MRI) or computed tomography. All slices taken, including those of the pituitary gland, trace the outer surface of the tumor, so the area and volume were calculated virtually using an image evaluation system. The measurements were performed at Weeks 12 and 24 (or at study treatment completion before Week 12 or 24) for all participants, at visit of “before surgery” for the preoperative LAN-ATG, and every 24 weeks after Week 24 for the long-term LAN-ATG. The severity of seven clinical symptoms (palpitations, tachycardia, hyperhidrosis, weight loss, diffuse goiter, headache, and visual impairment) [23] were scored (none = 0, mild = 1, moderate = 2, and severe = 3) and summed to obtain a total score.
The exploratory endpoints were 24-hour Holter electrocardiogram (ECG) analyzed by independent review for detecting tachycardia and arrhythmia due to the cardiac effects of thyroid hormones, and other serum hormone concentrations including GH and insulin-like growth factor-I (IGF-I).
Safety was assessed by presence or absence of AEs based on the results of clinical evaluations including physical examinations, vital signs, ECG, and laboratory tests. Abdominal ultrasound was performed every 6 months to detect gallbladder dysfunction. AEs were coded using the terms of the Medical Dictionary for Regulatory Activities, version 22.0 [24]. The severity (mild, moderate, and severe) of AEs and the relationship between AEs and treatment were assessed. An AE was classified as an adverse drug reaction (ADR) if the causal relationship was a reasonable possibility with study treatment. Serious AEs (SAEs), which were defined as events that result in death, initial or prolonged hospitalization, disability, or congenital anomaly and are life-threatening, were assessed. Anti-lanreotide antibodies were examined every 4 weeks until Week 24 (or study treatment completion before Week 24) for all participants, and afterwards, every 24 weeks for the long-term LAN-ATG. Anti-lanreotide antibodies were tested in two stages, screening and confirmation, using ECLIA. If the screening test yielded a specific positive result, antibody titers were determined by serial dilutions. To assess the pharmacokinetics of the 24-week treatment period, the trough concentration of serum lanreotide (Cmin) was measured using liquid chromatography-tandem mass spectrometry.
Statistical analysisBased on the intention-to-treat principle, efficacy analyses were conducted on the full analysis set (FAS), which consisted of all patients who received the study treatment at least once and had an efficacy evaluation. For the primary endpoint for long-term LAN-ATG, the analysis was also conducted on the per-protocol set (PPS), which consisted of all patients in the FAS, excluding those for whom treatment had deviated significantly from the study protocol. The safety analysis set included all patients who received the study treatment at least once and had any safety assessment. At the time of study protocol development, the total number of registered patients with TSHoma in Japan was 157 [25]. Considering the rarity of the disease and the feasibility of the study, the sample size was determined to be eight or more patients including three or more for preoperative LAN-ATG.
A descriptive analysis was performed: categorical variables were calculated as frequency and proportion, while continuous variables were calculated as mean, SD or standard error, or median and interquartile range (IQR). Percent changes in pituitary tumor size as well as serum concentrations of TSH, FT3, and FT4 from baseline were calculated. As a post hoc analysis, the mean serum concentrations of TSH, FT3, and FT4 between the baseline and final assessment were compared using a two-sided paired t test. Missing data were not imputed. Statistical analyses were performed using SAS version 9.3 (SAS Institute, the United States).
A total of 13 patients with TSHoma were enrolled at 10 institutions in Japan between February and December 2018; all patients started treatment with LAN-ATG 90 mg and were included in the FAS and safety analysis set. Data collected until November 2019 were analyzed. A total of six patients received preoperative LAN-ATG, and all patients underwent surgery for TSHoma and completed the study. They were all cured by surgery, and none of them restarted LAN-ATG treatment. Meanwhile, seven patients received long-term LAN-ATG: none of the patients discontinued study treatment, and all were on the study at the time of this analysis. Two patients with long-term LAN-ATG (patients No. 10 and 13) were excluded from the FAS (N = 11; 6 patients received preoperative LAN-ATG, and 5 received long-term LAN-ATG) due to the concomitant use of restricted drugs.
The TSH, FT3, and FT4 concentrations at baseline were similar between patients who received preoperative LAN-ATG and those who received long-term LAN-ATG (Table 1). The data on thyroid hormones and pituitary tumor size were expressed as medians because the value reported in one patient each was much higher than that reported in other patients. Overall, one patient had microadenoma and 12 had macroadenoma. Three patients presented with abnormal GH secretion and expression: two had acromegaly and one had pituitary adenoma co-secreting TSH and GH. Ten patients were naive to treatment for TSHoma, and the remaining three patients who previously underwent treatment for TSHoma received long-term LAN-ATG: pharmacotherapy (octreotide subcutaneous injection) in one patient, pharmacotherapy (potassium iodide) and pituitary adenomectomy in one, and pharmacotherapy (octreotide long-acting release [LAR], hydrocortisone, and thiamazole), pituitary adenomectomy, and radiation therapy (13 years before enrollment) in one.
| Preoperative LAN-ATG N = 6 | Long-term LAN-ATG N = 7 | |
|---|---|---|
| Sex, male/female, n (%) | 2 (33.3)/4 (66.7) | 3 (42.9)/4 (57.1) |
| Age (years) | 43.7 ± 17.1 | 60.4 ± 10.4 |
| 41.5 (24–71) | 59.0 (44–77) | |
| Time to diagnosis (months) | 22.0 ± 47.9 | 54.7 ± 59.2 |
| 3.0 (0–120) | 49.9 (1–170) | |
| TSH (μIU/mL) | 4.78 ± 3.24 | 4.79 ± 3.43 |
| 3.59 (1.5–10.2) | 3.08 (0.7–10.2) | |
| FT3 (pg/mL) | 6.02 ± 1.27 | 6.88 ± 5.20 |
| 5.80 (4.7–8.2) | 4.86 (4.1–18.5) | |
| FT4 (ng/dL) | 2.42 ± 0.44 | 2.56 ± 1.85 |
| 2.44 (1.9–3.0) | 1.85 (1.4–6.6) | |
| Co-secretion of TSH and GH, n (%) | 1 (16.7) | 2 (28.6) |
| Size of pituitary tumor (cm3) | 3.08 ± 3.37 | 12.77 ± 30.47 |
| 1.37 (0.2–8.7) | 0.96 (0.4–81.8) | |
| Previous therapy for TSHomaa, n (%) | ||
| Medication | 0 (0.0) | 3 (42.9) |
| Surgery | 0 (0.0) | 2 (28.6) |
| Others | 0 (0.0) | 1 (14.3) |
Results are mean ± SD and median (range) unless otherwise stated.
a Three patients received previous pharmacotherapy; of these, two patients had previously received surgery (including one with radiation therapy).
FT3, free T3; FT4, free T4; LAN-ATG, lanreotide autogel; TSHoma, TSH-secreting pituitary adenoma.
Of those who received preoperative LAN-ATG, four patients received 90 mg once and underwent surgery, while two patients received 90 mg four times and underwent surgery. Of those who received long-term LAN-ATG, two patients received 90 mg throughout the treatment period, four patients received a dose reduction to 60 mg at or after Week 16, and one patient received a dose increase to 120 mg at Week 48. The pharmacokinetic data for LAN-ATG administered at a dose of 90 mg are shown in Supplementary Table 1. None of the patients received any of the prohibited concomitant drugs. Two patients received the restricted concomitant drugs: one received dopamine hydrochloride once as treatment for an SAE of cardiac tamponade that occurred after Week 20, while the other started treatment with thiamazole for coexistent Graves’ disease diagnosed at Week 20.
Efficacy Primary endpointsIn patients receiving preoperative LAN-ATG (n = 6), the median serum concentrations of TSH, FT3, and FT4 decreased after initiation of LAN-ATG; the values at all measurement points from Week 4 through final assessment (before surgery) were lower than those at baseline (Fig. 2). The mean ± SD percent changes in serum concentrations of TSH, FT3, and FT4 from baseline at final assessment (before surgery) were –72.9% ± 22.5%, –45.7% ± 15.9%, and –39.6% ± 12.1%, respectively. In all patients, serum TSH concentrations decreased after initiation of LAN-ATG (Supplementary Fig. 1A). The numbers of patients whose FT3 and FT4 concentrations were within the reference range was 0 at baseline, but 4 and 5, respectively, at final assessment (Supplementary Fig. 1B and 1C). None of the patients developed a thyroid storm during the perioperative period. The mean differences in the changes in TSH, FT3, and FT4 from baseline to before surgery were –3.40 μIU/mL (95% confidence interval [CI], –5.42 to –1.38; p = 0.008), –2.82 pg/mL (95% CI, –4.40 to –1.24; p = 0.006), and –0.98 ng/dL (95% CI, –1.45 to –0.51; p = 0.003), respectively.

Median (±quartiles) serum (A) TSH, (B) FT3, and (C) FT4 concentrations in patients with TSHoma during preoperative treatment with LAN-ATG (full analysis set, N = 6)
The dotted horizontal lines show the reference ranges: reference ranges for serum TSH, FT3, and FT4 were 0.5–5.0 μIU/mL, 2.3–4.3 pg/mL, 0.9–1.7 ng/dL, respectively. Serum TSH, FT3, and FT4 concentrations were assessed by the median, as one patient had a significantly larger baseline value than the other patients.
FT3, free T3; FT4, free T4; LAN-ATG, lanreotide autogel; TSHoma, TSH-secreting pituitary adenoma.
In patients receiving long-term LAN-ATG (n = 7), median TSH concentrations remained stable throughout the treatment period (Fig. 3A). Median serum concentrations of FT3 and FT4 decreased after initial administration of LAN-ATG; the values at all measurement points from Week 4 through Week 48 were lower than those at baseline (Fig. 3B and 3C). The mean ± SD percent changes in serum concentrations of TSH, FT3, and FT4 from baseline at final assessment were –37.4% ± 37.0%, –23.3% ± 19.8%, and –24.6% ± 12.3%, respectively. The TSH concentrations at final assessment after initiation of LAN-ATG were lower than those at baseline in all but one patient (Supplementary Fig. 2A). The numbers of patients whose FT3 and FT4 concentrations were within the reference range was 1 and 2, respectively, at baseline, but 5 and 5, respectively, at final assessment (Supplementary Fig. 2B and 2C). The mean differences in the changes in TSH, FT3, and FT4 from baseline to final assessment were –1.65 μIU/mL (95% CI, –4.06 to 0.76; p = 0.145), –1.21 pg/mL (95% CI, –2.22 to –0.19; p = 0.027), and –0.53 ng/dL (95% CI, –0.75 to –0.30; p = 0.001), respectively. In patients receiving long-term LAN-ATG in the PPS (n = 5), the median serum concentrations of TSH, FT3, and FT4 at all measurement points from Week 4 through Week 48 were lower than those at baseline and within the reference ranges (Supplementary Fig. 3).

Median (±quartiles) serum (A) TSH, (B) FT3, and (C) FT4 concentrations in patients with TSHoma during long-term treatment with LAN-ATG (full analysis set, N = 7)
The dotted horizontal lines show the reference ranges: reference ranges for serum TSH, FT3, and FT4 were 0.5–5.0 μIU/mL, 2.3–4.3 pg/mL, 0.9–1.7 ng/dL, respectively. Serum TSH, FT3, and FT4 concentrations were assessed by the median, as one patient had a significantly larger baseline value than the other patients.
FT3, free T3; FT4, free T4; LAN-ATG, lanreotide autogel; TSHoma, TSH-secreting pituitary adenoma.
Median (IQR) values of pituitary tumor size for all 13 patients were 1.14 (0.51, 3.59) cm3 at baseline and 0.88 (0.40, 3.12) cm3 at final assessment; the median (IQR) of the percent change was –23.82% (–38.11%, –19.78%). Pituitary tumor size reduced until the time of surgery in all six patients receiving preoperative LAN-ATG (Fig. 4A); meanwhile, the tumor size remained at a low level until Week 48 in all seven patients receiving long-term LAN-ATG (Fig. 4B). Representative MRIs showing pituitary tumors in two patients (one received preoperative LAN-ATG and another received long-term LAN-ATG) among those who showed tumor shrinkage following therapy are presented in Supplementary Fig. 4 to illustrate the actual tracing and measurements of the tumors. An improvement in the total score of the clinical symptoms associated with TSHoma was observed from Week 4. The mean ± SD total score of the clinical symptoms of all patients (n = 13) improved from 2.7 ± 2.4 at baseline to 1.4 ± 2.0 at final assessment.

Percent change in pituitary tumor size in patients with TSHoma during (A) preoperative treatment (full analysis set, N = 6) and (B) long-term treatment (full analysis set, N = 7) with LAN-ATG
The pituitary tumor sizes (patient No.) at baseline were as follows: 1.1 cm3 (No. 1), 1.6 cm3 (No. 2), 1.1 cm3 (No. 3), 5.7 cm3 (No. 4), 8.7 cm3 (No. 5), and 0.2 cm3 (No. 6) in patients with preoperative LAN-ATG, and 3.6 cm3 (No. 7), 0.5 cm3 (No. 8, post-pituitary adenomectomy), 81.8 cm3 (No. 9), 1.6 cm3 (No. 10), 1.0 cm3 (No. 11, post-pituitary adenomectomy), 0.5 cm3 (No. 12), and 0.4 cm3 (No. 13) in patients with long-term LAN-ATG. An asterisk indicates overlapping of two patients; the percent changes in pituitary tumor size in each patient from baseline at Week 4 were –45.4% (yellow, patient No. 2) and –45.8% (black, patient No. 4), respectively.
LAN-ATG, lanreotide autogel; TSHoma, TSH-secreting pituitary adenoma.
Holter ECG monitoring showed that the heart rate during sleep and the 24-hour heart rate decreased after initiation of LAN-ATG, and the values at all assessments up to Week 24 were lower than those at baseline (Supplementary Table 2). Mean ± SD heart rate during sleep was 67.7 ± 13.1 beats/minute at baseline and 57.5 ± 11.9 beats/minute at final assessment. The mean ± SD 24-hour heart rate was 81.8 ± 13.8 beats/minute at baseline and 69.7 ± 12.2 beats/minute at final assessment.
The GH concentrations were within the reference range at baseline in all 13 patients (Supplementary Fig. 5A). The IGF-I concentrations decreased after initiation of LAN-ATG in all but one patient (Supplementary Fig. 5B). Changes in GH and IGF-I concentrations were not considered as AEs by the investigators. Three patients who had abnormal GH secretion and GH expression in the tissue had decreases not only in TSH but also in GH and IGF-I concentrations after initiation of LAN-ATG. There were no clinically significant changes in other serum hormone concentrations.
SafetyDuring the study, AEs were observed in 12 (92.3%) of the 13 patients, with a total of 105 AEs; of these, 78 AEs in 12 patients were classified as ADRs. All AEs were considered mild (74 AEs in 2/13 patients [15.4%]) or moderate (31 AEs in 10/13 patients [76.9%]), and none of the patients had severe AEs. The frequency of AEs did not increase during long-term treatment with lanreotide ATG (Table 2). The most common AEs were gastrointestinal disorders (50 AEs in 12/13 patients [92.3%]), which included diarrhoea (10/13 patients [76.9%]), faeces pale (5/13 [38.5%]), and faeces soft (2 [15.4%]). Other AEs that occurred in at least two patients were nasopharyngitis (3/13 patients [23.1%]), headache, malaise, and glycosylated haemoglobin increased (2/13, [15.4%] each). Notable AEs were administration site reactions (5/13 patients [38.5%]), which included 12 AEs of induration, pruritus, and pain at the injection or administration site. Cholelithiasis was observed in 3 (23.1%) of the 13 patients. One patient receiving preoperative LAN-ATG had biliary sludge detected before surgery, but completed surgery for TSHoma. The other two patients, receiving long-term LAN-ATG, had biliary sludge or cholelithiasis at Week 48.
| Patients, n (%) | Onset of AE (weeks) | ||||
|---|---|---|---|---|---|
| 1–12 | 13–24 | 25–36 | 37–48 | 49–52 | |
| N = 13 | N = 9 | N = 7 | N = 7 | N = 7 | |
| Diarrhoea | 10 (76.9) | 0 | 0 | 0 | 0 |
| Faeces pale | 4 (30.8) | 0 | 0 | 1 (14.3) | 0 |
| Injection site induration | 3 (23.1) | 0 | 0 | 0 | 0 |
| Headache | 2 (15.4) | 0 | 0 | 0 | 0 |
| Injection site pruritus | 2 (15.4) | 0 | 0 | 0 | 0 |
| Nasopharyngitis | 1 (7.7) | 2 (22.2) | 0 | 0 | 0 |
| Cholelithiasis | 1 (7.7) | 0 | 0 | 1 (14.3) | 1 (14.3) |
| Malaise | 1 (7.7) | 1 (11.1) | 0 | 0 | 0 |
| Faeces soft | 1 (7.7) | 0 | 0 | 1 (14.3) | 0 |
| Glycosylated haemoglobin increased | 0 | 2 (22.2)a | 0 | 0 | 0 |
AEs were coded using the terms of the Medical Dictionary for Regulatory Activities, version 22.0.
a The glycosylated haemoglobin values at baseline (central measurement) and at the time AE was judged (local measurement) were 6.4% and 6.5% (at Day 113) in one patient and 5.5% and 6.3% (at Day 141) in the other patient.
AE, adverse event; LAN-ATG, lanreotide autogel.
None of the patients died or discontinued study treatment due to AEs. A total of four SAEs were observed in three (23.1%) of the 13 patients: cardiac ablation and cardiac tamponade in one patient receiving long-term LAN-ATG, acute abdomen (sudden abdominal pain) in one patient receiving long-term LAN-ATG, and hyponatremia in one patient receiving preoperative LAN-ATG (after completion of the study treatment, possibly related to pituitary surgery). Causal relationship was not found between the study treatment and any SAEs, and all patients recovered from these SAEs.
Anti-lanreotide antibodies were reported in three (antibody titers were 1:32 in one patient, and 1:2 in two patients) of the 13 patients during treatment with LAN-ATG up to Week 52. Lack of drug efficacy or occurrence of ADRs such as hypersensitivity due to anti-drug antibodies were not reported. The antibodies had no influence on the pharmacokinetics of lanreotide (data not shown).
This study is, to our knowledge, the first clinical trial of LAN-ATG in patients with TSHoma. The study results suggest that treatment with LAN-ATG was well tolerated and effective in controlling thyroid function, clinical symptoms, and tumor size in patients with TSHoma. Most patients receiving LAN-ATG became euthyroid and showed improvement in thyrotoxicosis within 1 month after initial administration of LAN-ATG, irrespective of primary or postoperative treatment. These clinical and hormonal improvements were maintained throughout the treatment period for at least 1 year. Our results were consistent with those of the studies conducted in patients with TSHoma treated with LAN-PR for 6 months [8, 9]. Moreover, TSHoma appeared to be controlled by treatment with LAN-ATG alone without surgery in the majority of patients (5/7 patients received long-term LAN-ATG) in our study, suggesting that primary treatment with LAN-ATG may be applied in patients with contraindication to surgery or refusal of surgery.
Our study reported that most patients receiving LAN-ATG experienced a reduction in tumor size, which was maintained during long-term LAN-ATG. SSAs have a potential antitumor effect by suppressing proliferation [4, 15, 26], and a clinical trial of octreotide LAR reported a reduction in tumor size [10]. While the doses of LAN-PR evaluated in previous studies might not be high enough to elicit an antitumor effect [8, 9], the doses of LAN-ATG administered in our study could have been sufficient to inhibit cell proliferation and then reduce the tumor size. Our results are also consistent with the previous case reports that four patients with preoperative or postoperative residual/recurrent TSHoma treated with LAN-ATG 90 to 120 mg/month showed a reduction in tumor size (including tumor disappearance in one patient) [27, 28]. The remission rate of TSHoma after surgery is approximately 60%, and the postoperative recurrence rate in the long term is approximately 20% [14]. Two patients with a history of pituitary adenomectomy were included in the group receiving long-term LAN-ATG, suggesting that LAN-ATG might also be beneficial for patients with postoperative residual or recurrent tumor.
In our study, patients receiving preoperative LAN-ATG showed rapid improvement in their thyrotoxicosis and could undergo surgery without the occurrence of a thyroid storm. There have been reports proposing the importance of controlling thyroid function using SSAs or antithyroid drugs before surgery [6, 7]; however, no clinical trials have yet been conducted on this issue. In Japan, a retrospective observational study of 43 patients with TSHoma preoperatively treated with octreotide (LAR or subcutaneous injection) showed that FT4 normalized in 84% of patients, tumor size decreased in 61% of patients, and none of the patients developed a thyroid storm [13]. In our study, FT4 normalized in five of the six patients receiving preoperative LAN-ATG, and tumor size decreased in all patients. In the previous case reports [6, 7], the concentrations of preoperative thyroid hormones in two patients with TSHoma who did not receive any preoperative SSAs and developed thyroid storm were far above the upper limit of the normal range. In our study, the thyroid hormone concentrations at baseline were above the reference range in most patients, but the values decreased to the reference range after initiation of LAN-ATG in almost all the individuals. Although we have not evaluated the efficacy of antithyroid drugs, such as iodine, thiamazole, or propylthiouracil as the preoperative treatment, our results suggest the potential clinical utility of preoperative treatment with LAN-ATG in patients with TSHoma.
The following exploratory findings regarding treatment with LAN-ATG for TSHoma were obtained. Firstly, decrease in heart rate was observed during treatment, which was concurrent with the decrease in thyroid hormones and improvement in clinical symptoms of TSHoma. Some patients in this study experienced a decrease in pulse rate without significant changes in the concentrations of FT3 and FT4, which was considered partly attributable to the suppressive action of the lanreotide on the sympathetic nerve system through SSTR2 [29-31]. To date, only a few studies have quantitatively evaluated heart rate during treatment with SSAs using Holter ECG [32]. Secondly, the GH and IGF-I concentrations tended to decrease during treatment with LAN-ATG. Since IGF-I is influenced both by thyroid function and by GH secretory kinetics [33, 34], the reduction of IGF-I might be associated with improvement of thyroid hormone level and suppression of GH secretion. A previous study reported that acromegaly was observed in 16% of TSHoma patients [12]. In our study, not only TSH but also GH and IGF-I seemed to be controlled after initiation of LAN-ATG in three patients with abnormal GH secretion. One of these patients had coexistent Graves’ disease, and a combination of LAN-ATG and antithyroid drug was effective in controlling her thyroid function and pituitary tumor size. Although GH co-secretion was reported to be one of the unfavorable factors affecting the surgical outcomes of TSHoma [12], SSAs with high affinity for SSTR2 are considered to be effective for TSH and GH co-secreting adenoma [17]. Lastly, with regard to the pharmacokinetics, the Cmin after the fourth administration of LAN-ATG in patients with TSHoma in our study was similar to the Cmin during treatment with LAN-ATG in Japanese and non-Japanese patients with acromegaly [19, 35], suggesting that a steady state was almost reached. Although accumulation of serum lanreotide was not clearly observed during the 24 weeks, the effects of excessive lanreotide in the long-term treatment period cannot necessarily be ruled out.
SSAs generally have a good tolerability profile, and the major ADRs are gastrointestinal symptoms and injection site reactions [4, 15]. The most common AEs in our study were gastrointestinal symptoms, such as diarrhea and soft stool, and injection site reactions; these were consistent with previous studies on LAN-PR for TSHoma [8, 9] and information on ADRs of LAN-ATG for other indications [21, 22]. In our study, no AEs led to discontinuation of the study as in previous studies [8, 9]. When an AE occurred, the dose was adjusted as needed, and all patients were able to continue treatment with LAN-ATG. Cholelithiasis, sinus bradycardia, bradycardia, changes in blood glucose, and thyroid dysfunction are AEs of interest that have been reported during continuous treatment with SSAs [4, 15, 21]. New gallstones did not appear during LAN-PR treatment in previous studies [8, 9]; however, in our study, three patients developed biliary sludge or cholelithiasis, although none were symptomatic or discontinued treatment. The complications at baseline among patients who experienced the AEs of bradycardia or changes in blood glucose during LAN-ATG treatment were as follows: patient No. 11 who experienced sinus bradycardia had hypertension and right bundle branch block; patient No. 8 who experienced bradycardia had hypertension; patient No. 7 who experienced an increase in glycosylated haemoglobin had an impaired glucose tolerance and a serum HbA1c level 6.4% at baseline; patient No. 9 who experienced an increase in blood glucose level did not have any baseline complications associated with changes in blood glucose but later developed an increase in glycosylated haemoglobin. Of note, neither bradycardia nor changes in blood glucose influenced the continuation of treatment with LAN-ATG. Furthermore, no patients worsened thyroid dysfunction during the study. Tolerance of SSAs may improve progressively with continuation of treatment [8, 15]. In our study, delayed AEs were not observed in patients receiving LAN-ATG up to Week 52. There were no safety concerns associated with the anti-lanreotide antibodies that developed in three patients during the study. Based on these results, no new safety issues related to the administration of LAN-ATG were identified.
It is sometimes difficult to differentiate TSHoma from resistance to thyroid hormone (RTH) syndrome. Furthermore, several cases of RTH had been reported to coexist with pituitary incidentaloma or even TSHoma [36, 37]. Mannavola et al. reported that 2-months’ administration of long-acting SSA could differentiate between TSHoma and RTH, since FT3 and FT4 levels were markedly reduced in TSHomas, while no normalization of FT3 and FT4 could be seen in RTH [38]. Campi et al. have recently substantiated these findings [39]. Although we could not examine the patients with RTH, LAN-ATG–induced pituitary tumor shrinkage and normalization of FT3 and FT4 in most TSHomas in our study may suggest the usefulness of preoperative LAN-ATG treatment for differentiating RTH complicated with pituitary incidentaloma.
This study has some limitations. The sample size was small because of the rarity of the disease. Due to the limited patient numbers, we performed this trial without a control group. Despite these limitations, the efficacy of LAN-ATG for TSHoma was suggested in the within-group comparison and intra-patient comparison before and after treatment, in which thyroid hormones rapidly normalized through the action of the SSA. Moreover, we were able to describe the tolerability of this treatment in patients with TSHoma. Further accumulation of data is anticipated if clinical approval is granted for LAN-ATG for TSHoma in order to obtain better knowledge on its effectiveness and safety.
In conclusion, preoperative, or primary or postoperative long-term treatment with LAN-ATG appeared to be effective in controlling thyroid function and pituitary tumor size in Japanese patients with TSHoma with no clinically relevant AEs observed. The clinical approval of LAN-ATG for TSHoma in Japan is hoped to provide a first-line pharmacotherapy and contribute to the multidisciplinary treatment of this rare disease.
While the revised manuscript has been under review, LAN-ATG has been approved for medical treatment against the TSH-secreting pituitary adenoma in Japan, as of Dec. 25, 2020.
ADR, adverse drug reaction; AE, adverse event; CI, confidence interval; ECG, electrocardiogram; ECLIA, electrochemiluminescence immunoassay; FAS, full analysis set; FT3, free triiodothyronine; FT4, free thyroxine; HbA1c, hemoglobin A1c; IGF-I, insulin-like growth factor-I; IQR, interquartile range; LAN-ATG, lanreotide autogel; LAN-PR, lanreotide prolonged release; LAR, long-acting release; MRI, magnetic resonance imaging; PPS, per-protocol set; RTH, resistance to thyroid hormone; SAE, serious adverse event; SITSH, syndrome of inappropriate secretion of TSH; SSA, somatostatin analog; SSTR, somatostatin receptor; TSHoma, TSH-secreting pituitary adenoma
This study was supported by Teijin Pharma Limited. We would like to thank all patients and investigators who participated in this study as well as Dr. Ichiro Fujisawa of Kishiwada City Hospital and Dr. Shuzo Ikuta of Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital for performing the central radiology reviews. Kunihito Watanabe (Medical Science Department) and Yuzuru Sasaki (Pharmaceutical Development Department) of Teijin Pharma Limited assisted with the writing, preparation, and publication of this manuscript, and Ipsen provided comments on a draft of the publication prior to author approval. Statcom Co., Ltd. provided medical writing and publication support services funded by Teijin Pharma Limited; Kae Uetani of Statcom Co., Ltd. assisted in writing the manuscript.
Akira Shimatsu received lecture fees from Teijin Pharma Limited and served as a medical advisor to Teijin Pharma Limited. Yutaka Takahashi received research funding from Teijin Pharma Limited. Fumitoshi Satoh received research funding from FUJIREBIO Inc., FUJIFILM Wako Pure Chemical Corporation, and Hitachi Chemical Diagnostics Systems Co., Ltd. Hiroshi Nishioka received honoraria and research funding from Teijin Pharma Limited. Koji Takano received funding for basic research from Teijin Pharma Limited. Shohei Tateishi, and Yusaku Matsushita are employees of Teijin Pharma Limited. Akinobu Nakamura, Shingo Fujio, Shigeyuki Tahara, Miho Yamashita, Hiroshi Arima, and Atsushi Tominaga have no conflicts of interest.
Akinobu Nakamura (Hokkaido University Hospital), Fumitoshi Satoh (Tohoku University Hospital), Masanobu Yamada (Gunma University Hospital), Hiroshi Nishioka (Toranomon Hospital), Shigeyuki Tahara (Nippon Medical School Hospital), Koji Takano (Kitasato University Hospital), Miho Yamashita (Hamamatsu University Hospital), Hiroshi Arima (Nagoya University Hospital), Noriyuki Suzaki (Nagoya Medical Center), Tetsuya Tagami (Kyoto Medical Center), Youichi Saitoh (Osaka University Hospital), Yutaka Takahashi (Kobe University Hospital), Atsushi Tominaga (Hiroshima Prefectural Hospital), Masatoshi Nomura (Kurume University Hospital), Shingo Fujio (Kagoshima University Hospital).
Takahiro Takase and Hiraku Kameda (Hokkaido University Hospital), Ryo Morimoto and Masataka Kudo (Tohoku University Hospital), Yutaka Oki (Hamamatsu University Hospital), Hiroshi Takagi and Kazuhito Takeuchi (Nagoya University Hospital), Hidenori Fukuoka (Kobe University Hospital).
| Study visit | Serum lanreotide concentration (ng/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| na | Mean | SD | CV (%)b | Min | Median | Max | mc | Geometric mean | Geometric SD | Geometric CV (%)d | |
| Pre dosee | 13 | NC | NC | NC | NC | NC | NC | 0 | NC | NC | NC |
| Week 4 | 13f | 1.4323 | 0.9516 | 66.4 | 0.000 | 1.1800 | 3.340 | 12 | 1.3288 | 1.8168 | 65.4 |
| Week 8 | 9 | 1.5902 | 1.0099 | 63.5 | 0.187 | 1.8200 | 3.240 | 9 | 1.1974 | 2.5127 | 115.6 |
| Week 12 | 9 | 2.5078 | 0.8971 | 35.8 | 1.770 | 2.3000 | 4.720 | 9 | 2.3997 | 1.3455 | 30.3 |
| Week 16 | 7 | 2.4311 | 1.0504 | 43.2 | 0.608 | 2.3000 | 3.770 | 7 | 2.1504 | 1.8366 | 66.9 |
| Week 20 | 4 | 3.7950 | 1.3299 | 35.0 | 2.330 | 3.6850 | 5.480 | 4 | 3.6188 | 1.4330 | 37.2 |
| Week 24 | 4 | 4.0725 | 1.1395 | 28.0 | 3.110 | 3.8950 | 5.390 | 4 | 3.9543 | 1.3232 | 28.6 |
a Number of patients who had no missing value.
b CV (%): SD/mean × 100; the SD and mean were calculated using untransformed values.
c Number of patients who had no missing value and BLQ.
d Geometric CV (%): [exp (SD2) – 1]1/2 × 100; SD was calculated using natural log-transformed values.
e Descriptive statistics were not calculated at “pre dose” as the serum lanreotide concentration was BLQ in all patients.
f As for one patient whose serum lanreotide concentration was BLQ, the BLQ was replaced with 0 ng/mL in the analysis.
BLQ, below the lower limit of quantification; CV, coefficient of variation; LAN-ATG, lanreotide autogel; Max, maximum; Min, minimum; NC, not calculated.
| Study visit | n | Heart rate during sleep (beats/minute) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | SE | Min | Median | Max | ||
| Pre dose | 13 | 67.7 | 13.1 | 3.6 | 49 | 65.4 | 97 |
| Week 12 | 9 | 57.3 | 12.2 | 4.1 | 45 | 52.1 | 82 |
| Week 24 | 6 | 53.7 | 8.3 | 3.4 | 43 | 56.5 | 64 |
| Final assessment | 13 | 57.5 | 11.9 | 3.3 | 43 | 57.1 | 82 |
| Study visit | n | Heart rate (beats/day) | |||||
| Mean | SD | SE | Min | Median | Max | ||
| Pre dose | 13 | 117,787.3 | 19,832.0 | 5,500.4 | 90,110 | 116,856.0 | 150,989 |
| Week 12 | 9 | 99,225.4 | 17,073.8 | 5,691.3 | 79,904 | 102,078.0 | 135,270 |
| Week 24 | 6 | 92,376.0 | 10,358.0 | 4,228.6 | 78,613 | 97,272.0 | 101,762 |
| Final assessment | 13 | 100,301.2 | 17,577.7 | 4,875.2 | 78,613 | 99,576.0 | 135,270 |
LAN-ATG, lanreotide autogel; Max, maximum; Min, minimum; SE, standard error; TSHoma, TSH-secreting pituitary adenoma.

Serum (A) TSH, (B) FT3, and (C) FT4 concentrations at baseline and final assessment in each patient with TSHoma during preoperative treatment with LAN-ATG (full analysis set, N = 6)
The dotted horizontal lines show the reference ranges: reference ranges for serum TSH, FT3, and FT4 were 0.5–5.0 μIU/mL, 2.3–4.3 pg/mL, 0.9–1.7 ng/dL, respectively.
FT3, free T3; FT4, free T4; LAN-ATG, lanreotide autogel; TSHoma, TSH-secreting pituitary adenoma.

Serum (A) TSH, (B) FT3, and (C) FT4 concentrations at baseline and final assessment in each patient with TSHoma during long-term treatment with LAN-ATG (full analysis set, N = 7)
The dotted horizontal lines show the reference ranges: reference ranges for serum TSH, FT3, and FT4 were 0.5–5.0 μIU/mL, 2.3–4.3 pg/mL, 0.9–1.7 ng/dL, respectively. Patient No. 13 was the patient with coexistent Graves’ disease.
FT3, free T3; FT4, free T4; LAN-ATG, lanreotide autogel; TSHoma, TSH-secreting pituitary adenoma.

Median (±quartiles) serum (A) TSH, (B) FT3, and (C) FT4 concentrations in patients with TSHoma during long-term treatment with LAN-ATG (per-protocol set, N = 5)
The dotted horizontal lines show the reference ranges: reference ranges for serum TSH, FT3, and FT4 were 0.5–5.0 μIU/mL, 2.3–4.3 pg/mL, 0.9–1.7 ng/dL, respectively.
FT3, free T3; FT4, free T4; LAN-ATG, lanreotide autogel; TSHoma, TSH-secreting pituitary adenoma.

Raw and annotation images of sagittal MRI section of TSHomas from (A) patient No. 4 (preoperative LAN-ATG) and (B) patient No. 9 (long-term LAN-ATG)
LAN-ATG, lanreotide autogel; MRI, magnetic resonance image; TSHoma, TSH-secreting pituitary adenoma.
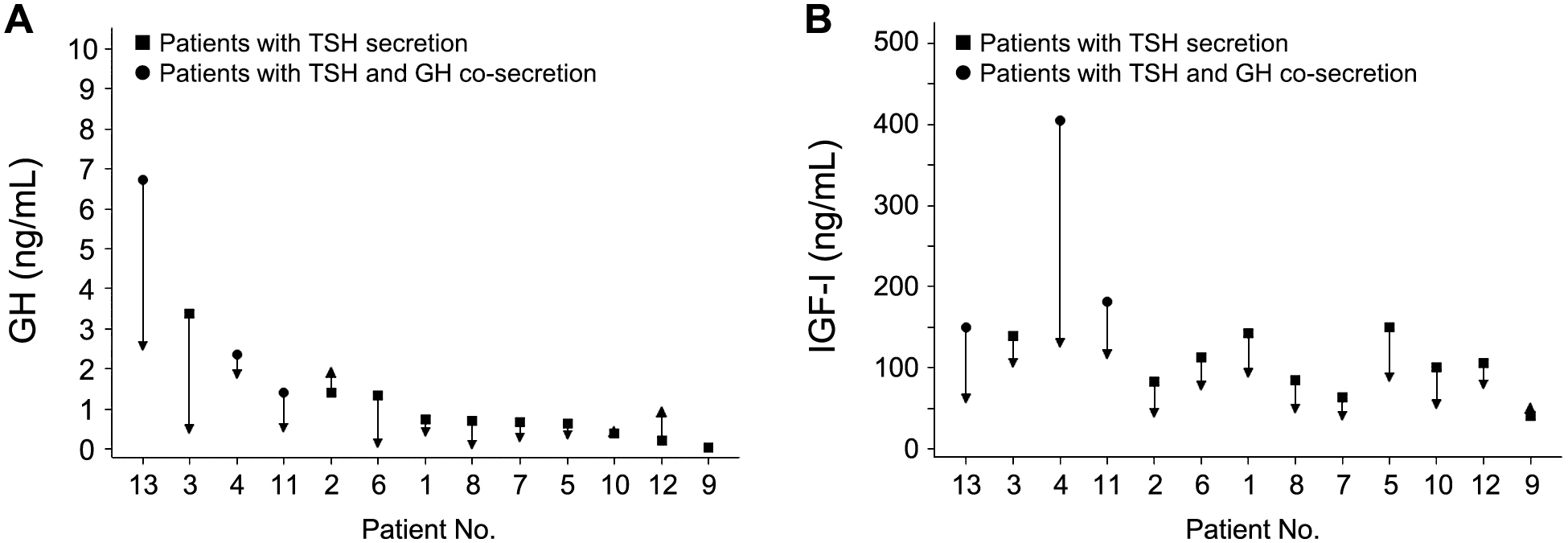
Serum (A) GH and (B) IGF-I concentrations at baseline and final assessment in each patient with TSHoma during treatment with LAN-ATG (full analysis set, N = 13)
The reference ranges for serum GH concentration were ≤2.47 ng/mL for men and 0.13–9.88 ng/mL for women. Patient Nos. 1 to 6 represent patients who received preoperative treatment with LAN-ATG, and patient Nos. 7 to 13 represent patients who received primary or postoperative long-term treatment with LAN-ATG. Patient No. 13 was the patient with coexistent Graves’ disease.
IGF-I, insulin-like growth factor I; LAN-ATG, lanreotide autogel; TSHoma, TSH-secreting pituitary adenoma.