2023 年 70 巻 12 号 p. 1123-1130
2023 年 70 巻 12 号 p. 1123-1130
In adrenal fasciculata cells stimulated by ACTH, Ca2+ and cAMP play indispensable roles as second messengers in cortisol production. However, whether their second messengers cooperatively or independently participate in steroid production remains unclear. We focused on the roles of Ca2+ and cAMP in cortisol production in bovine adrenal fasciculata cells stimulated by ACTH for a relatively short period (1 h). Incubation of the cells with 100 pM ACTH in Ca2+-containing (normal) medium for 1 h increased cortisol production without affecting cAMP content. In contrast, treatment of the cells with the peptide at a higher concentration (1 nM) significantly augmented both cortisol production and cAMP content. However, ACTH did not increase either of them in the Ca2+-free medium. ACTH rapidly increased the intracellular free Ca2+ concentration ([Ca2+]i) in the normal medium, but did not influence [Ca2+]i in the Ca2+-free medium, indicating that ACTH caused Ca2+ influx into the cells. ACTH-induced Ca2+ influx and cortisol production were suppressed by a voltage-sensitive L-type Ca2+ channel blocker but not by a T-type, N-type, or P-type Ca2+ channel blocker. In contrast, dibutyryl cAMP, a cell-permeable cAMP analog, greatly enhanced cortisol production in the normal or Ca2+-free medium and slowly caused Ca2+ influx into the cells. These results strongly suggest that Ca2+, as a second messenger, is more critical than cAMP for cortisol production. However, both second messengers jointly participate in the production in adrenal fasciculata cells stimulated by ACTH.
THE ADRENAL GLAND, which consists of the cortex and medulla, is one of the most critical endocrine tissues and produces and secretes vital hormones to maintain somatic homeostasis. The adrenal cortex produces and secretes steroid hormones, typically the glucocorticoid cortisol in the zona fasciculata, the mineralocorticoid aldosterone in the zona glomerulosa, and a sex steroid hormone dehydroepiandrosterone in the zona reticularis. The medulla secretes catecholamines, which are noradrenaline, adrenaline, and dopamine. Under excessive or long-term stressful conditions, the adrenal gland secretes a large volume of hormones and helps cope with stress [1-3].
In adrenal cortisol production, it is generally accepted that a primary physiological secretagogue, ACTH, is secreted from the anterior pituitary, and binds to Gs protein-coupled melanocortin 2 receptors, increasing cAMP via the activation of adenylate cyclase in adrenal fasciculata cells [4, 5]. Consequently, enzymes and proteins involved in steroidogenesis are activated and/or induced via the cAMP-protein kinase A system, and cortisol production is enhanced [6-8]. Thus, cAMP is a critical second messenger involved in ACTH-induced cortisol production in the adrenal fasciculata cells. Ca2+ has also been reported to be a crucial second messenger in cortisol production [9, 10]. The activation of peptide receptors by ACTH depolarizes cell membranes by inhibiting the TREK-1 channel, a K+ channel, which leads to Ca2+ influx into the cells through voltage-sensitive Ca2+ channels, increasing the intracellular free Ca2+ concentration ([Ca2+]i) [11, 12]. In turn, Ca2+-dependent phosphorylation of steroidogenic proteins and transcription of genes encoding the proteins are stimulated, leading to increased cortisol production [13-15].
Thus, Ca2+ and cAMP are indispensable messengers in ACTH-stimulated cortisol production. However, whether their second messengers independently or cooperatively regulate steroid production under normal physiological or stressful conditions remains unclear. Therefore, in bovine adrenal fasciculata cells stimulated by ACTH for a relatively short period, we investigated the roles of Ca2+ and cAMP in cortisol production.
Nifedipine was obtained from Wako Pure Chemical Industries (Osaka, Japan). ω-Agatoxin IVA and NNC 55-0396 dihydrochloride were purchased from Tocris Bioscience (Bristol, UK). ω-Conotoxin GVIA was purchased from Smartox Biotechnology (Saint Martin D’Hères, France). Alamethicin, dibutyryl cAMP (db-cAMP), and Fura-2-AM were purchased from Sigma-Aldrich (St. Louis, MO, USA). The agents were dissolved in dimethyl sulfoxide (DMSO). The concentration of DMSO in the reaction medium was 0.5% and did not affect the production of cortisol and cAMP, as well as the change in [Ca2+ ]i in bovine adrenal fasciculata cells under the conditions used in this study. Human ACTH (1–24) was obtained from Seikagaku Kogyo (Tokyo, Japan). Dulbecco’s modified Eagle’s medium (DMEM) and F-12 Nutrient Mixture (Ham) were obtained from Life Technologies (Grand Island, NY, USA), and fetal bovine serum was obtained from JRH Biosciences (Lenexa, KS, USA). Oxygenated Krebs–Ringer–HEPES (N-[2-hydroxyethyl] piperazine-N'-[2-ethanesulfonic acid]) (KRH) buffer (pH 7.4) was used as the reaction medium and was composed of 125 mM NaCl, 4.8 mM KCl, 2.6 mM CaCl2, 1.2 mM MgSO4, 25 mM HEPES, 5.6 mM glucose, and 0.1% bovine serum albumin. In Ca2+-free KRH buffer, 0.5 mM EGTA instead of CaCl2 was added. The tissue culture instruments were obtained from Falcon Plastics Co. (Cockeysville, MD, USA). The cAMP enzyme immunoassay system was purchased from GE Healthcare (Buckinghamshire, UK). Poly-L-lysine coated cover glass was purchased from AGC Techno Glass Co., Ltd. (Tokyo, Japan).
Methods Isolation and primary culture of bovine adrenal fasciculata cellsThe Kanagawa Meat Center (Atsugi, Japan) generously provided bovine adrenal glands. As previously described, adrenal fasciculata cells were prepared by collagenase digestion [16, 17]. The isolated cells were suspended in DMEM–Ham’s F-12 (1:1) containing 10% fetal bovine serum, cytosine arabinoside (3 μM), and antibiotics (100 units/mL penicillin, 50 μg/mL streptomycin, 100 μg/mL gentamicin, 100 μg/mL neomycin, and 25 μg/mL amphotericin B) and were maintained as a monolayer culture in plates with 15-mm diameter wells at a density of 5 × 105 cells. The cells were cultured at 37°C in a CO2 incubator (95% air/5% CO2).
Measurement of cortisol from bovine fasciculata cellsAfter four days of culture, the cells were washed twice with prewarmed KRH buffer and then incubated with or without the test agents in the presence or absence of ACTH or other stimuli in 1 mL of Ca2+-free or Ca2+-containing KRH buffer for 1 h at 37°C. The reaction was terminated by transferring the reaction medium to tubes in an ice-cold bath. Cortisol production in the medium was extracted with dichloromethane and quantified by the sulfonic acid condensation method using a fluorescence spectrophotometer (650-10S, Hitachi, Tokyo, Japan) at excitation and emission wavelengths of 470 and 520 nm, respectively [18]. The amount of cortisol produced from the cells was expressed as ng/well/h.
Measurement of cAMP level in bovine fasciculata cellsAfter incubating the cells with or without ACTH in Ca2+-free or 2.6 mM Ca2+-containing KRH buffer for 1 h, the reaction medium was removed, and the cells were immediately sonicated with 65% ethanol. The samples were centrifuged at 20,000 × g for 20 min, and the supernatant was collected and dried. The assay buffer was added, and cAMP levels were measured using an enzyme immunoassay [19]. The amount of cAMP was expressed as pmol/well/h.
Measurement of intracellular free Ca2+ concentration ([Ca2+ ]i)Fasciculata cells were loaded with Fura-2 by modifying the method described by Grynkiewicz et al. [20]. The isolated cells were cultured for 24 h on poly-L-lysine-coated coverslips cut to fit into a spectrofluorometer cuvette. The cultured cells on the coverslip were incubated with 5 μM Fura-2-AM, 0.04% Pluronic F-127, and 1.25 mM probenecid in KRH buffer for 3 h at 37°C, followed by washing three times with KRH buffer. The coverslip was placed in a cuvette and preincubated with Ca2+-free or 2.6 mM Ca2+-containing KRH buffer for 2 min at 37°C in a fluorescence meter (CAF-100, JASCO CO. Tokyo, Japan). The test agents were then added to the cuvette, and increases and decreases in a fluorescence induced by the Fura-2-Ca2+ complex were recorded simultaneously at excitation wavelengths of 340 and 380 nm, respectively, and at an emission wavelength of 500 nm. The change in [Ca2+]i was expressed as the ratio of fluorescence at excitation wavelengths of 340 and 380 nm.
Statistical analysisData were expressed as mean ± standard error of the mean (S.E.M.) for each group. One-way analysis of variance (ANOVA) for multiple comparisons followed by Tukey-Kramer’s test was used to determine the statistical significance of differences between experimental groups. A t-test was used to compare the significance of the differences between the two groups. A p value <0.05 was considered to indicate statistical significance.
To explore the role of extracellular Ca2+ in cortisol production and cAMP content in adrenal fasciculata cells stimulated by ACTH, the cells were incubated with various concentrations of ACTH (10 pM–10 nM) in Ca2+-free or 2.6 mM Ca2+-containing (normal) KRH buffer for 1 h (Fig. 1A). ACTH increased cortisol production in a concentration-dependent manner in normal KRH buffer. A slight but significant increase in cortisol production was detected at 10 pM ACTH, and steroid production was almost maximal at 1 nM ACTH. In contrast, ACTH at higher concentrations (1–10 nM) augmented the intracellular cAMP content, but at lower concentrations (10–100 pM), the peptide did not increase the cAMP content (Fig. 1B). Under extracellular Ca2+-free conditions, ACTH did not increase cortisol production or cAMP levels. Thus, the increases in both cortisol production and cAMP content were dependent on the presence of extracellular Ca2+.

Effects of extracellular Ca2+ on cortisol production and cAMP content in bovine adrenal fasciculata cells. (A, B and D) Cultured adrenal fasciculata cells were incubated without (control (Cont)) or with various concentrations of ACTH (10 pM–10 nM) or 1 mM db-cAMP in Ca2+-free or 2.6 mM Ca2+-containing KRH buffer for 1 h at 37°C. (C) Cultured cells were incubated with or without 100 pM ACTH in various concentrations of Ca2+ (0–2.6 mM) -containing KRH buffer. (A, C and D) The cortisol production was expressed as ng/well/h. (B) The cAMP content in the cells was expressed as pmol/well/h. Values represent means ± S.E.M. from at least four experiments. *p < 0.01, significantly different from the control. #p < 0.01, significantly different from ACTH-induced cortisol production.
As shown in Fig. 1C, at a lower ACTH concentration (10 pM), cortisol production was enhanced with an increase in the external Ca2+ concentrations (0–2.6 mM). In 0.26 mM Ca2+, ACTH significantly increased cortisol production. These increases in cortisol production were not observed in Ca2+-free KRH buffer. In contrast, db-cAMP at 1mM, a cell-permeable cAMP analog, significantly induced cortisol production in normal KRH and Ca2+-free KRH buffer (Fig. 1D).
Effect of alamethicin on cortisol production in adrenal fasciculata cellsTo determine the role of extracellular Ca2+ influx into adrenal fasciculata cells in cortisol production, we investigated the effect of alamethicin, a Ca2+ channel-forming peptide that acts as a potent Ca2+ ionophore [21, 22], on steroid production (Fig. 2). Cells were incubated with various concentrations of alamethicin (1–10 μM) for 1 h in normal or Ca2+-free KRH buffer. Alamethicin produced cortisol in a concentration-dependent manner in normal buffer. Steroid production was significantly increased by 1 μM and maximally increased by 5 μM alamethicin, but was attenuated by 10 μM alamethicin. High concentrations of Ca2+ channel-forming peptides impair biomembranes [22]. In contrast, in the Ca2+-free buffer, the alamethicin-induced cortisol production was not observed even at 5 μM.

Effect of alamethicin on cortisol production in bovine adrenal fasciculata cells. Cultured adrenal fasciculata cells were incubated with different concentrations of alamethicin (0–10 μM) in Ca2+-free or 2.6 mM Ca2+-containing KRH buffer for 1 h at 37°C. The cortisol production was expressed as ng/well/h. Values represent means ± S.E.M. from at least four experiments. *p < 0.01, significantly different from the control.
Since cortisol production induced by ACTH was found to be dependent on extracellular Ca2+, to examine external Ca2+ influx into adrenal fasciculata cells stimulated by various stimuli, we measured [Ca2+]i using Fura-2-AM (Fig. 3). When Fura-2-loaded fasciculata cells were stimulated with 100 pM ACTH, an increase in [Ca2+]i was observed after approximately 2 min and became pronounced after 3 min in normal buffer. However, it was not detected in Ca2+-free KRH buffer (Fig. 3A), indicating that ACTH induces the influx of external Ca2+ into the cells. Alamethicin at 5 μM induced an increase in [Ca2+]i in normal medium in a manner similar to ACTH, but did not have a stimulatory effect on [Ca2+]i in Ca2+-free buffer (Fig. 3B). In contrast, db-cAMP slowly increased [Ca2+]i. The db-cAMP effect was detected 8 min after adding the analog, whereas it was not detected in the Ca2+-free KRH buffer (Fig. 3C), suggesting that alamethicin and the cAMP analog introduce external Ca2+ into the cells.

Effects of various stimuli on intracellular free Ca2+ concentration ([Ca2+]i) in bovine adrenal fasciculata cells. Fura-2-loaded adrenal fasciculata cells were preincubated with Ca2+-free or 2.6 mM Ca2+-containing KRH buffer for 2 min at 37°C. Then the cells were incubated with 100 pM ACTH (A), 5 μM alamethicin (B) or 1 mM db-cAMP (C) in Ca2+-free or 2.6 mM Ca2+-containing KRH buffer. The fluorescence was recorded after the addition of each stimulus. The change in [Ca2+]i was expressed as the ratio of the fluorescence at excitation wavelengths of 340 and 380 nm. Data are from a representative sample of at least four experiments.
Next, we evaluated the effects of various voltage-sensitive Ca2+ channel blockers on both the [Ca2+]i (Ca2+influx) and ACTH-induced cortisol production. Nifedipine (Nif) [23], NNC 55-0396 (NNC) [24], ω-conotoxin GVIA (Cono) [25], and ω-agatoxin IVA (Aga) [26] were used as L-type, T-type, N-type, and P/Q-type voltage-sensitive Ca2+ channel blockers, respectively. The Fura-2-loaded cells were preincubated with or without 30 μM Nif, 10 μM NNC, 3 μM Cono, or 20 nM Aga for 2 min and then stimulated with 100 pM ACTH. Nif significantly reduced the increase in Ca2+ influx into the cells induced by ACTH (Fig. 4A). However, NNC and Cono did not affect the ACTH-induced Ca2+ influx (Fig. 4B and C). On the other hand, Aga further enhanced the ACTH-induced Ca2+ influx (Fig. 4D). Voltage-sensitive Ca2+ channel blockers did not affect basal [Ca2+]i (data not shown).
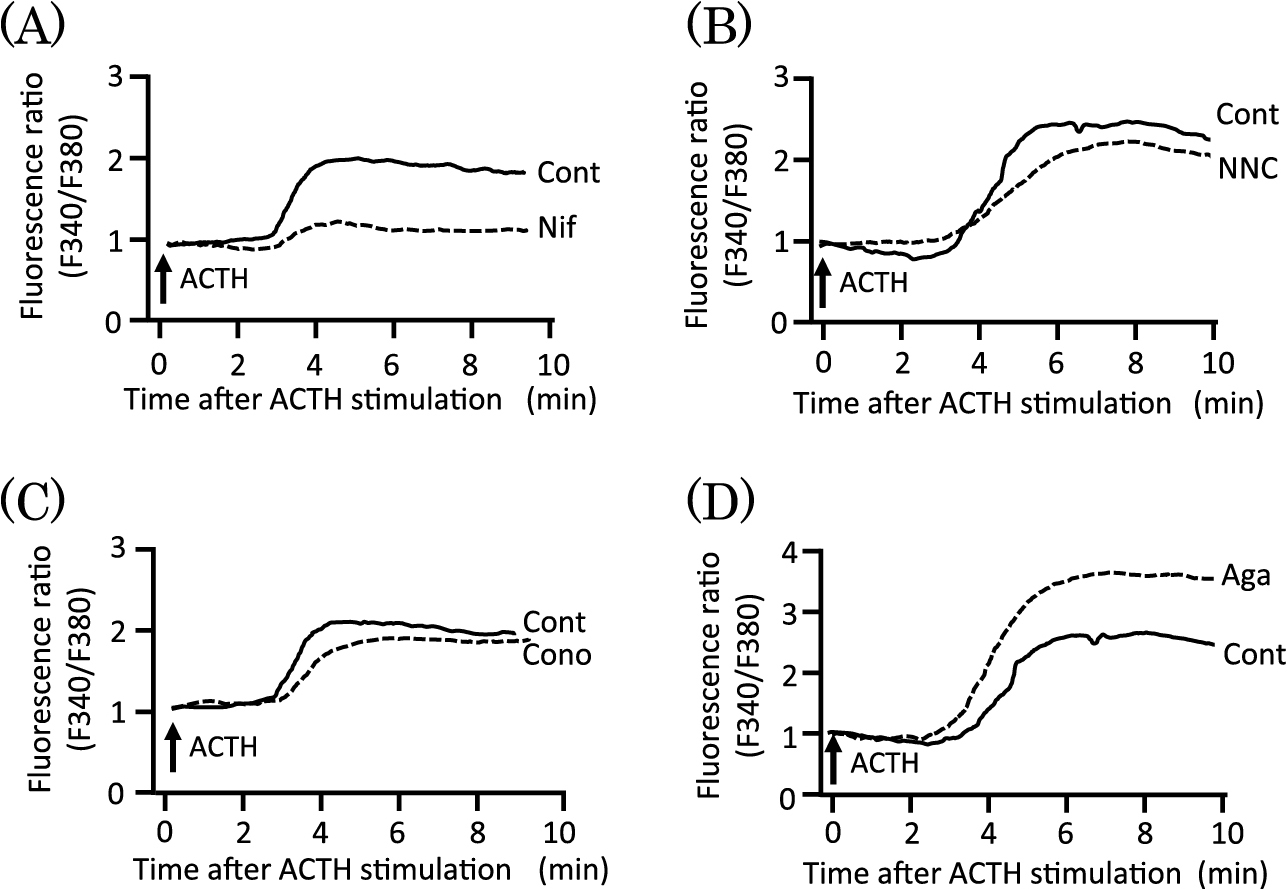
Effects of various voltage-sensitive Ca2+ channel blockers on ACTH-induced increase in [Ca2+]i in bovine adrenal fasciculata cells. Fura-2-loaded adrenal fasciculata cells were preincubated with or without 30 μM nifedipine (Nif) (A), 10 μM NNC 55-0396 (NNC) (B), 3 μM ω-conotoxin GVIA (Cono) (C) or 20 nM ω-agatoxin IVA (Aga) (D) in KRH buffer for 2 min at 37°C. Subsequently, the cells were incubated without (control (Cont)) or with the same Ca2+ channel blockers in the presence of 100 pM ACTH. The fluorescence was recorded after the addition of the stimulus. The change in [Ca2+]i was expressed as the ratio of the fluorescence at excitation wavelengths of 340 and 380 nm. Data are from a representative sample of at least four experiments.
The cells were incubated with various voltage-sensitive Ca2+ channel blockers or NiCl2 for 15 min and then stimulated with 100 pM ACTH for 1 h. Nif at 30 μM significantly inhibited ACTH-induced cortisol production, whereas 10 μM NNC, 3 μM Cono, and 20 nM Aga did not affect it (Fig. 5). NiCl2 at 400 μM, another blocker of T-type voltage-sensitive Ca2+ channels, did not affect ACTH-induced cortisol production. Upon measuring [Ca2+]i using Fura-2, we could not utilize Ni2+ as a T-type blocker because Ni2+ interferes with the interaction between Fura-2 and Ca2+.

Effects of voltage-sensitive Ca2+ channel blockers on cortisol production in bovine adrenal fasciculata cells. Cultured adrenal fasciculata cells were preincubated with or without 30 μM nifedipine (Nif), 10 μM NNC 55-0396 (NNC), 400 μM NiCl2, 3 μM ω-conotoxin GVIA (Cono) or 20 nM ω-agatoxin IVA (Aga) in KRH buffer for 10 min at 37°C. Next, the cells were incubated with or without the same Ca2+ channel blockers in the absence (control (Cont)) or presence of 100 pM ACTH. The cortisol production was expressed as ng/well/h. Values represent means ± S.E.M. from at least four experiments. *p < 0.01, significantly different from basal cortisol production. #p < 0.01, significantly different from ACTH-induced cortisol production.
Voltage-sensitive Ca2+ channel blockers did not affect the basal cortisol production (data not shown).
It is well known that both Ca2+ and cAMP are crucial second messengers involved in intracellular signaling of various tissues. In adrenal fasciculata cells, stimulation with ACTH, a physiological secretagogue, produces the glucocorticoid cortisol via an increase in intracellular Ca2+ concentration [9, 10, 27] and cAMP content [4, 5]. However, the relationship between their second messengers in adrenal steroidogenesis is not completely understood. The present study clarified this correlation.
It appears that Ca2+ is more critical than cAMP for cortisol production in the bovine adrenal fasciculata cells. In the absence of extracellular Ca2+, ACTH did not increase cortisol production or cAMP levels in the cells (Fig. 1). Thus, ACTH-induced increases in cortisol production and cAMP levels were completely dependent on the presence of extracellular Ca2+. Furthermore, lower concentrations of ACTH (10–100 pM) induced Ca2+ influx into the cells and led to increased cortisol production without affecting cellular cAMP content (Figs. 1A, B and 3A). Besides alamethicin, a Ca2+ channel-forming peptide, which is a potent Ca2+ ionophore [21, 22], caused Ca2+ influx into the cells and cortisol production (Figs. 2 and 3B). Therefore, Ca2+ is thought to be the second messenger responsible for cortisol production induced by ACTH, especially at lower concentrations. Presumably, extracellular Ca2+ enters the cells through voltage-sensitive Ca2+ channels across the plasma membranes depolarized by ACTH stimulation, and [Ca2+]i increases. Consequently, steroid production via Ca2+-dependent protein phosphorylation is augmented [13-15]. Indeed, ACTH has been reported to cause Ca2+ influx into adrenal fasciculata cells through voltage-sensitive Ca2+ channels [11, 27-29].
In this study, ACTH was also found to stimulate Ca2+ influx into cells through voltage-sensitive Ca2+ channels, which were the L-type (Figs. 4 and 5). In contrast, Enyeart et al. have shown electrophysiologically and biochemically that they are T- and two L-type voltage-sensitive Ca2+ channels [11]. However, in our study, not only ACTH-induced Ca2+ influx was unaffected by NNC, a potent, selective blocker of T-type channels (Cav3.1) [24, 30], but also ACTH-induced cortisol production was not influenced by NNC and Ni2+, another blocker of T-type channels [28]. This suggests that the channels for Ca2+ influx are only the L-type voltage-sensitive Ca2+ channels. Although the discrepancy between their results and ours cannot be explained, there may be differences in some factors such as cell culture conditions and bovine species. In fact, in the response of adrenal fasciculata cells to secretagogues, the amounts of cortisol production induced by 100 pM and 10 nM ACTH were 120 and 210 ng/106 cells/h, respectively, in the study by Enyeart et al. In contrast, in our study, the amounts were 190 and 1,350 ng/5 × 105 cells/h, respectively. Furthermore, the amounts induced by the cAMP analogs (at 0.6 mM in their study and 1 mM in our study) were approximately 900 ng/106 cells/2 h and 1,200 ng/5 × 105 cells/h, respectively. Our adrenal fasciculata cells produced much more cortisol than the cells prepared by Enyeart et al., when stimulated with ACTH or cAMP analogs. Thus, the responses of the cells prepared by Enyeart et al. to secretagogues differed significantly from those reported in this study. Furthermore, the bovine adrenal glands used by Enyeart et al. were from the steer species (castrated male calf), while those used by us were from castrated Holstein male adults. Therefore, some signaling pathways for cortisol production in cells are considered to be different. These major differences may explain the discrepancy between their results and ours; however, further studies are required. Aga, a blocker of P/Q-type voltage-sensitive Ca2+ channels, did not affect ACTH-induced cortisol production (Fig. 5), indicating a low involvement of P/Q-type channels in steroid production. However, Aga potentiated the ACTH-induced Ca2+ influx into the cells (Fig. 4D). The underlying reason remains unclear, but so far, it has not been reported that P/Q-type voltage-sensitive Ca2+ channels are involved in Ca2+ influx into fasciculata cells. However, this discrepancy in the effects of Aga on Ca2+ influx and cortisol production requires further investigation.
cAMP is also an important second messenger in cortisol production in bovine adrenal fasciculata cells. This argument is supported by several reports demonstrating that cAMP mediates steroidogenesis [4-8]. In this study, ACTH at higher (1–10 nM) but not at lower concentrations (10–100 pM) elevated cAMP levels in the cells and greatly increased steroid production (Fig. 1A and B). This ACTH-induced increase in cAMP levels in cells required the presence of extracellular Ca2+ (Fig. 1B). In contrast, db-cAMP, a cell permeable cAMP analog, augmented cortisol production regardless of the presence or absence of extracellular Ca2+ (Fig. 1D). It is probable that the increase in [Ca2+]i induced by ACTH stimulated adenylate cyclase. In fact, the Ca2+-activated isoform of adenylate cyclase AC1 has been found in human fasciculata cells [31]. It is likely that ACTH binds to melanocortin 2 receptors, inducing Ca2+ influx into the cells through voltage-sensitive L-type Ca2+ channels, and activates AC1 via Gs protein along with Ca2+. Consequently, increased cAMP levels activate protein kinase A, which phosphorylates and/or stimulates the functions of proteins and enzymes involved in steroidogenesis.
db-cAMP increased Ca2+ influx into the cells, but it required more than 8 min to observe an increase in [Ca2+]i after the addition of the analog (Fig. 3C). Protein kinase A phosphorylates L-type voltage-sensitive Ca2+ channels and facilitates Ca2+ influx in human and bovine glomerulosa cells, but not in fasciculata cells [32, 33]. Accordingly, the additional Ca2+ influx into the cells through channels phosphorylated by the cAMP-protein kinase A system also contributes to sustained cortisol production. These results strongly indicate that steroidogenesis in adrenal fasciculata cells stimulated by ACTH at higher concentrations is mediated by both Ca2+ and cAMP.
Although alamethicin induced Ca2+ influx into the cells and increased cortisol production, the amount of steroid production was slight but significant in a manner similar to that induced by ACTH at lower concentrations (Figs. 1A and 2). It has been reported that serum ACTH levels are approximately 60 fold higher in stress-loaded rats (immobilized) than in non-treated rats [34]. Furthermore, in humans, they have been approximately 3 pM under normal body conditions, whereas they have been approximately 22 pM under stressful conditions (strong excise) [35]. Thus, ACTH levels are greatly increased under acute stress conditions. Therefore, it is considered that under normal body conditions, cortisol production is preferentially mediated by Ca2+. However, under stressful conditions (when large amounts of ACTH are secreted), not only Ca2+ but also cAMP, as second messengers, participate in the overproduction of steroids to cope with stress.
This study was partly sponsored by a grant from the Prof. and Ms. Tetsuro Fujita Foundation. We would like to thank Ms. Miki Akiyama and Ms. Tamamo Kurihara for their experimental assistance, and Editage for the English language editing.
The authors declare that there are no conflicts of interest that would prejudice the impartiality of this study.