2022 年 69 巻 5 号 p. 585-596
2022 年 69 巻 5 号 p. 585-596
Whole-body vibration (WBV) can improve skeletal muscle function in aging mice, but whether the effect on young and aging skeletal muscle is consistent has not been studied. We selected C57BL/6J mouse models, which were divided into young control group (YC), young vibration group (YV), aging control group (AC) and aging vibration group (AV). After 12 weeks of WBV, we found that compared with the YC group, the pathways of linoleic acid metabolism, biosynthesis of unsaturated fatty acids, arachidonic acid metabolism, nicotinate and nicotinamide metabolism, glycine, serine and threonine metabolism, and arginine and proline metabolism improved significantly in the YV group. Compared with the AC group, the pathways of arachidonic acid metabolism, alpha-linolenic acid metabolism, biosynthesis of unsaturated fatty acids, pentose and glucuronate interconversions and pentose phosphate pathway improved significantly in the AV group. Furthermore, we found that WBV decreased triglyceride (TG), total cholesterol (TC), and free fatty acid (FFA) levels in aging mice, improved mitochondrial membrane potential, and increased the expression of phosphorylated activated protein kinase (p-AMPK), peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) and carnitine palmitoyl transferase 1B (CPT1B) in the skeletal muscle of young and aging mice. Our study revealed that WBV mainly improved lipid metabolism and amino acid metabolism pathways of skeletal muscle in young mice and mainly improved lipid metabolism and glucose metabolism pathways of skeletal muscle in aging mice. WBV can activate the AMPK/CPT1 signaling pathway and improve mitochondrial function in skeletal muscle in both young and aging mice.
AGING is an inevitable process of gradual degradation of the body. Aging can cause changes in body composition, resulting in fat accumulation and an increased fat content in muscle, resulting in the decline of muscle quality and the damage of corresponding organ function [1, 2]. As one of the most important organs of the human body, skeletal muscle plays a significant role in energy metabolism and exercise [3]. Physical exercise is considered an important intervention to increase the life span and promote healthy aging [4], and it is an effective method for elderly individuals to reduce the risk of various diseases and injuries [5]. Studies have shown that exercise can delay some changes associated with aging [6], and endurance sports can enhance mitochondrial biosynthesis of skeletal muscle and insulin sensitivity in elderly people [7]. However, due to the decline in physical ability, walking obstacles and lack of companionship, many elderly people cannot do physical exercises. As a passive way of movement, whole body vibration (WBV) is an effective training method to improve muscle movement and is especially suitable for elderly and disabled people [8]. Studies have shown that WBV can improve muscle strength, reduce muscle atrophy and reduce the risk of falls in elderly people [9]. Whole body vibration can reduce the area of the aortic pulse of atherosclerotic mice via insulin-like growth factor 1 [10]. WBV may prevent or reverse age-related skeletal muscle weight loss [11].
Our previous study found that whole-body vibration exercise can improve the lipid metabolism level of skeletal muscle in aging mice, but whether the effect of vibration exercise on young and aging skeletal muscle is consistent has not been studied thus far. Metabolomics is a modern analytical technique with high throughput and high sensitivity. Thus, we use metabolomics to further explore. As an energy receptor in eukaryotic cells, the expression of AMP-activated protein kinase (AMPK) in skeletal muscle can be activated by exercise [12, 13]. Activation of the AMPK-carnitine palmitoyl transferase 1 (CPT1) signaling pathway can reduce lipid deposition and improve lipid metabolism. Inhibition of CPT1 can lead to mitochondrial dysfunction [14, 15]. The mitochondrial function of skeletal muscle is impaired during aging [7].Therefore, we hypothesized that WBV can enhance the mitochondrial function of skeletal muscle by activating the AMPK-CPT1 signaling pathway in aging mice. For this purpose, we selected C57BL/6J mouse models to further explore the influence of WBV on skeletal muscle in young and aging mice by metabolomics to provide a reliable basis for improving skeletal muscle metabolism in elderly individuals.
According to the requirements of the experimental design, the experimental animals were prepared using C57BL/6J mice (2 months and 18 months). All mice were maintained in the Animal Center of Jinzhou Medical University. Mice was given free access to food and water. The experimental protocols were approved by the Ethics Committee of Jinzhou Medical University.
Experimental protocolsAll mice were given one week to allow acclimatization to the environment, after which the young mice were randomly divided into a young control group (YC, n = 16) and young + vibration group (YV, n = 16) and the aging mice were randomly divided into an aging control group (AC, n = 16) and aging + vibration group (AV, n = 16). According to a literature review and previous experiments, the mice in the YV and AV groups were treated with whole-body vibration for 30 min once a day, 6 days per week (frequency: 15 Hz; amplitude: 2 mm; and acceleration: 0.68 g) [10, 16, 17]. Weight and fasting blood glucose (FBS) were measured once a month in each mouse that was fasted overnight.
Blood collection and tissue preparationAfter 12 weeks of vibration, the mice in each group were sacrificed, and blood samples and gastrocnemius muscles were collected. Blood was immediately centrifuged to obtain serum, which was stored at –80°C for further examination. The skeletal muscle samples were divided into two parts. One part was stored directly at –80°C, and the other part was stored in 4% paraformaldehyde for further histological examination.
Extraction and detection of metabolitesSkeletal muscle samples were taken from a –80°C refrigerator, defrosted at 4°C, weighed with an electronic balance, then added 10 times the mass of 50% methanol and fully homogenized. Then, 100 μL from each sample was added to 400 μL of acetonitrile and shaken and mixed well overnight at –20°C. On the second day, all samples were centrifuged at 20,000 g for 10 min, the supernatant was removed and lyophilized, and 50 μL of complex solution (water: acetonitrile = 1:1) was added to each sample. After mixing, in order to monitor the stability and repeatability of instrumental analysis, 5 μL of each sample was taken for QC. QC samples were inserted regularly and analyzed every 3 samples. All samples were collected by an LC-MS system according to the instrument instructions. First, an ultrahigh-performance liquid chromatography (UPLC) system (SCIEX) was used for chromatographic separation, and an XBridge Beh C18 column (3.5 μm, 2.1 mm × 100 mm, Waters, UK) was used for reversed-phase separation. The metabolites were detected by a triplet high-resolution tandem mass spectrometer 5600 (SCIEX). IDA mode was used for mass spectrometry data acquisition. The software used for peak extraction was XCMS, and its processing includes peak alignment, peak extraction, normalization, deconvolution and other steps. In this study, adduct ions were identified based on the HMDB database, and the extracted peak list was used for subsequent statistical analysis.
Hematoxylin and Eosin stainingThe gastrocnemius muscles were removed from 4% paraformaldehyde, dehydrated in gradient alcohol, cleared with xylene, embedded in prepared paraffin, and finally placed in a finished paraffin block on the machine to make 4–6-μm-thick slices. Then, HE staining was performed with a hematoxylin eosin (HE) staining kit.
Immunofluorescence stainingSkeletal muscle fixed in 4% paraformaldehyde was made into frozen slices. The slices were dried and rinsed with phosphate buffer solution (PBS), incubated with Triton X-100 for 30 minutes, washed with PBS three times, and incubated with goat serum for 2 hours. Then, the primary antibodies were added, and the slices were placed in a damp box at 4°C overnight. The next day, the primary antibody was discarded, the slices were washed three times with PBS, and then secondary antibodies were added. After 2 hours, the secondary antibodies were washed off. Finally, the slices were sealed with DAPI, and pictures were taken under a fluorescence microscope.
Western blot (WB) analysisThe muscles were placed on ice, sheared using RIPA lysis buffer, and centrifuged at 4°C to obtain proteins, which were quantified by BCA reagents and processed for Western blotting as described previously [16]. The main primary antibodies used in our experiment were as follows: Anti-AMPKα (1:1,000, Affinity, USA), Anti-phospho-AMPKα (1:1,000, Affinity, USA), Anti-CPT1B (1:1,000, Affinity, USA), Anti-PGC-1α (1:1,000, Abcam, UK), and Anti-GAPDH (1:1,000; Proteintech, USA).
Electron microscopyFresh muscles were cut into slices smaller than 1 mm × 1 mm × 1 mm and fixed with electron microscope fixative at 4°C, rinsed, dehydrated, embedded and made ultrathin, and then observed by transmission electron microscopy.
Measurement of mitochondrial membrane potentialThe fresh gastrocnemius muscle of the mice was weighed in a 1.5-mL centrifuge tube; the weight of each sample was approximately 100 mg and it was washed with PBS once. The centrifuge tube was put on ice, and the tissue was cut into small pieces with scissors. Then, 10 volumes of precooled PBS were added and incubated in a bath for 3 minutes. A total of 600 g was centrifuged for 15 seconds, the tissue sample was precipitated, and the supernatant was discarded. Then, 8 volumes of precooled trypsin digestive solution were added and placed in an ice bath for 20 minutes. A total of 600 g was centrifuged for 15 seconds, the tissue sample was precipitated, and the supernatant was discarded. Two volumes of mitochondrial separation reagent was added, and the tissue was resuspended to wash away the residual trypsin. A total of 600 g was centrifuged for 15 seconds, the tissue sample was precipitated, and the supernatant was discarded. Eight volumes of precooled corresponding mitochondrial separation reagent were added and homogenized in an ice bath 20–30 times. We centrifuged 600 g for 5 minutes at 4°C. The supernatant was carefully transferred to another centrifuge tube and centrifuged at 11,000 g for 10 minutes at 4°C. We carefully removed the supernatant. The precipitate was the isolated mitochondria. Then, 40 μL of the corresponding mitochondrial storage solution was added and resuspended. Then, according to the mitochondrial membrane potential assay kit with JC-1, JC-1 working fluid was prepared, and isolated mitochondria were added. The results were detected by fluorescence microplating. Finally, the ratio of red fluorescence to green fluorescence was calculated.
Oil red O stainingThe gastrocnemius muscles were made into frozen sections. The slices were treated with PBS 3 times, added to 60% isopropanol for 1 minute, stained with oil red O working solution for 20 minutes, washed with distilled water, added to hematoxylin for 1 minute, washed with distilled water and sealed with neutral resin. Lipid deposition in skeletal muscle was observed under a microscope and photographed.
Statistical analysesMetabolomics statistics of the skeletal muscle were analyzed via univariate analysis (t-test and variance multiple analysis), multivariate analysis (principal component analysis (PCA) and partial least squares discrimination analysis (PLS-DA)). The analysis of metabolic pathways in this study was based on the KEGG database and HMDB database.
The other data were expressed as the means ± SD and analysed by SPSS 20.0. One-way analysis of variance (ANOVA) was used. When the variance was equal, Bonferroni’s post hoc test was used, and when the variance was uneven, a Kruskal-Wallis test was used. A p < 0.05 indicated a significant difference.
In our metabolomics study, PCA in unsupervised mode and PLS-DA in supervised mode were used. PCA is an unsupervised multidimensional statistical analysis method that is mainly used to observe the separation trend between groups in the experimental model and whether there are abnormal points. At the same time, it reflects the variability between groups and within groups from the original data. The abscissa represents the first principal component PC1, and the ordinate represents the second principal component PC2. Each point in the figure represents a sample, and different colors represent different groups. The better the sample aggregation, the more stable the detection state of the instrument and the better quality of the collected data. PCA score plots demonstrated that the YC group and YV group were separated, the YC group and AC group were separated, and the AC group and AV group were separated (Fig. 1A–1C). PLS-DA is a supervised discriminant analysis statistical method that can reflect the differences between classification groups to the greatest extent. This method uses partial least squares regression to establish the relationship model between metabolite expression and sample categories to realize the modeling and prediction of sample categories. The abscissa represents the first principal component PC1, and the ordinate represents the second principal component PC2. Each point in the figure represents a sample, and the dispersion degree of different color symbols represents the distribution trend of the two groups of samples on the PC1 and PC2 axes. The PLS-DA models showed that the samples from the YC group and YV group were significantly separated (R2 = 0.9794, Q2 = 0.9329), the samples from the YC group and AC groups were significantly separated (R2 = 0.9400, Q2 = 0.9757), and the samples from the AC group and AV groups were significantly separated (R2 = 0.9626, Q2 = 0.9460) (Fig. 1D–1F).

Multivariate statistical analysis of results (n = 6). (A) PCA score plot (comparison of YC and YV). (B) PCA score plot (comparison of YC and AC). (C) PCA score plot (comparison of AC and AV). (D) PLS-DA scatter (comparison of YC and YV). (E) PLS-DA scatter (comparison of YC and AC). (F) PLS-DA scatter (comparison of AC and AV). YC, young control group; YV, young vibration group; AC, aging control group; AV, aging vibration group. Red dot represents young control group (YC). Magenta dot represents young vibration group (YV). Blue dot represents aging control group (AC). Green dot represents aging vibration group (AV).
The purpose of a heat map is to show the screening results of different ions among different groups and the clustering relationship among different ions more intuitively. The color block in the graph represents the abundance. Blue represents low abundance, and red represents high abundance. The deeper the color, the higher the abundance. To identify small molecule metabolites that are affected by WBV and to observe whether the effects of whole body vibration exercise on skeletal muscle in young mice and aging mice are different in our experiment, 17 different metabolites were identified between the YC group and YV group, 22 different metabolites were identified between the YC group and AC group, and 17 different metabolites were identified between the AC group and AV group (Fig. 2A, C, E).
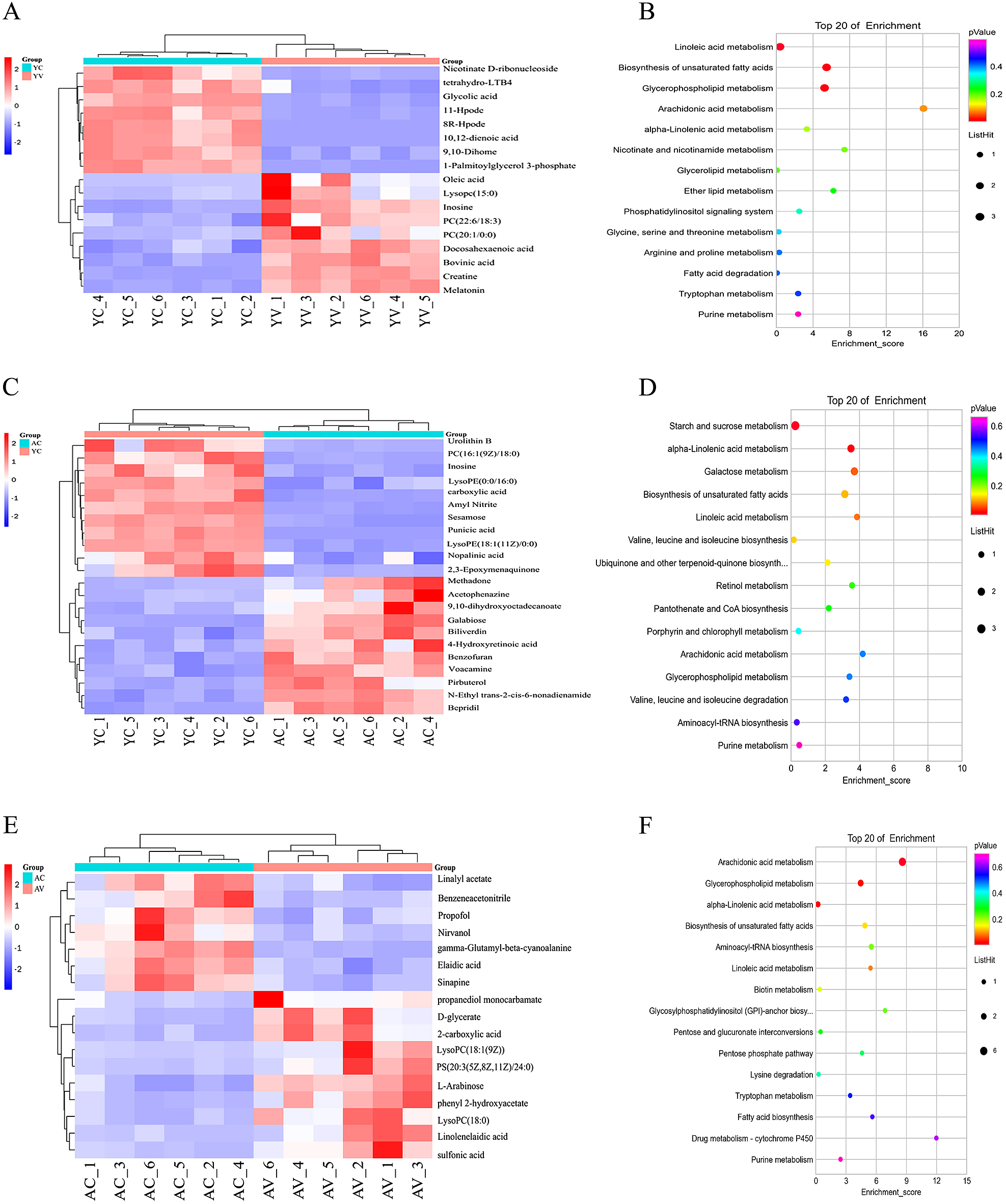
Analysis of biological functions of differential metabolites (n = 6). (A) Heatmap analysis (comparison of YC and YV). (B) Metabolic pathway analysis (comparison of YC and YV). (C) Heatmap analysis (comparison of YC and AC). (D) Metabolic pathway analysis (comparison of YC and AC). (E) Heatmap analysis (comparison of AC and AV). (F) Metabolic pathway analysis (comparison of AC and AV). YC, young control group; YV, young vibration group; AC, aging control group; AV, aging vibration group.
In this study, a KEGG pathway database was used to annotate the pathways of different metabolites. Through pathway enrichment analysis of all skeletal muscle differential metabolites, a pathway enrichment analysis bubble diagram was drawn. The Y-axis shows the KEGG pathway enriched by metabolites, and the X-axis shows the enrichment score. The size of the dot indicates the number of metabolites, and the color of the dot indicates the level of p value. After 12 weeks of WBV, we found that compared with the YC group, the pathways of linoleic acid metabolism, biosynthesis of unsaturated fatty acids, arachidonic acid metabolism, nicotinate and nicotinamide metabolism, glycine, serine and threonine metabolism, and arginine and proline metabolism changed significantly in the YV group, and the pathways of starch and sucrose metabolism, alpha-linolenic acid metabolism, biosynthesis of unsaturated fatty acids, valine, leucine and isoleucine biosynthesis and arachidonic acid metabolism changed significantly in the AC group. Compared with the AC group, the pathways of arachidonic acid metabolism, alpha-linolenic acid metabolism, biosynthesis of unsaturated fatty acids, pentose and glucuronate interconversions and pentose phosphate pathway changed significantly in the AV group (Fig. 2B, D, F).
WBV had no obvious effect on body weight or blood sugar in either young or old miceAfter WBV, there was no difference in body weight between the YC group and the YV group. Compared with the YC group, body weight increased in the AC group. There was no difference in body weight between the AC group and AV group. There was no difference in fasting blood glucose (FBS) between the YC group and the YV group. There was no difference in FBS between the AC group and AV group (Fig. 3A, B). However, compared with the YC group, the ratio of gastrocnemius muscle to body weight increased in the YV group and decreased in the AC group. Compared with the AC group (p < 0.05), the ratio of gastrocnemius muscle to body weight increased in the AV group (p < 0.01) (Fig. 3C).

Body weight, fasting blood glucose, the level of TC, TG and FFA in blood, HE staining in skeletal muscle after vibration for 12 weeks. (A) Body Weight at every month (n = 8, each group). (B) Fasting Blood Glucose at every month (n = 8, each group). (C) The ratio of gastrocnemius muscle to body weight (%) (n = 8, each group). (D) Total Cholesterol (TC) (n = 8, each group). (E) Triglyceride (TG) (n = 8, each group). (F) Free fatty acid (FFA) (n = 8, each group). (G) HE staining of skeletal muscle. (H) Skeletal muscle bundle space (n = 3, each group). Data were expressed as means ± SD (one-way ANOVA followed by Bonferroni’s post hoc test); * p < 0.05; ** p < 0.01. YC, young control group; YV, young vibration group; AC, aging control group; AV, aging vibration group.
After 12 weeks of WBV, there was no significant difference of triglyceride (TG), total cholesterol (TC), or free fatty acid (FFA) between the YC and YV groups, while TG, TC and FFA were significantly higher in the AC group than in the YC group (p < 0.01). The levels of TG, TC and FFA were lower in the AV group than in the AC group, and the difference was significant (p < 0.05) (Fig. 3D–3F).
Hematoxylin-eosin (HE) stainingThe HE staining results of skeletal muscle indicated that the fibers were arranged in clusters in both the YC group and the YV group. The arrangement was loose, and the bundle spacing was significantly increased in the AC group compared with that in the YC group (p < 0.01). After WBV, the arrangement of muscle fibers in the AV group was orderly, and the bundle spacing was significantly decreased compared with that in the AC group (p < 0.01) (Fig. 3G, H).
WBV improved the mitochondrial function of skeletal muscleElectron microscopy showed that in the YC and YV groups, the arrangement of muscle fibers was complete without damage to the cell membrane, the Z-line was clear and continuous without fracture, the M-line and light dark band were clear and clear, and long oval mitochondria could be seen near the Z-line. Compared with the YC group, there was no significant increase in the number of mitochondria in the YV group. In the AC group, there was more muscle fiber breakage, Z-line breakage, obvious swelling and vacuoles of mitochondria, the number of mitochondria per unit area was found to be 52% lower in the AC group than in the YC group (p < 0.01). Compared with the AC group, sarcomeres were arranged more orderly, myofibril breakage was reduced, there were fewer vacuoles, and the number of mitochondria was increased 34% in the AV group than in the AC group (p < 0.01). Compared with the YC group, the mitochondrial densities per unit area increased 5% in the YV group (p < 0.05) and decreased 54% in the AC group (p < 0.01). Compared with the AC group, the mitochondrial densities per unit area increased 31% in the AV group (p < 0.01) (Fig. 4A–4C). Mitochondrial membrane potential showed that compared with the YC group, mitochondrial membrane potential increased in the YV group (p < 0.01) and decreased in the AC group (p < 0.01). The mitochondrial membrane potential increased in the AV group after WBV (p < 0.01) (Fig. 4D).

Electron microscopy, measurement of mitochondrial membrane potential and Oil red O staining in skeletal muscle after vibration for 12 weeks. (A) Electron microscopy of skeletal muscle. (B) Mitochondrial densities per unit area in skeletal muscle. (C) Number of mitochondria per unit area in skeletal muscle. (D) Measurement of mitochondrial membrane potential in skeletal muscle. (E) Oil red O staining in skeletal muscle. (F) Area of oil red O staining in skeletal muscle. Data were expressed as means ± SD (n = 3, each group, one-way ANOVA followed by Bonferroni’s post hoc test); * p < 0.05; ** p < 0.01. YC, young control group; YV, young vibration group; AC, aging control group; AV, aging vibration group.
Oil red O staining showed that there was no difference in the area of oil red O staining between the YC and the YV groups. Compared with the YC group, the area of oil red O staining increased in the AV group (p < 0.01), and compared with the AC group, the area of oil red O staining decreased in the AV group (p < 0.01) (Fig. 4E, F).
WBV increased the expression of p-AMPK in skeletal muscle of young and aging miceTo verify whether WBV can activate the expression of AMPK, we measured the protein levels of p-AMPK and AMPK in skeletal muscle. Compared with that in the YC group, the ratio of p-AMPK to AMPK was increased in the YV group (p < 0.01) and was suppressed in the AC group (p < 0.01). The ratio of p-AMPK to AMPK increased in the AV group after WBV (p < 0.05). The results suggested that WBV can increase the phosphorylation of AMPK in skeletal muscle in both young and aging mice (Fig. 5A, B).

Protein expression of p-AMPK, AMPK, CPT1B and PGC1-α in skeletal muscle after vibration for 12 weeks. (A) Western blots analysis of p-AMPK and AMPK. (B) Relative intensity of p-AMPK/AMPK in different groups. (C) Western blots analysis of CPT1B and PGC1-α in skeletal muscle. (D) Relative intensity of CPT1B protein/GAPDH in different groups. (E) Relative intensity of PGC1-α protein/GAPDH in different groups. (F) Immunofluorescence image of CPT1B. (G) Percentage of CPT1B positive cell. (H) Immunofluorescence image of PGC1-α. (I) Percentage of PGC1-αpositive cell. Data were expressed as means ± SD (n = 3, each group, one-way ANOVA followed by Bonferroni’s post hoc test); * p < 0.05; ** p < 0.01. YC, young control group; YV, young vibration group; AC, aging control group; AV, aging vibration group.
To investigate whether AMPK can activate the expression of CPT1 after WBV to improve lipid metabolism in skeletal muscle, we performed further research. The Western blot results showed that the expression of peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) (p < 0.05) and CPT1B (p < 0.01) was higher in the YV group than in the YC group and was suppressed in the AC group compared with the YC group (p < 0.01). The expression of PGC-1α and CPT1B increased in the AV group after WBV (p < 0.05). Immunofluorescence staining showed that the percentages of PGC-1α (p < 0.01)- and CPT1B (p < 0.01)-positive cells were higher in the YV group than in the YC group and lower in the AC group than in the YC group (p < 0.01). The percentages of PGC-1α- and CPT1B-positive cells increased in the AV group after WBV (p < 0.01) (Fig. 5C–5I).
Metabolomics is used to study the levels of small molecule metabolites in organisms and can effectively reflect changes in metabolism-related pathways [18]. In this study, we used nontarget metabolomics. We found that with increasing age, the pathways related to glucose metabolism, lipid metabolism and amino acid metabolism changed. As passive exercise, WBV mainly improved lipid metabolism and amino acid metabolism pathways of skeletal muscle in young mice and mainly improved lipid metabolism and glucose metabolism pathways of skeletal muscle in aging mice. Therefore, WBV had an obvious effect on the lipid metabolism pathway of skeletal muscle in both young and aging mice. Regardless of the process of aging or after whole-body vibration exercise, the biosynthesis pathways of unsaturated fatty acids and arachidonic acid metabolism changed obviously. Unsaturated fatty acids are fatty acids that constitute body fat, and indispensable fatty acids for the human body and are divided into monounsaturated fatty acids and polyunsaturated fatty acids. Arachidonic acid is a polyunsaturated fatty acid and is esterified in the form of phospholipids in the cell membrane. As an important component of phospholipids, phosphatidylcholine (PC) and its degradation product lysophosphatidylcholine (Lyso PC) can maintain the stability of the cell membrane. After WBV, the metabolites oleic acid, docosahexaenoic acid, PC (22:6/18:3), linolenelaidic acid and lyso PC increased, which suggested that WBV improved the unsaturated fatty acid metabolic pathway of skeletal muscle in mice. Polyunsaturated fatty acids in the mitochondrial membrane are very important to maintain the integrity of the mitochondrial membrane and the normal function of mitochondria. When mitochondrial lipids are oxidized, the integrity and function of mitochondria may be damaged, resulting in mitochondrial dysfunction [19]. Elevated plasma free fatty acids can lead to mitochondrial disorder in skeletal muscle [20]; thus, we performed further research.
Exercise can reduce lipid deposition in skeletal muscle and increase the mass of skeletal muscle [7]. Studies have also shown that vibration training can increase energy metabolism and reduce the percentage of body fat [21]. WBV did not change the body weight of mice, which may be related to the increase in muscle mass and the decrease in fat content.
Our electron microscopy showed that WBV improved the structure of skeletal muscle mitochondria in aging mice, and the expression of PGC-1α increased in both young and aging mice after WBV. Mitochondrial dysfunction is the core of aging theory, and mitochondrial dysfunction is one of the phenotypic and aging characteristics of age-related diseases [22, 23]. One of the key mediators of mitochondrial function is PGC-1α, and its expression and activity are associated with diseases of multiple ages. PGC-1α can regulate mitochondrial synthesis and function and regulate the energy metabolism of adipose tissue [24]. PGC-1α can prevent the decline in mitochondrial enzyme content related to aging, and it is also necessary for sports training [25]. Endurance training can increase PGC-1α levels in the skeletal muscle of mice and improve mitochondrial function [26]. We also found that the number of mitochondria in skeletal muscle of young mice did not increase after low-frequency and long-duration whole-body vibration exercise. Studies have reported that endurance training regulates mitochondrial biosynthesis in skeletal muscle, replacing old mitochondria with new mitochondria, improving mitochondrial function and increasing the activities of mitochondrial-related enzymes without increasing the number of mitochondria [27].
Aging is thought to be related to the decline in mitochondrial function and the decrease in mitochondrial ATP synthesis induced by AMPK [28]. AMPK is an energy receptor in eukaryotic cells and can improve the ability to adapt to changes in the internal and external environment to maintain the stability of cells and the whole body [29, 30]. Activated AMPK inhibits anabolic pathways (such as adipogenesis) and enhances catabolic pathways (such as adipogenesis or fatty acid oxidation) that produce adenosine triphosphate (ATP) [13]. The study reported that the expression of AMPK in the liver of aging rats induced by D-galactose was decreased due to lipid deposition [31]. Aging affects the AMPK signaling pathway of the heart. Activation of the AMPK signaling pathway can significantly reduce myocardial injury in the elderly heart. AMPK can delay aging [32, 33]. Exercise can activate the expression of AMPK in skeletal muscle and induce energy imbalance in muscles, leading to increased intracellular AMP and ADP concentrations. The muscle energy turnover rate increases greatly, which changes the nucleotide state and indirectly activates AMPK [34, 35]. After 12 weeks of WBV, AMPK was activated in skeletal muscle. Our study confirmed that as a passive movement, WBV can also activate the expression of AMPK in skeletal muscle.
After 12 weeks of WBV, the expression of CPT1B in skeletal muscle of young and aging mice increased. CPT1 is the key rate-limiting enzyme of fatty acid oxidation, which is located in the outer membrane of mitochondria and represents the initiation and regulation steps of β-oxidation of fatty acid [36]. AMPK can decrease the inhibition of CPT1 and increase fatty acid oxidation through Acetyl-CoA Carboxylase inactivation [14, 15]. CPT1B is specifically overexpressed in skeletal muscle and myocardium and can convert intracellular lipoyl coenzyme A into coenzyme A and lipoyl carnitine and increase the oxidation rate of long-chain fatty acyl coenzyme A into mitochondrial fatty acids [37]. Upregulation of CPT1 can enhance skeletal muscle mitochondrial β oxidation, improve the utilization of FFA as an energy source, reduce the accumulation of lipid metabolites, and thus reduce skeletal muscle lipotoxicity [38]. The changes in CPT1 indicated that WBV improved the fatty acid metabolism of skeletal muscle in both young and aging mice.
Our study also found that although WBV activated AMPK, the blood lipid level of young mice did not change. Studies have reported that AMPK is related to the expression of CD36 and/or the content of fatty acid binding protein, CD36 is involved in fatty acid uptake, and fatty acid binding protein is considered an important regulator of fatty acid uptake and oxidation [39]. It has also been reported that exercise could improve the existence of CD36 and fatty acid binding protein on the plasma membrane without changing the protein expression [40]. However, the specific reasons are not clear, and further research is needed.
Based on this metabolomics study, we found that glucose metabolism, lipid metabolism and amino acid metabolism pathways changed in aging mice, as passive exercise WBV primarily improved lipid metabolism and amino acid metabolism pathways of the skeletal muscle in young mice and improved lipid metabolism and glucose metabolism pathways of the skeletal muscle in aging mice. WBV can activate the AMPK/CPT1 signaling pathway and improve mitochondrial function in skeletal muscle in both young and aging mice.
guarantor of integrity of the entire study: Ding-wen Jiang
study design: Ding-wen Jiang; Chang Liu
literature research: Ding-wen Jiang; Dan-meng Zheng
experimental studies: Ding-wen Jiang; Xue-jiao Xing
data acquisition: Ye Chen
data analysis: Ding-wen Jiang; Ye Chen
statistical analysis: Ding-wen Jiang; Ye Chen
manuscript preparation: Ding-wen Jiang;
manuscript editing: Ding-wen Jiang
manuscript review: Chang Liu
This work was supported by National Natural Science Foundation of China (82072076), General Project of Liaoning Provincial Department of Education of China (Grant No. JYTJCZR2020045), Scientific research fund project of Liaoning Provincial Department of Education (Grant No. JJL 202015401), Liaoning Revitalization Talents Program (Grant No. XLYC2002037) and 2020 national innovation and entrepreneurship training program for College Students of China (Grant No. 1271 and Grant No. 1272)
The authors declare that they have no conflict of interest.