2022 年 69 巻 3 号 p. 283-290
2022 年 69 巻 3 号 p. 283-290
Gonadotropin-releasing hormone (GnRH) stimulation of annexin A1 (ANXA1) and A5 (ANXA5) mRNA expression was analyzed in LβT2 gonadotrope cells. Quantitative polymerase chain reaction results showed that a GnRH analog (GnRHa) stimulated the expression of both ANXA1 and A5 mRNA with a peak at 12 h of incubation; however, ANXA1 mRNA was extremely stimulated (60 folds). Immunocytochemical analysis confirmed these findings. A GnRH antagonist inhibited the effect of GnRHa. ANXA1 and A5 mRNA levels were significantly increased by protein kinase C (PKC) activator (12-O-Tetradecanoylphorbol-13-acetate; TPA), but not by dibutyryl cAMP. GnRHa-stimulated induction of ANXA1 and A5 mRNA was inhibited by PKC (GF109203) and MEK inhibitors (PD98059). TPA increased ANXA1 and A5 mRNA expression in a dose-dependent manner (1 nM to 10 μM), while the extent of the increase was much greater in ANXA1. After stimulation with 10 nM or 1 μM TPA, ANXA1 and A5 mRNA levels were increased at 6 h. ANXA1 mRNA levels were higher in the 1 μM TPA than in the 10 nM TPA treatment, whereas 1 μM TPA did not show further stimulation of ANXA5 mRNA compared to 10 nM TPA. These results clearly show that ANXA1 mRNA expression is stimulated by GnRH through PKC like ANXA5, and the response of ANXA1 is much larger than that of ANXA5. A close relationship between these annexins and a significant role for ANXA1 in GnRH action at gonadotropes is suggested.
GONADOTROPIN-RELEASING HORMONE (GnRH) is a hypothalamic decapeptide hormone that is delivered to the anterior pituitary gland through the pituitary portal system [1]. GnRH stimulates the expression of multiple genes in the gonadotropes, including the gonadotropin subunits, such as the α and β subunits of luteinizing hormone (LH) and follicle stimulating hormone (FSH), and its own receptor [2, 3]. The GnRH receptor is a member of the G protein-coupled receptor superfamily; it is mainly coupled with Gαq/11 and activates phospholipase C [4]. Phospholipase C converts the membrane phospholipid, phosphatidyl inositol (4,5)-bis phosphate, to inositol 3 phosphate, which releases calcium ions, and diacylglycerol to stimulate protein kinase C (PKC). In addition to Gαq/11, Gαs, Gαi and mitogen-activated protein kinase (MAPK) are also involved in signal transduction of the GnRH receptor [5-7].
Annexins constitute a family of structurally similar proteins that exhibit the common characteristic of calcium-dependent phospholipid binding [8, 9]. In vertebrates, there are 12 annexins, such as annexin A (ANXA), which are named ANXA1–11 and A13 [10]. ANXAs consist of a conserved C-terminal core domain, four (eight for ANXA6) ~60 amino acid sequence repeats, and a variable N-terminus [8]. Annexins have various functions, including membrane repair, signaling, hormone secretion, inhibition of blood coagulation, regulation of inflammation, and biomarkers of various pathophysiological changes [8, 9, 11]. Despite various observations on annexins, their physiological functions remain obscure.
We previously demonstrated that GnRH stimulates mRNA expression of ANXA5 through the GnRH receptor-MAPK cascade in a mouse gonadotrope-derived cell line (LβT2) [12-15]. In ovariectomized rats, expression of ANXA5 increases in the gonadotropes, which can be inhibited by estradiol administration [12]. Thus, pituitary ANXA5 expression is physiologically regulated by hypothalamic GnRH. In addition, we demonstrated that ANXA5 participates in the control of GnRH-stimulated gonadotropin secretion [13, 15]. ANXA5 itself stimulates both LH and FSH release, whereas an antisense-oligodeoxynucleotide for ANXA5 mRNA inhibits GnRH stimulation of LH in primary cultures of rat anterior pituitary cells [13]. These findings suggest that ANXA5 is a prerequisite for GnRH stimulation of gonadotropin secretion.
Recently, we detected ANXA4, A5, A6, A7, and A11 mRNAs in LβT2 cells and showed that a GnRH agonist (GnRHa) increased mRNA levels of only ANXA1 and A5 among all annexins in LβT2 cells [16]. GnRHa dramatically increased ANXA1 mRNA levels at trace amounts, without GnRH [16]. ANXA1 promotes LH secretion in LβT2 cells [17]. These results suggest that ANXA1 plays an important role in the regulation of gonadotropin secretion in the pituitary gland, in addition to ANXA5. Hence, it is important to clarify the difference in the responses of these two annexins to explore the mechanism by which GnRH enhances gonadotropin secretion. Our results show a higher responsiveness of ANXA1 to GnRH receptor-PKC-MEK signaling.
A GnRHa (Des-Gly10 [Pro9]-GnRH ethylamide; Conceral) was obtained from Nagase Pharmaceuticals Co., Ltd. (Osaka, Japan). The GnRH receptor antagonist, Cetrorelix, was kindly provided by Zentaris GmbH (Frankfurt, Germany). An inhibitor of PKC, bisindolylmaleimide GF109203, was acquired from Calbiochem (San Diego, CA, USA). The MAPK kinase (MEK) inhibitor PD98059, dibutyryl cAMP (dbcAMP), and the PKC activator (12-O-Tetradecanoylphorbol-13-acetate; TPA) were purchased from FUJIFILM Wako Pure Chemicals (Osaka, Japan), Sigma-Aldrich (St. Louis, MO, USA), and Cayman Chemical (Ann Arbor, MI, USA), respectively. Rabbit polyclonal antibody against recombinant rat ANXA5 (AB_2533983) was produced as previously described [18]. Rabbit anti-ANXA1 antibody (AB_2533983) and donkey anti-rabbit IgG (H+L) antibody, Alexa Fluor 488 conjugated (AB_2535792) were purchased from Thermo Fisher Scientific (Waltham, MA, USA) and Molecular Dynamics (Chatsworth, CA, USA), respectively.
Cell cultureThe LβT2 cell line was kindly provided by Professor P. Mellon (University of California, San Diego, CA, USA). LβT2 cells express all gonadotropin subunit genes and GnRH receptors [19]. The cells were grown in 75 cm2 flasks containing Dulbecco’s modified Eagle’s medium with high glucose (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Biowest, Nuaillé, France) and an antibiotic-antimycotic mixture (penicillin 100 U/mL, streptomycin 100 μg/mL, and amphotericin B 250 ng/mL; Invitrogen). The culture was maintained in an atmosphere of 95% air, 5% CO2 and 100% humidity. The cells were subcultured before they became confluent. Cells (106) in 2 mL medium were plated in 35-mm plastic dishes, and experiments were performed two days later.
Gene expression experimentsTo examine the effect of continuous stimulation with GnRHa, the cells were harvested from three dishes at 1, 3, 6, 12, 24, and 48 h after changing to a medium containing 10 nM GnRHa. Next, to examine the specific effects of GnRHa, cells were treated for 2 h with 1 μM cetrorelix, a GnRH receptor blocker, after which the medium was supplemented with 10 nM GnRHa, and the cells were incubated for an additional 2 h.
The role of PKC and the cyclic AMP pathway was investigated by stimulating the cells with 1 μM TPA or 500 μM dbcAMP for 2 h. In another experiment, cells were treated with 5 μM bisindolylmaleimide (a PKC inhibitor, GF109203) for 2 h, after which the medium was supplemented with 10 nM GnRHa, and the cells were incubated for an additional 2 h.
To further examine the role of PKC, cells were treated with different concentrations of TPA (1 nM to 10 μM) for 2 h. In another experiment, the cells were harvested from three dishes at 1, 3, 6, 12, 24, and 48 h after changing to a medium containing 10 nM or 1 μM TPA.
The role of the MAPK system in GnRH receptor signal transduction was investigated by treating cells with PD98059, a MAPK kinase (MEK) inhibitor. Cells were treated with 50 μM PD98059 for 2 h, after which the medium was supplemented with 10 nM GnRHa, and the cells were incubated for an additional 2 h.
RNA extraction and complementary DNA synthesisTotal RNA was extracted using the acid guanidinium thiocyanate-phenol-chloroform extraction method with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Briefly, the cell growth medium was removed, and 500 μL of TRIzol reagent was added. The cell suspension was transferred to a plastic 1.5 mL centrifuge tube, and 100 μL of chloroform was added. The mixture was centrifuged at 12,000 × g for 15 min. The aqueous phase was transferred to a new tube, and the RNA was precipitated using isopropanol. Total RNA (1 μg) was treated with RNase-free DNase I (Invitrogen) to exclude genomic DNA and was reverse-transcribed using 200 U of ReverTra Ace (TOYOBO, Osaka, Japan) and 10 pmol of random primers (Invitrogen).
Real-time polymerase chain reaction (PCR)Real-time PCR was performed using SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) and QuantStudio (Applied Biosystems). Previously described primers (ANXA1, 5'-ATGTTGCTGCCTTGCACAAA-3' and 5'-CCAAGGGCTTTCCATTCTCCT-3'; ANXA5, 5'-CTCTGTTTGGCAGGGACCTT-3' and 5'-GGCATCGTAGAGTCGTGAGG-3'; and RPL19, 5'-CTGATCAAGGATGGGCTGAT-3' and 5'-TTCAGCTTGTGGATGTGCTC-3') were used for each PCR assay [16]. ANXA1 and A5 mRNA levels were standardized by dividing by the RPL19 mRNA level in the same sample.
ImmunocytochemistryImmunocytochemistry for ANXA1 and A5 was performed after 12 h of incubation with GnRHa. LβT2 cells were seeded onto coverslips coated with poly-l-lysine in 35 mm dishes at a density of 3 × 105 cells/dish. Three days later, the subculture medium was changed to that containing GnRHa (10 nM) or that containing no GnRHa. The medium was removed 12 h later, and 2 mL of methanol (–20°C) was added. The cells were then left for 10 min, dried, and subjected to immunocytochemistry. The cells were first incubated with antibody binding buffer (ABBB, 150 mM NaCl, 5 mM EDTA, 50 mM Tris, 0.25% gelatin, 0.05% NP40, pH 7.4) for 2 h. The cells on coverslips were incubated with ABBB supplemented with 3% fetal calf serum for 15 min and then incubated with anti-ANXA1 antibody (1:1,000, AB_2533983) or anti-ANXA5 antibody (1:10,000, AB_2827744) overnight at 4°C. The cells were then incubated for 2 h with the secondary antibody (Alexa 488-labeled anti-rabbit IgG donkey serum; AB_2535792). The cells were observed using a confocal laser microscope (Zeiss LSM 880, Tokyo, Japan).
Statistical analysisData were calculated by dividing the value of each sample by the mean value of the corresponding control group to obtain the relative amounts. The statistical analyses were performed using KaleidaGraph version 4.5 software (Synergy Software, Reading, PA, USA). Data are expressed as the mean ± standard error of the mean (SEM) and were statistically evaluated using Tukey’s multiple comparison test. Statistical significance was set at p < 0.05.
Continuous stimulation with 10 nM GnRHa significantly increased the mRNA levels of both ANXA1 and A5 (Fig. 1A). The fold increase in ANXA1 expression is extremely high. Therefore, the changes in ANXA5 mRNA were also observed with different ordinates (Fig. 1B). Graphs show significant increases in ANXA1 and A5 mRNA at 3 and 1 h, respectively. Both mRNA levels continued to increase for 6 h and remained high until 12 h. They maintained at these significantly high levels at 24 h and returned to basal levels after 48 h. The time course of changes in ANXA1 mRNA following GnRHa stimulation was similar to that of ANXA5 mRNA. The rate of mRNA increase of ANXA1 was much higher than that of ANXA5 (60 times higher rate, Fig. 1A).

Changes in ANXA1 (A) and A5 (A, B) mRNA levels in LβT2 cells after GnRH stimulation
The cells were treated with GnRH agonist (10 nM) and were harvested after 0, 1, 3, 6, 12, 24, and 48 h of incubation. Values represent the mean ± SEM (n = 3) and are presented as the ratio to the 0 h value. Asterisk (*) indicates a significant difference from the 0 h value of each group (p < 0.05, by Tukey’s multiple comparison test).
Immunocytochemistry revealed a dotted distribution of ANXA1 and A5. Immunofluorescent signals for both ANXA1 and A5 were spread throughout the cells (Fig. 2). GnRHa stimulation for 12 h increased the expression of both ANXA1 and A5.

Immunocytochemical analysis of ANXA1 and A5 expression in LβT2 cells
The cells were grown on a coverslip and challenged with the GnRHa (10 nM) for 12 h. ANXA1 (A) and A5 (B) expressions were analyzed with anti-ANXA1 and anti-ANXA5 antibodies, respectively. ANXA1 and A5 are shown with green fluorescence. DAPI was used to stain the nucleus (blue). Scale bars indicate 10 μm.
When the GnRH receptor antagonist, Cetrorelix, was added along with GnRHa, the increase in mRNA levels of ANXA1 and A5 was completely suppressed (Fig. 3), indicating that they were stimulated by GnRHa via the GnRH receptor.

Effects of GnRH receptor antagonist on GnRHa stimulation of ANXA1 and A5 mRNA levels in LβT2 cells
The cells were untreated or treated with GnRH receptor antagonist cetrorelix (1 μM) for 2 h. The medium was then replaced by medium supplemented with 10 nM GnRHa, and the incubation was continued for 2 h. Values represent the mean ± SEM (n = 3) and are presented as the ratio to the control value. Asterisk (*) indicates a significant difference between bracketed measurements (p < 0.05, by Tukey’s multiple comparison test).
The PKC-activating phorbol ester TPA significantly increased the mRNA expression of ANXA1 and A5 after 2 h of incubation, whereas dbcAMP did not affect the mRNA expression of ANXA1 or A5 (Fig. 4). In addition, both the PKC inhibitor bisindolylmaleimide GF109203 (Fig. 5A, B) and the MEK inhibitor PD98059 (Fig. 5C, D) significantly suppressed GnRH stimulation of ANXA1 and A5 mRNA expression. The results observed for ANXA5 are consistent with those of a previous study [15].

Effect of TPA and dbcAMP on ANXA1 and A5 mRNA levels in LβT2 cells
The cells were treated with TPA (1 μM) or dbcAMP (100 μM) for 2 h, Values represent the mean ± SEM (n = 3) and are presented as the ratio to the control value. Asterisk (*) indicates a significant difference from control value (p < 0.05, by Tukey’s multiple comparison test).
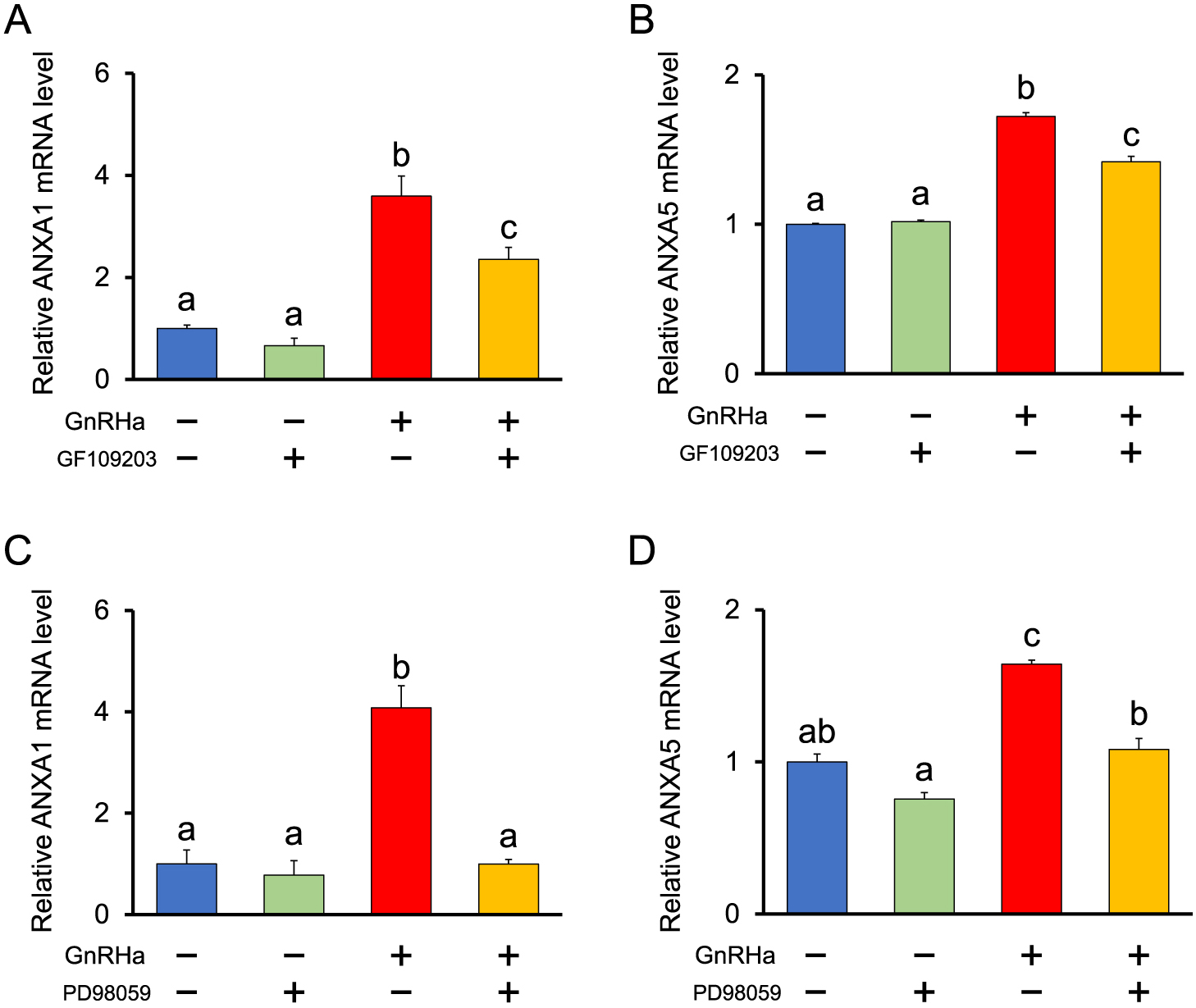
Effects of PKC inhibitor GF109203 and MEK inhibitor PD98059 on GnRHa stimulation of ANXA1 and A5 mRNA levels in LβT2 cells
The cells were untreated or treated with GF109203 (5 μM) or PD98059 (50 μM) for 2 h. The medium was then replaced by medium supplemented with 10 nM GnRHa, and the incubation was continued for 2 h. Values represent the mean ± SEM (n = 3) and are presented as the ratio to the control value. Data labeled with different letters are significantly different from each other (p < 0.05, by Tukey’s multiple comparison test).
Next, the effect of TPA dose on the mRNA expression of ANXA1 and A5 was examined. The mRNA levels of ANXA1 significantly increased from 100 nM to 10 μM in a dose-dependent manner, whereas those of ANXA5 mRNA significantly increased from 10 nM to 1 μM (Fig. 6A, B).
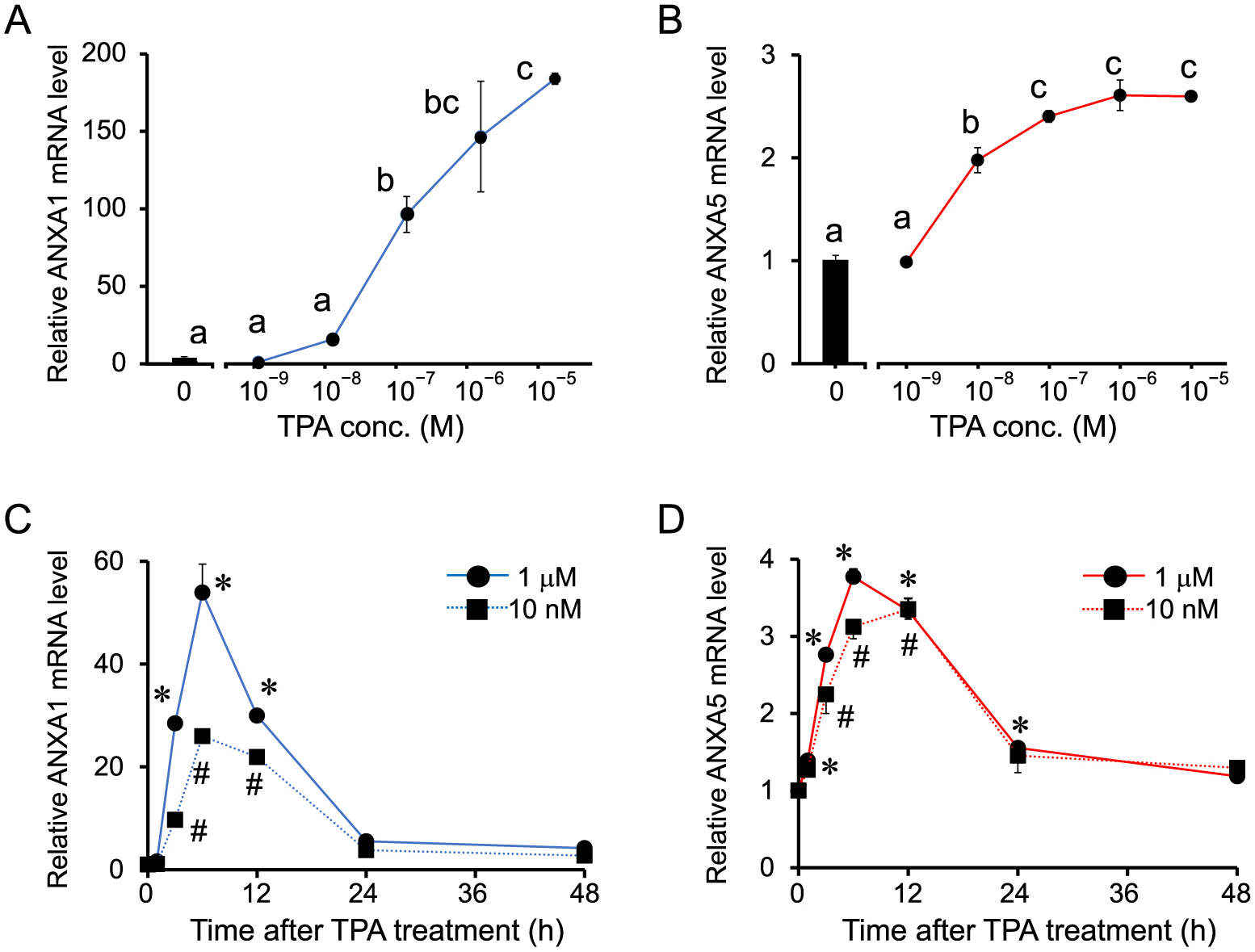
Effects of PKC activator TPA on ANXA1 and A5 mRNA levels in LβT2 cells
The cells were treated with 10–9, 10–8, 10–7, 10–6 or 10–5 M TPA for 2 h (A, B) and were treated with 10 nM or 1 μM TPA for 0, 1, 3, 6, 12, 24 and 48 h (C, D). Values represent the mean ± SEM (n = 3) and are presented as the ratio to the control value. Data labeled with different letters are significantly different from each other (A, B). * and # symbols indicate a significant difference from the 0 h value of each group (p < 0.05, by Tukey’s multiple comparison test).
The time course of ANXA1 and A5 mRNA expression following TPA stimulation was examined. The cells were treated with two different doses of TPA (10 nM and 1 μM) for 48 h. Changes in these mRNA levels were similar to those observed following GnRH stimulation, as they began to significantly increase from 1 or 3 h, and continued to increase until they reached peak levels between 6–12 h, and returned to the basal levels by 48 h (Fig. 6C, D). The time course of ANXA1 mRNA expression at 1 μM was also similar to that of cells treated with GnRHa, but the peak was observed at ~6 h (Fig. 6C). Although the stimulatory effect of 1 μM TPA treatment was greater than that of 1 nM, as reflected by the peak mRNA levels of ANXA1 (6–12 h), this was not observed for ANXA5 (Fig. 6).
We have demonstrated that ANXA5 at least partly regulate GnRH-stimulated gonadotropin secretion in pituitary gonadotropes [12-15]. Recently, we found that GnRH significantly increased the expression of ANXA1 in LβT2 cells [16]. In addition, a preliminary experiment showed that ANXA1 promotes LH secretion in LβT2 cells [17]. Furthermore, western blot analyses showed that ovariectomy increased ANXA1 immunoreactivity in the rat pituitary gland [17]. Thus, ANXA1 is an annexin that regulates gonadotropin secretion in the pituitary and involves in hypothalamic-pituitary-ovarian axis, in addition to ANXA5. Therefore, comparison between ANXA1 and A5 is crucial for understanding their physiological roles in gonadotropin secretion.
The present results show that ANXA1 expression, similar to ANXA5, is stimulated by TPA and not by dbcAMP. Furthermore, GF109203, an inhibitor of PKC, suppressed the GnRH-stimulated increase in ANXA1 expression. These results indicate that the activation of PKC by GnRH is the main signaling pathway for the expression of both ANXA1 and A5 in LβT2 cells. GnRH has been reported to activate the MAPKs p38, JNK, and ERK1/2 [20-22]. In this study, we found that PD98059, an inhibitor of MEK-1-mediated activation of MAPK [23], suppresses GnRH-stimulation of ANXA1 and A5 mRNA expression, suggesting that the MEK-MAPK pathway is responsible for the expression of ANXA1 and A5.
The distribution of ANXA1 in LβT2 cells was similar to that observed in a previous report [16], even though the GnRHa concentration and incubation times were different. ANXA1 and A5 are both GnRH-inducible genes, but the response to GnRH is extremely high for ANXA1. Immunocytochemistry also showed a clear increase in the ANXA1 signal in LβT2 cells. The physiological functions of ANXA1 and A5 in gonadotropes under the effects of GnRH are currently being studied in our laboratory.
We also found that mRNA expression of ANXA1 and A5 following stimulation with GnRHa treatment appeared at 3 h, reached maximal levels between 6–12 h, declined within 24 h, and returned to basal levels by 48 h. Changes in the mRNA levels of ANXA1 and A5 after TPA treatment were almost similar to those induced by GnRHa. The GnRH receptor-PKC cascade appears to be central to the expression of ANXA1 and A5 following stimulation by GnRH. However, the amplitude of the response to TPA on the expression of ANXA1 and A5 was different. ANXA5 mRNA levels began to increase at low concentrations of TPA (10 nM) and reached a maximum at 1 μM after 2 h of incubation, whereas ANXA1 mRNA levels began to increase significantly at 100 nM and continued to increase until 10 μM. During a time-course experiment, the observed change in the mRNA level of ANXA5 was similar for 10 nM and 1 μM TPA stimulation, whereas ANXA1 levels increased at high concentrations of TPA (1 μM). These results suggest different mechanisms for the expression of ANXA1 and A5.
The present study demonstrated that TPA strongly promotes the expression of ANXA1 in LβT2 cells. Dexamethasone induces serine phosphorylation and membrane translocation of ANXA1 through the PKC-MAPK pathway in human folliculostellate cells [24] and increases serine phosphorylated ANXA1 levels on the cell surface, through the PKC pathway in rat pituitary in vitro [25]. In contrast, potential PKC phosphorylation sites similar to those found in other ANXAs are not present in ANXA5 [26], although a potential pseudo-substrate site for PKC was reported in ANXA5 [27]. While both ANXA1 and A5 expression are induced by the activation of PKC in the pituitary cells, as demonstrated in this study, other PKC mechanisms might be involved in the regulation of ANXA1 and A5. PKCα, PKCβII, PKCδ, and PKCε activation by TPA has been reported to mediate ERK2 and JNK1 expression in LβT2 cells [28]. Several PKCs involved in GnRH action might be key factor(s) to explain the different responses to TPA between ANXA1 and A5 expression.
Overall, ANXA1 and A5 expression was stimulated by GnRH through the activation of MEK-MAPK in LβT2 cells. Since LH secretion is stimulated by ANXA1 [17] and A5 [13], both are likely to be involved in GnRH-regulation of LH secretion from the gonadotropes. The difference in the response patterns of ANXA1 and A5 expression following GnRH and TPA stimulation suggests that the expression of these proteins is regulated by different intracellular mechanisms that share a common pathway—the PKC-MAPK pathway. Considering the Ct value obtained using real-time PCR, basal ANXA5 mRNA levels appear to be higher than basal ANXA1 mRNA levels. However, a sharp increase in ANXA1 mRNA levels induced by GnRH might suggest that the ANXA1 plays a different role in GnRH-regulating mechanism from ANXA5. This study demonstrates that ANXA1 is a novel target gene of GnRH.
This work was supported in part by the Japan Society for the Promotion of Science KAKENHI, Grant-in-Aid for Scientific Research (21K05964) to T.M. and (17K08128) to M.K. The authors would like to thank Professor P. Mellon for providing LβT2.
The authors declare no conflicts of interest associated with this research.