2022 年 69 巻 9 号 p. 1035-1042
2022 年 69 巻 9 号 p. 1035-1042
Continuous glucose monitoring (CGM) has been widely used in children and adolescents as well as adults with type 1 diabetes. CGM metrics include three key measurements of target glucose: time in range (TIR: 70–180 mg/dL), time below range (TBR: <70 mg/dL), and time above range (TAR: >180 mg/dL). The primary goal of optimal glycemic control is to increase TIR to more than 70%, while simultaneously reducing TBR to less than 4%, while minimizing severe hypoglycemia to less than 1%, as proposed by the Advanced Technologies and Treatments for Diabetes (ATTD) panel. However, several studies have indicated that the TIR goal is quite difficult to achieve in pediatric patients who have remarkable interindividual and day-to-day glycemic variation due to their irregular lifestyles. Previous studies have demonstrated that patients without an automated insulin delivery system are unlikely to attain the recommended glycemic goals. On the other hand, reduction of hypoglycemia, particularly minimizing severe hypoglycemia, is a critical issue in the effective management of children with type 1 diabetes. Frequent episodes of severe hypoglycemia and hypoglycemia can cause lasting neurological damage. Accordingly, we propose reducing the TBR to less than 5%, rather than just targeting the TIR to more than 70%. In CGM metrics this should be the cardinal glycemic goal for pediatric patients who are either being treated with multiple daily injections of insulin or a conventional insulin pump, but who are not using an automated insulin delivery system.
By providing real-time predictive glucose data, continuous glucose monitoring (CGM) has recently begun to replace the standard fingerstick-blood glucometer in the routine clinical setting. The average number of patients with type 1 diabetes who have used CGM increased from 7% in 2010–2012 to 30% in 2016–2018 [1]. The data from CGM allows the detection of glucose trends, the identification of asymptomatic hyper- and hypoglycemic events, and the review of glucose variability during a certain period. The CGM data are interpreted in an ambulatory glucose profile (AGP), which provides a graphic quantitative display of glucose trends throughout the day. In a composite profile of dynamic glucose activities, AGP provides information on important metrics for glycemic management such as the periods of appropriate glucose and high and low blood glucose levels. In February 2019, the Advanced Technologies and Treatments for Diabetes (ATTD) Congress organized an international panel of researchers and clinicians with abundant knowledge and expertise in CGM technology to standardize the CGM metrics [2]. The metrics included three key measurements of target glucose levels over a 24-hour period: the average time in range (TIR: 70–180 mg/dL), time below range (TBR: <70 mg/dL), and time above range (TAR: >180 mg/dL). TBR and TAR are subdivided into two severity levels, with level 2 being the most severe events of hypo- and hyperglycemia, respectively. Level 2 in TBR, sensor glucose level <54 mg/dL, is linked to the decreased awareness and impaired consciousness of hypoglycemia, which is associated with damage to the central nervous system and mortality. Level 2 in TAR, sensor glucose level >250 mg/dL, is associated with increased risk of developing ketoacidosis and long-term vascular complications [3]. The target ranges and time goals defined for each patient are shown in Table 1. The ATTD panel arrived at a consensus on the glycemic cutoff point of TIR of 70–180 mg/dL for patients with type 1 or 2 diabetes for all age groups. The target ranges and time goals should also be individualized based on the population being treated, with older patients and those with a high risk of hypoglycemia having lower TBR goals and higher TAR ones, and pregnant women having a more strictly-defined TIR of 63–140 mg/dL with a strong focus on the reduction of TAR [2].
| Target range | Time goal for Patients with Standard Risk | Time goal for Patients with High Risk* and Older Patients |
|---|---|---|
| TAR level 2: >250 mg/dL | <5% (<1 h 12 min)** | <10% (<2 h 24 min) |
| TAR level 1: >180 mg/dL | <25% (<6 h) | <50% (<12 h) |
| TIR: 70–180 mg/dL | >70% (>16 h 48 min) | >50% (>12 h) |
| TBR level 1: <70 mg/dL | <4% (<58 min) | <1% (<14 min) |
| TBR level 2: <54 mg/dL | <1% (<14 min) | 0 (0 min) |
| GMI (%)*** | <7 | <8 |
CGM: continuous glucose monitoring, TIR: time in range, TAR: time above range, TBR: time below range, GMI: Glucose management indicator
* High-risk individuals include those with a higher risk of complications, comorbid conditions (e.g., cognitive deficits, renal disease, joint disease, osteoporosis, fracture, and/or cardiovascular disease), and those requiring assistance of care, which can complicate treatment regimens [2].
** Time in parentheses indicates target time/day
*** GMI (%): GMI was calculated as 3.31 + 0.02392 × mean glucose (mg/dL) [41].
The primary goal for optimal glycemic control is to increase TIR to more than 70%, while simultaneously reducing TBR to less than 4% and minimizing extreme hypoglycemia (level 2 in TBR, glucose range <54 mg/dL) to less than 1%. In a study on the relationship between glycosylated hemoglobin A1c (HbA1c) and TIR, four randomized clinical trials, involving 545 adults with type 1 diabetes who had central laboratory HbA1c measurements, showed that TIR (70–180 mg/dL) of 70% and 50% strongly corresponded with an HbA1c of approximately 7% and 8%, respectively [4]. An increase in TIR of 10% corresponded with a decrease in HbA1c of approximately 0.5%. Similar correlations were observed in an analysis of 18 randomized controlled trials [5], which included more than 2,500 patients with type 1 diabetes and type 2 diabetes over a wide range of ages and HbA1c levels. It showed that every 10% change in TIR was equivalent to an inverse change of –0.8% in HbA1c.
In CGM metrics, achieving the glycemic goals for both TIR and TBR would result in a concomitant decrease in TAR, thereby improving glycemic outcome. Therefore, the first therapeutic objective should be to minimize TBR to target levels, followed by addressing TIR and TAR targets [2]. Acute hypoglycemia is a common complication in the management of type 1 diabetes. Reduction of hypoglycemia, particularly the minimization of severe hypoglycemia, is a critical issue in the management of type 1 diabetes [6]. Hypoglycemia is associated with damage of central nervous system and is particularly problematic in young children with type 1 diabetes. The ISPAD (International Society for Pediatric and Adolescent Diabetes) Clinical Practice Consensus Guidelines 2018 has recommended that the HbA1c target to be less than 7.0% in children and adolescents of all ages who have access to the advanced technologies of insulin treatment and have the potential to use CGM [7]. Careful attention must be paid to avoid severe hypoglycemia in patients with risk factors for severe hypoglycemia, such as young children or individuals with irregular lifestyles and eating habits [6]. Therefore, reduction in level 1 TBR to less than 4% and minimizing level 2 TBR to the extent possible, which also contributes to improvement of TIR and TAR, are the two most important issues in the management of pediatric patients with type 1 diabetes.
Although the ATTD panel recommended personalizing CGM metrics to match the needs of patients with diabetes, several studies have demonstrated that the potential to achieve the glycemic goals differed according to insulin-treatment regimen. Patients treated with multiple daily injections of insulin (MDI) tend to show lower TIR, whereas those treated with an automated insulin delivery system, such as an insulin pump with a low glucose suspension system or a closed-loop insulin delivery, are more likely to achieve greater TIR and more satisfactory glycemic control [8].
There are two types of CGM: professional CGM, in which patients are blinded to the results at time of measurement and the glucose data are retrospectively reviewed; and personal CGM, in which patients are required to be more engaged with their glycemic management data according to the glucose trends they are experiencing in real time. Moreover, there are two types of devices used for personal CGM: intermittently scanned CGM (isCGM) and real-time CGM (rtCGM). Patients on isCGM can monitor their glucose levels by scanning the sensor transmitter with a receiver or a smartphone without the burden of frequent finger-sticking. They can obtain more detailed information about their glucose trends by scanning the sensor at any time, including when they recognize symptoms of an oncoming hyper- or hypoglycemic episode or exhibit fluctuating glucose profiles such as on days when they fall ill. However, several studies have demonstrated that frequent scanning with isCGM may affect glycemic control [5, 9-14]. Some studies showed that the mean scanning frequency was approximately 15 times/day in type 1 diabetes [9, 12], whereas it was approximately 8–10 times/day in type 2 diabetes treated with insulin [10]. A European analysis in a large population for type 1 diabetes found a high scanning frequency of 16 scans/day in over 60 million glucose tests [11]. We also reported that the mean scanning frequency was 11.5 times/day in Japanese children and adolescents with type 1 diabetes with isCGM [15]. On the other hand, in a European analysis involving seven countries, Dunn et al. [11] demonstrated that a higher scanning frequency contributed to improved HbA1c, and was linked to increased TIR and decreased TAB and TBR. These results were shown to be consistent across different countries. In our previous study, using icCGM with algorithm of reading data prior to the autumn of 2021, a higher scanning frequency had a significant inverse correlation with HbA1c levels (r = –0.815, p < 0.0001) (Fig. 1), positive correlation with TIR (r = 0.719, p < 0.0001) (Fig. 2a), and inverse correlation with TAR (r = –0.743, p < 0.0001) (Fig. 2b), whereas it had no correlation with TBR (Fig. 2c) [15]. One possible reason for the lack of a correlation with TBR may be that the patients usually start to scan more frequently when they experience the onset of a hypoglycemic episode and continue to scan until they recover from it. These findings suggest that the increased frequency of scans indicates patients’ awareness of their blood glucose levels, and that, as more scans provide more information about those levels, it leads them to achieve improved glycemic control [15, 16]. However, the most important point about CGM is that it allows patients to see their recent glucose trends over a period of time along with their real-time glucose levels and lets them adjust their insulin doses accordingly [2]. Medical staff need to train their patients, family members, and caregivers to apply the CGM data appropriately rather than merely allowing patients to scan more frequently.

Association between scanning frequency and mean HbA1c level.
HbA1c: glycosylated hemoglobin A1c
Study subjects included 85 children with type 1 diabetes.
Analysis was conducted by Pearson’s correlation coefficient. A p < 0.05 was considered as statistically significant.
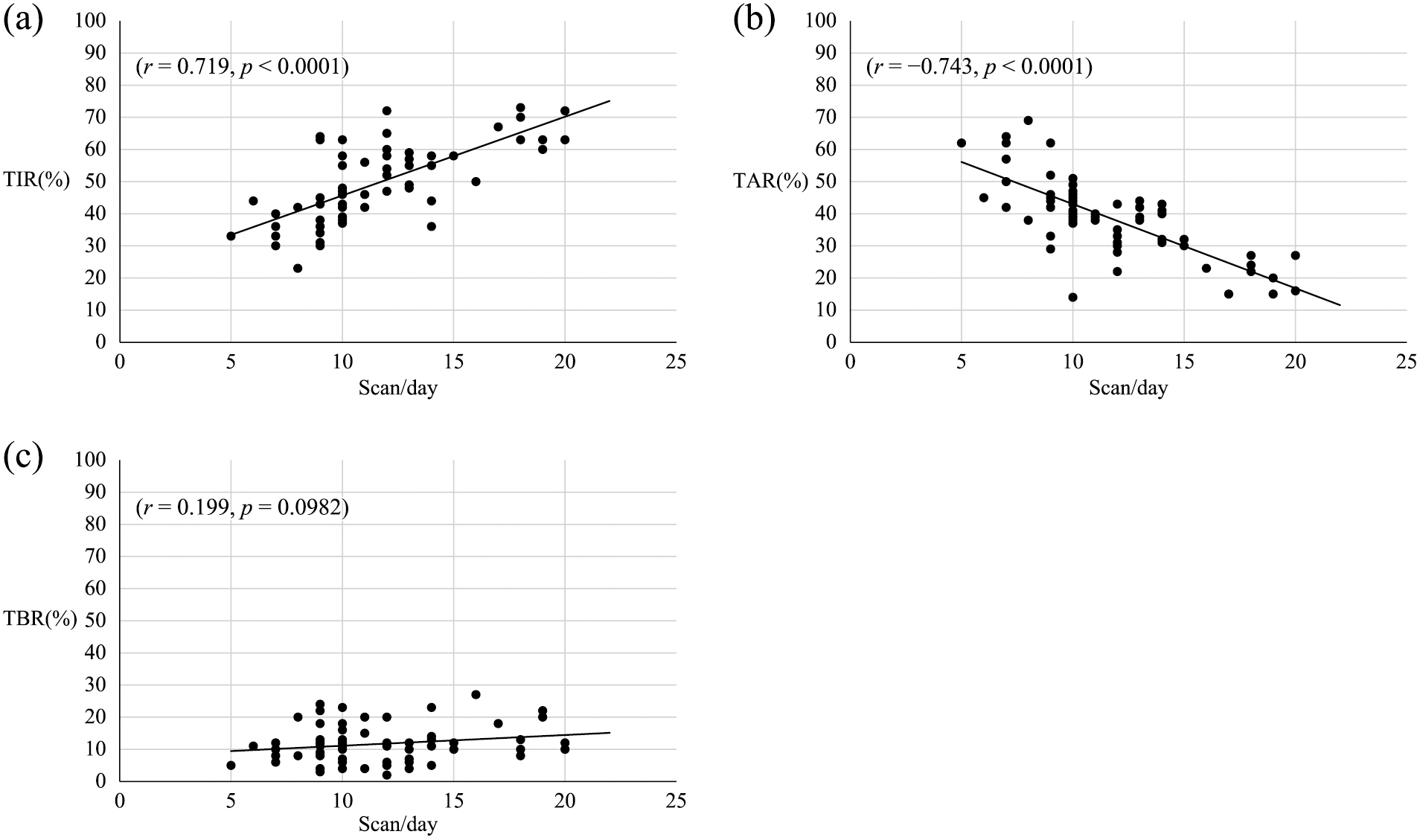
(a) Association between scanning frequency and TIR.
TIR: time in range (70–180 mg/dL)
Study subjects included 85 children with type 1 diabetes.
Analysis was conducted by Pearson’s correlation coefficient. A p < 0.05 was considered as statistically significant.
(b) Association between scanning frequency and TAR
TAR: time above range (>180 mg/dL).
Study subjects included 85 children with type 1 diabetes.
Analysis was conducted by Pearson’s correlation coefficient. A p < 0.05 was considered as statistically significant.
(c) Association between scanning frequency and TBR.
TBR: time below range (<70 mg/dL)
Study subjects included 85 children with type 1 diabetes.
Analysis was conducted by Pearson’s correlation coefficient. A p < 0.05 was considered as statistically significant.
Several studies have demonstrated that both isCGM and rtCGM are superior to self-monitoring of blood glucose (SMBG) for achieving optimal glycemic control and improving the quality of life in patients treated with MDI or an insulin pump [9, 17-19], whereas some studies have indicated that rtCGM is better than isCGM in terms of reduction of hypoglycemia and improvement of glycemic control in adults with type 1 diabetes [20-24]. Reddy et al. [20] and Préau et al. [23] reported that switching from isCGM to rtCGM decreased TBR and simultaneously increased TIR in patients with type 1 diabetes. They suggested switching from isCGM to rtCGM as an alternative to changing the insulin delivery system in patients with suboptimal glycemic control. Maiorino et al. [25] performed a meta-analysis of randomized controlled trials of the effects of CGM on glycemic control in patients with type 1 and type 2 diabetes. They found that TBR was significantly lower and TIR was significantly higher in patients on rtCGM than in patients on isCGM, except for studies in patients treated with a sensor-augmented insulin pump. We also [26] demonstrated that the use of rtCGM is more effective than isCGM with algorithm of reading data prior to the autumn of 2021, with regard to reducing TBR (4.3 ± 2.7% vs. 10.2 ± 5.4%, p < 0.001) and increasing TIR (57.7 ± 12.3% vs. 52.3 ± 12.3%, p = 0.0368) in children and adolescents with type 1 diabetes. The real-time alert/alarm system for hyper- and hypoglycemia with rtCGM may be the main reason for the greater effectiveness of rtCGM. Reduction of the number of hypoglycemic events and prevention of the occurrence of severe hypoglycemia are critical issues for glycemic management in pediatric patients with type 1 diabetes. An alert/alarm system against hypoglycemia warns patients of immediate or impending hypoglycemic events. Although isCGM is useful for adjusting insulin doses according to real-time glucose levels with scanning to improve TIR and TBR, rtCGM with a low-glucose alert/alarm system may be superior to reduce the unpredictable hypoglycemic events. The appropriate adjustment of basal and bolus insulin doses will also reduce TBR.
In our first report [27], we demonstrated the mean frequencies of TIR and TBR in children and adolescents with type 1 diabetes using isCGM were 50.7 ± 12.2% (23–75%), and 11.8 ± 5.8% (2–27%), respectively. The TIR was highly correlated with TAR (r = –0.862, p < 0.0001), and HbA1c level (r = –0.869, p < 0.0001), but not with TBR (r = –0.130, p = 0.2836) [7]. We also reported the mean frequency of TIR as 50.7 ± 12.2% [15] and 52.3 ± 12.3% [26] on isCGM, and 57.7 ± 12.3% [26] on rtCGM in pediatric patients with type 1 diabetes. These data were from CGM with algorithm of reading data prior to the autumn of 2021, and the majority of the subjects were treated with MDI, not with CSII. These TIR results were apparently lower than 70%, which is the primary goal for TIR recommended by the ATTD panel [26]. In the previous reports, Edge et al. [28] showed that the mean frequency of TIR was 50% in pediatric patients aged 4–17 years with type 1 and type 2 diabetes, and Campbell et al. [29] showed that the mean frequency of TIR was 46% in pediatric patients aged 4–17 years with type 1 diabetes. Recent report of an 11-center cross-sectional study in a large group of children with type 1 diabetes using CGM with nonautomated insulin delivery systems demonstrated that the median frequencies of TIR were 49%, 56%, 56%, and 61%. in patients on isCGM with MDI, on rtCGM with MDI, on isCGM with a insulin pump, and on rtCGM with a insulin pump, respectively [30]. It is also reported that among nonautomated insulin delivery systems, simultaneous use of rtCGM and a insulin pump was related to a higher frequency of TIR and a lower frequency of TAR, and a lower value of HbA1c [30]. In these studies, there was a wide interindividual variation in the frequencies of TIR. However, it seems difficult to achieve the recommended TIR goal of more than 70% in pediatric patients treated with nonautomated insulin delivery systems. Children and adolescents with type 1 diabetes are likely to have high magnitudes in postprandial glucose levels and remarkable interindividual and day-to-day glycemic variations. Therefore, it may be substantially difficult to maintain the glucose level of 70–180 mg/dL to more than 70% in the management of pediatric patients with type 1 diabetes. The recommended TIR target proposed by the ATTD panel is more frequently achieved with an automated insulin delivery system: i.e., a sensor-augmented insulin pump with a low-glucose suspension system or a closed-loop insulin delivery with a more accurate glucose sensor and a more sensitive alert/alarm system for hyper- and hypoglycemia. Several reports have demonstrated that closed-loop insulin delivery is substantially superior for achieving better glycemic control while simultaneously reducing hypoglycemia. Jiao et al. [31] evaluated the effectiveness of a closed-loop insulin delivery using systematic review and meta-analysis. Eleven RCTs (817 patients) that satisfied the eligibility criteria were included in the meta-analysis. The analysis indicated that the mean frequency of TIR was 10.3% (95% CI: 8.7–12.0%, p < 0.00001) higher in closed-loop insulin delivery compared with controls (mean frequency of TIR and mean value of HbA1c: a closed-loop insulin delivery: 61.2–71%, 7–7.6% vs. controls: 51.6–60.4%, 7.2–7.9%, respectively). Study subjects reported by the ATTD panel for the CGM-metrics targets [2] mostly consisted of Caucasian pediatric patients, and they were treated with an automated insulin delivery system. By contrast, in Japanese children and adolescents with type 1 diabetes, MDI is still a main means for insulin treatment, and less than 40% of the patients are treated with a insulin pump [32] and few use a sensor-augmented insulin pump or a closed-loop insulin delivery. On the other hand, we reported that HbA1c level of 7.0%, which is an essential worldwide goal even for management of pediatric patients [7], corresponded with a TIR of 55.1% (95% CI: 53.7–56.5%) (Fig. 3a), and a TIR of 70% corresponded with a HbA1c level of 6.1% (95% CI: 5.9–6.3%) (Fig. 3b) [7] in Japanese children and adolescesnts with type 1 diabetes on CGM. Therefore, the glycemic goal should be individualized according to the patients’ characteristics and lifestyles particularly in pediatric patients with type 1 diabetes, who are mainly treated with MDI and a conventional insulin pump although many are also using CGM.

(a) Frequency of TIR corresponded with an HbA1c of 7.0%.
HbA1c: glycosylated hemoglobin A1c, TIR: time in range.
Study subjects included 85 children with type 1 diabetes.
(b) HbA1c level corresponded with TIR of 70%.
HbA1c: glycosylated hemoglobin A1c, TIR: time in range.
Study subjects included 85 children with type 1 diabetes.
With regard to TBR, we reported that the mean frequencies of TBR were 11.8 ± 5.8% [27] and 10.2 ± 5.4% [26] on isCGM with algorithm of reading data prior to the autumn of 2021, while it was 4.3% ± 2.7% on rtCGM with an alert/alarm system [26]. Campbell et al. [29] reported that the mean frequency of TBR as 5% in children aged 4–17 years with type 1 diabetes. In a multinational study that was conducted in seven European countries, Dunn et al. [11] analyzed the isCGM data in more than 60 million glucose tests and reported that the mean frequency of TBR was 6.1–7.0%. Frequent episodes of mild hypoglycemia, particularly with impaired awareness of hypoglycemia can subsequently lead to severe hypoglycemia, with resultant neurological damage [33, 34]. Severe hypoglycemia is known to be a strong risk factor for neurological dysfunction [35, 36] and structural brain abnormalities [37, 38] in children with type 1 diabetes. We demonstrated that most episodes of hypoglycemia occurred during the night [39], and several studies have reported that approximately half of the nocturnal hypoglycemia episodes go unnoticed by the patients themselves and their caregivers [6, 40]. Reduction of hypoglycemia, particularly minimization of severe hypoglycemia, is a critical issue in the management of type 1 diabetes [6], and the ATTD panel emphasized the reduction of TBR to target levels and minimization of level 2 in TBR, followed by addressing TIR and TAR targets [2]. Consequently, reducing the TBR to less than 5%, rather than just targeting the TIR to more than 70%, should be a cardinal glycemic goal for pediatric patients although it ideally needs to be achieved using a nonautomated insulin delivery system. rtCGM with an alert/alarm system should be introduced to pediatric patients at high risk of frequent episodes of hypoglycemia, because isCGM can neither detect nor reduce the unpredictable hypoglycemic events since it has no real-time alert/alarm system.
CGM metrics should be individualized according to the age, level of comprehension, treatment options, and the needs of each patient [2, 27]. Nevertheless, an automated insulin delivery system, such as a sensor-augmented CSII with a hypoglycemia-suspension system or a closed-loop insulin delivery, can help to reduce dysglycemia in pediatric patients with type 1 diabetes. Patients without an automated insulin delivery system have difficulty attaining the ATTD recommended goals: to increase TIR to more than 70% and reduce TBR to less than 5%. Nevertheless, the CGM-metrics goals proposed by the ATTD panel of TIR of 70% and TBR of 5.0% should be adapted according to the patients’ characteristics and needs. Use of CGM is currently popular even in pediatric patients. On the other hand, reduction of hypoglycemia, particularly minimization of severe hypoglycemia, is a critical issue in the management of children with type 1 diabetes. Therefore, using CGM to reduce the TBR to less than 5% should be the cardinal glycemic goal in pediatric patients treated with MDI or a conventional insulin pump, who are not using an automated insulin delivery system.
Tatsuhiko Urakami has received honoraria from Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., Terumo Corp., and JCR Pharmaceuticals Co., Ltd.