2023 年 70 巻 4 号 p. 419-426
2023 年 70 巻 4 号 p. 419-426
Acquired fibroblast growth factor (FGF) 23-related hypophosphatemic osteomalacia is characterized clinically by muscle weakness, bone pain, and fractures. Its biochemical features include hypophosphatemia, caused by renal phosphate wasting, and inappropriately normal or low 1,25-dihydroxy-vitamin D levels. Recently, burosumab, a fully human monoclonal antibody targeting FGF23, was approved for the treatment of FGF23-related hypophosphatemic rickets and osteomalacia. We report the case of a 75-year-old Japanese woman with decompensated liver cirrhosis and hepatic encephalopathy, caused by primary biliary cholangitis, who complained of back pain and limited mobility resulting from multiple vertebral fractures. She was not receiving iron infusion therapy and denied alcohol consumption. The patient exhibited hypophosphatemia with a low tubular maximum reabsorption of phosphate per unit glomerular filtration rate (TmP/GFR) and a high circulating concentration of FGF23. Conventional therapy with alfacalcidol and oral phosphate slightly improved her serum phosphate concentration and back pain, but she experienced a hip fracture, causing her to become wheelchair-dependent. Burosumab was initiated 8 weeks after the hip fracture, which increased her serum phosphate concentration and TmP/GFR. Her mobility gradually improved, such that she could walk without a cane after 16 weeks of treatment. Her lumbar bone mineral density increased after 48 weeks. Hepatic encephalopathy developed once before the initiation of treatment and twice after the initiation of the therapy, but her liver function was preserved. This is the first study to report the efficacy and safety of burosumab treatment for FGF23-related hypophosphatemic osteomalacia with decompensated liver cirrhosis.
FIBROBLAST GROWTH FACTOR (FGF) 23-related hypophosphatemic osteomalacia is a rare metabolic bone disease. An excess of FGF23 causes hypophosphatemia because of a reduction in the maximum tubular reabsorption of phosphate per unit glomerular filtration rate (TmP/GFR) and inhibits the intestinal absorption of phosphate because of a reduction in the synthesis of active vitamin D [1]. The resulting chronic hypophosphatemia causes insufficient bone matrix mineralization, leading to osteomalacia, fractures, and pseudo-fractures [2].
A major cause of acquired FGF23-related hypophosphatemic osteomalacia is tumor-induced osteomalacia (TIO), which is characterized by the paraneoplastic secretion of FGF23 [3]. The tumors associated with TIO are usually benign and small, which renders them difficult to identify using standard imaging techniques [4]. A complete surgical resection of causative tumors represents the best treatment option [3]; however, in some cases, a tumor cannot be identified using imaging techniques or resected because of poor liver function. In patients with TIO who have tumors that cannot be resected, conventional treatments are used, such as oral inorganic phosphate and/or active vitamin D preparations [4, 5]. However, the efficacy of such treatments is limited and their use may be associated with gastrointestinal side effects, secondary hyperparathyroidism, renal calcification, ureter stones and renal insufficiency [6].
Recently, burosumab, a recombinant fully human IgG1 monoclonal antibody that targets FGF-23, was approved for the treatment of FGF23-related hypophosphatemic rickets and osteomalacia, including X-linked hypophosphatemia and TIO. In this case report, we report an improvement in mobility following burosumab treatment in a patient with FGF23-related hypophosphatemic osteomalacia and decompensated liver cirrhosis in the absence of treatment-emergent adverse events.
A 75-year-old Japanese woman with liver cirrhosis was referred to our hospital because she had experienced multiple vertebral fractures, including of Th5, Th11, Th12, and L4. She had a history of osteoporosis, osteoarthritis of the knee, and cervical spinal canal stenosis. The underlying pathology for her liver cirrhosis was primary biliary cholangitis (PBC), and she had Child-Pugh grade C liver function. She had poor mobility, and could only walk indoors with a cane, because of back pain and poor lower limb strength.
The patient was prescribed tolvaptan at 7.5 mg once daily, spironolactone at 50 mg twice daily, torasemide at 8 mg once daily, ursodeoxycholic acid at 200 mg three times daily, oral sachets of branched-chain amino acid (BCAA) granules (L-isoleucine, 0.952 g; L-leucine, 1.904 g; and L-valine, 1.144 g per sachet) three times daily, and an oral BCAA-enriched nutrient (Aminoleban EN, Otsuka Pharmaceutical Factory, Inc, Tokyo, Japan) at 50 g once daily. Additionally, she received intravenous nutrition with BCAA-enriched solutions three times a week because of recurrent episodes of hepatic encephalopathy. She was not administered iron infusion therapy, and denied alcohol consumption. She also had no family history of rickets or other metabolic bone disease.
Laboratory analyses revealed hypophosphatemia (1.0 mg/dL phosphate; normal range, 2.5–4.5 mg/dL) and low maximum tubular reabsorption of phosphate per unit glomerular filtration rate (TmP/GFR, 0.580 mg/dL) (Tables 1 and 2). She also had a high serum intact FGF23 concentration (987 pg/mL; normal range, <30 pg/mL; Minaris Medical Co., Ltd, Tokyo, Japan), suggesting that she had FGF23-induced hypophosphatemia. 99mTc bone scintigraphy revealed substantial uptake at multiple foci, including in the vertebral column, ribs, and left femoral neck (Fig. 1A). In addition, her alkaline phosphatase (ALP-IFCC; 439 U/L, normal range, 38–113 U/L; Fujifilm Wako Pure Medical Co., Ltd, Osaka, Japan), and bone-specific alkaline phosphatase (BAP; 114.8 μg/L; normal range, 3.8–22.6 μg/L; Beckman Coulter, Brea, CA, USA) activities were high, but her serum osteocalcin concentration (bone Gla protein, BGP; 15.0 ng/mL; normal range, 8.3–32.7 ng/mL; Tosoh Corporation, Toyama, Japan) was not high, suggesting osteomalacia [7].
| Complete blood count | (normal range) | Biochemistry | (normal range) | ||
|---|---|---|---|---|---|
| WBC | 3,700/μL | 4,300–8,000 | UA | 3.6 mg/dL | 2.6–5.5 |
| RBC | 316 × 104/μL | 395–465 | Na | 137 mEq/L | 138–145 |
| Hb | 11.4 g/dL | 11.3–14.9 | K | 4.1 mEq/L | 3.6–4.8 |
| Ht | 33.5% | 36.0–47.0 | Cl | 108 mEq/L | 101–108 |
| Plt | 22.4 × 104/μL | 18.0–34.0 | Ca | 7.7 mg/dL | 8.8–10.1 |
| Coagulation | (normal range) | Pi | 1.0 mg/dL | 2.5–4.3 | |
| PT | 57% | 70–130 | Mg | 2.0 mg/dL | 1.8–2.4 |
| APTT | 41.4 sec | 24.0–34.0 | FPG | 87 mg/dL | 73–109 |
| Biochemistry | (normal range) | HbA1c | 4.5% | 4.6–6.2 | |
| TP | 5.8 g/dL | 6.6–8.1 | CRP | 2.26 mg/dL | 0–0.40 |
| Alb | 2.5 g/dL | 3.5–5.0 | iron | 54 μg/dL | 40–188 |
| T-bil | 1.6 mg/dL | 0.2–1.0 | TIBC | 132 μg/dL | 251–398 |
| AST | 31 U/L | 13–30 | ferritin | 180.9 ng/mL | 6.2–138 |
| ALT | 14 U/L | 6–27 | Immunochemistry | (normal range) | |
| FIB-4 index | 2.52 | <1.45 | IgG | 1,347 mg/dL | 861–1,747 |
| ALP | 439 U/L | 38–113 | IgA | 426 mg/dL | 93–393 |
| γ-GTP | 9 U/L | 9–32 | IgM | 147 mg/dL | 50–269 |
| ChE | 124 U/L | 201–421 | ANA | 1:1,280 | <1:40 |
| NH3 | 60 μg/dL | 0–70 | anti-M Ab | 1:20 | <1:20 |
| BUN | 15 mg/dL | 8–20 | anti-M2 Ab | <1.5 | 0–6.0 |
| Cr | 0.66 mg/dL | 0.40–0.90 | type Ⅳ collagen | 8.1 ng/mL | 0–6.0 |
| eGFR | 65.42 mL/min/1.73 m2 | 60≤ | hyaluronic acid | 159 ng/mL | 0–50 |
Abbreviations: WBC, white blood cell count; RBC, red blood cell count; Hb, hemoglobin; Ht, hematocrit; Plt, platelet count; PT, prothrombin time; APTT, activated partial thromboplastin time; TP, total protein; Alb, albumin; T-bil, total bilirubin; AST, aspartate aminotransferase activity; ALT, alanine aminotransferase activity; FIB-4, fibrosis-4; ALP, alkaline phosphatase activity; γ-GTP, gamma-glutamyl transferase activity; ChE, cholinesterase activity; BUN, blood urea nitrogen; Cr, creatinine; eGFR, estimated glomerular filtration rate; UA, uric acid; Na, sodium; K, potassium; Cl, chloride; Ca, calcium; Pi, phosphorus; Mg, magnesium; NH3, ammonia; FPG, fasting plasma glucose; CRP, C-reactive protein; TIBC, total iron binding capacity; Ig, immunoglobulin; ANA, anti-nuclear antibody; anti-M Ab, anti-mitochondrial antibody; anti-M2 Ab, anti-mitochondrial antibody, M2 subtype.
| Endocrinological test data | (normal range) | Endocrinological test data | (normal range) | ||
|---|---|---|---|---|---|
| whole PTH | 46.1 pg/mL | 14.9–56.9 | F-T4 | 1.04 ng/dL | 0.90–1.70 |
| 1.25(OH)2D | 10 pg/mL | 20–60 | TSH | 8.420 μIU/mL | 0.500–5.000 |
| 25OHD | 14.7 ng/mL | 30≤ | ACTH | 67.3 pg/mL | 7.2–63.3 |
| BAP | 114.8 μg/L | 3.8–22.6 | Cortisol | 12.6 μg/dL | 3.7–19.4 |
| osteocalcin (BGP) | 15.0 ng/mL | 8.3–32.7 | |||
| TRACP-5b | 1,223 mU/dL | 120–420 | |||
| FGF23 | 987 pg/mL | <30 | |||
| Urinary test data | (normal range) | Arterial blood gas data (room air) | (normal range) | ||
| pH | 7.0 | 5.0–7.5 | pH | 7.486 | 7.350–7.450 |
| urinary gradient | 1.016 | 1.005–1.030 | PaCO2 | 34.8 mmHg | 35–45 |
| glucose | – | — | PaO2 | 85 mmHg | 80–100 |
| protein | ± | — | HCO3– | 26.0 mEq/L | 22.0–26.0 |
| blood | 1+ | — | BE | 2.9 mEq/L | 0 ± 2.0 |
| NAG | 109.7 IU/L | 0.7–11.2 | Anion gap | 6.8 | 12.0 ± 2.0 |
| β2MG | 611 μg/L | ≤340 | |||
| U-Ca/U-Cr | 0.07 | 0.05–0.15 | |||
| FECa | 0.59% | 2.0–4.0 | |||
| TmP/GFR | 0.580 mg/dL | 2.3–4.3 | |||
Abbreviations: PTH, parathyroid hormone; 1.25(OH)2D, 1.25-dihydroxyvitamin D; 25OHD, 25-hydroxyvitamin D; BAP, bone-type alkaline phosphatase activity; BGP, bone Gla protein; TRACP-5b, tartrate-resistant acid phosphatase-5b activity; FGF23, fibroblast growth factor-23; F-T4, free thyroxine; TSH, thyroid-stimulating hormone; ACTH, adrenocorticotropic hormone; NAG, N-acetyl-β-D-glucosaminidase; β2-MG, β2-microglobulin; TmP/GFR, maximum tubular reabsorption of phosphate per unit glomerular filtration rate; BE, base excess.
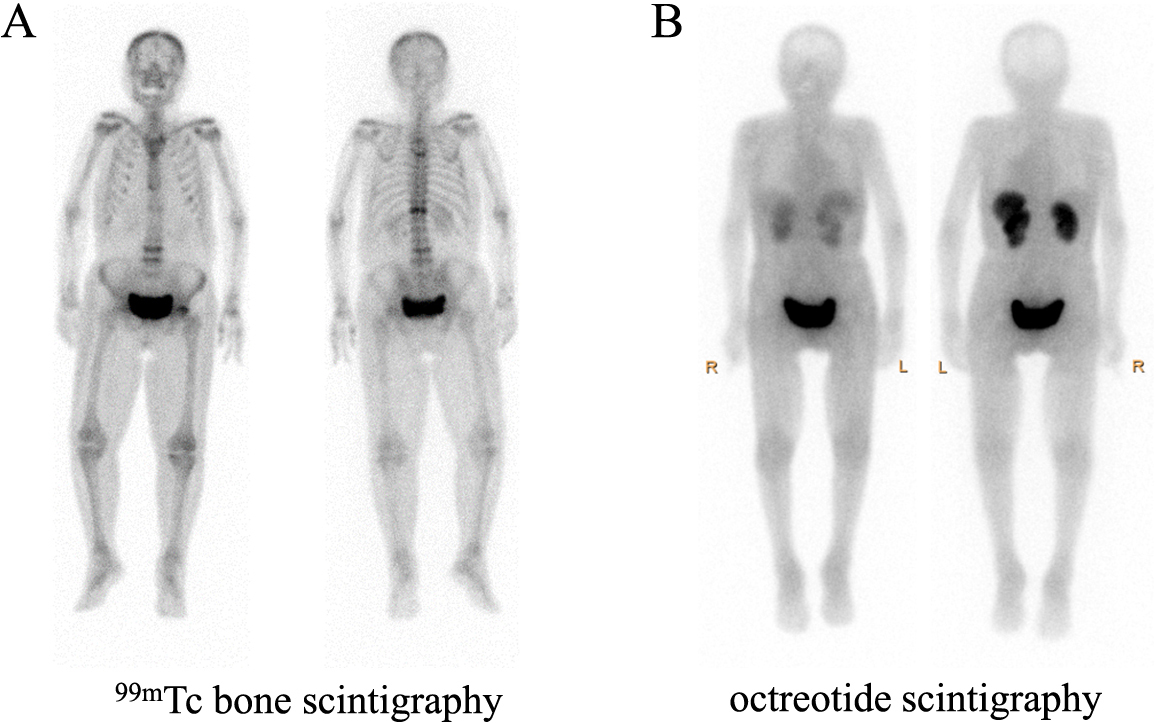
Marked uptake at multiple foci, including in the vertebral column, ribs, and left femoral neck, during 99mTc bone scintigraphy (A) and a lack of uptake during octreotide scintigraphy (B).
A bone biopsy was not performed because of the patient’s poor liver function. However, her circulating tartrate-resistant acid phosphatase-5b activity (TRACP-5b; 1,223 mU/dL; normal range, 120–420 mU/dL; Nittobo Medical, Fukushima, Japan) was high. In addition, her serum 25-hydroxyvitamin D (25OHD) concentration was 14.7 ng/mL (normal range, <30 ng/mL; DiaSorin S. P. A., Saluggia, Italy). Her serum 1,25-dihydroxyvitamin D concentration was low (1,25(OH)2D; 10 pg/mL; normal range, 20–60 pg/mL; Immunodiagnostic Systems, East Boldon, UK) because of the high FGF23 concentration [8]. Renal tubular acidosis (RTA) and Fanconi syndrome were absent. These data were compatible with a diagnosis of FGF23-related hypophosphatemic osteomalacia. At the first visit to our hospital, her CRP were mildly elevated, but before the visit and after admission to our hospital, CRP were not elevated. In addition, her iron status kept normal ranges before and after her admission to our hospital. These data suggested that she was unlikely to be autosomal dominant hypophosphatemic rickets (ADHR), who developed elevation of FGF23 and subsequent hypophosphatemia when they also suffer from iron deficiency or chronic inflammation [9]. The possibility of TIO was considered, and causative tumors were screened for using imaging techniques; however, no tumors were identified using magnetic resonance imaging of the patient’s head and limbs, and no uptake was identified on octreotide scintigraphy (Fig. 1B).
Because we could not find a causative tumor, we initiated conventional treatment with 1 μg/day alfacalcidol and oral phosphate supplementation for osteomalacia. Increasing the dose of alfacalcidol to 2 μg/day returned the patient’s serum phosphate concentration to within the normal range, but her TmP/GFR ratio remained low (Fig. 2). Thereafter, her back pain and physical activity improved slightly, but she developed a fracture of her left femoral neck (FN) 10 weeks after starting the conventional therapy. Although hip replacement was performed, her physical function markedly declined and she became wheelchair-dependent even after surgery and rehabilitation.

Clinical course of the patient, along with the pharmacodynamic parameters and activities of daily living, before and after the initiation of burosumab treatment.
Eight weeks after the patient developed the FN fracture, treatment with subcutaneous burosumab (0.3 mg/kg) every 4 weeks was initiated. Before this, her height was 143 cm, her body mass was 51 kg, and her body mass index (BMI) was 24.9 kg/m2. Her body temperature was 36.9°C, her blood pressure was 140/51 mmHg, and her pulse rate was 98 bpm. Laboratory examination revealed a serum phosphate concentration of 2.2 mg/dL, a TmP/GFR of 1.016 mg/dL, an ALP activity of 158 U/L, a TRACP-5b of 653 mU/dL, and low BGP and 1,25(OH)2D concentrations (7.4 ng/mL and 11 pg/mL, respectively). Bone mineral density (BMD), measured using dual energy X-ray absorptiometry (Horizon W DXA system, Hologic Inc., Marlborough, MA, USA) revealed osteoporosis in both her lumbar spine (LS; 0.624 g/cm2, T-score –3.2 SD) and FN (0.358 g/cm2, –3.9 SD).
Both her serum phosphate concentration and TmP/GFR ratio increased during the 48 week period following the initiation of burosumab treatment (Fig. 2). Serum Pi and TmP/GFR peaked after 8 weeks of treatment, at 3.8 mg/dL and 3.557 mg/dL, respectively, and stayed within their normal ranges subsequently. Notably, the patient’s serum ALP, BGP, and TRACP-5b activities peaked between 4 and 12 weeks of burosumab administration, and then gradually decreased (Table 3). By contrast, the serum concentration of 1,25(OH)2D rapidly increased, fell below the baseline concentration of 11 pg/mL after 4 weeks, but then gradually increased to 34 pg/mL (Fig. 2). In addition, the LS-BMD (T-score) increased from 0.624 g/cm2 (–3.2 SD) to 0.684 g/cm2 (–2.7 SD) during the 48 weeks following the initiation of burosumab treatment.

Although the patient was administered intravenous nutrition with BCAA-enriched solutions three times per week while undergoing conventional treatment, she developed hepatic encephalopathy. In addition, two episodes of hepatic encephalopathy occurred during the early phase of burosumab treatment, as indicated by high plasma ammonia concentrations, because of volume depletion and constipation. However, the patient did not experience subsequent episodes and showed no laboratory signs of deteriorating liver function, such as changes in albumin, bilirubin, or fibrosis-4 (FIB-4) index (Table 3). Before the initiation of burosumab treatment, the patient’s back pain, the result of multiple vertebral fractures, limited her physical activity to walking indoors with a cane, and her FN fracture rendered her wheelchair-dependent. However, she could walk freely inside and to use a walker outside without experiencing pain after 16 weeks of burosumab therapy and rehabilitation.
This is the first report of the successful use of burosumab for the treatment of FGF23-related hypophosphatemic osteomalacia and decompensated liver cirrhosis. After treatment, the patient’s serum phosphate concentration not only returned to within the normal range, but the treatment also improved her mobility, without affecting her liver function. TIO is a major cause of acquired FGF23-related hypophosphatemic osteomalacia [3]. The efficacy and safety of burosumab in patients with TIO have been demonstrated in two clinical trials performed in Asia [10] and the United States [11] and in three patients with TIO [12-14].
We have described the successful treatment of a patient with TIO and decompensated liver cirrhosis secondary to PBC with burosumab. Conventional therapy normalized the patient’s serum phosphate concentration, but not her TmP/GFR ratio, BGP, or 1,25(OH)2D. After she experienced a hip fracture, we switched her treatment to burosumab, and found that her serum phosphate and TmP/GFR ratio to within their normal ranges after 4 weeks, after which they remained stable. In addition, the patient’s LS-BMD increased alongside improvements in serum BGP and 1,25(OH)2D. Although she experienced two episodes of hepatic encephalopathy, there were no laboratory signs of deteriorating liver function, and her back pain and physical activity improved. Thus, this is the first report of the efficacy and safety of burosumab treatment of a patient with TIO and decompensated liver cirrhosis.
25OHD deficiency is the most common cause of osteomalacia [15]. Poor absorption of calcium and phosphate from the intestinal tract, with or without concomitant fat malabsorption, and low vitamin D 25-hydroxylase activity are features of PBC-associated osteomalacia progression [16]. However, osteomalacia is very uncommon in patients with PBC, despite most of them having osteoporosis or osteodystrophy [17]. In the present patient, PBC did not appear to be the principal cause of osteomalacia because the patient had neither RTA nor Fanconi syndrome and 25OHD deficiency was mild. The increase in serum Pi levels after alfacalcidol administration supports the finding that hepatic 25-hydroxylase activity was not impaired in this patient. Therefore, we concluded that hypophosphatemia, secondary to FGF23 overproduction, had exacerbated the osteomalacia.
Metabolic bone markers, such as ALP, BAP, BGP, N-terminal propeptide of type I procollagen (P1NP), and C-terminal cross-linking telopeptide of type I collagen (CTX), initially increase during burosumab administration, peak after 16 or 24 weeks, and then gradually decrease [10, 11]. Similarly, in the present patient, her serum ALP, BGP, and TRACP-5b peaked at between 4 and 12 weeks, and then gradually decreased. Her serum ALP activity closely reflected her bone metabolism because it did not increase during the episode of hepatic encephalopathy. It is thought that BGP reflects mineralization rather than osteoid synthesis because its synthesis and secretion are controlled by 1,25(OH)2D [18]. Therefore, the increase in serum BGP concentration is consistent with the improvement in the osteomalacia of the present patient. It has been reported that the low BGP in a patient with TIO increased during the 2 weeks following the removal of an FGF23-producing tumor, peaked after 8 weeks, and then gradually decreased [19]; and also that a patient with TIO who had not entered remission had a similar clinical course during burosumab treatment [12]. The present patient showed a similar improvement in her serum 1,25(OH)2D concentration, and her low serum BGP increased during the first 12 weeks of burosumab treatment, and then gradually decreased. We have also shown that burosumab may ameliorate impairments in bone mineralization, even in patients with decompensated liver cirrhosis.
There is no evidence that active vitamin D metabolites or analogs or oral phosphate supplementation increases the BMD of patients with TIO, but complete surgical removal of the causative tumor is known to increase BMD [20]. Previous studies have shown that LS-BMD increases after treatment with burosumab [10-12], but this has not been confirmed in larger numbers of patients. In addition, surgical resection and burosumab administration may accelerate the healing of fractures and pseudo-fractures, reduce the risk of new fractures, ameliorate pain, and improve physical function [3, 10, 11, 21-23]. Although the hip fracture that occurred in the present patient prevented accurate assessments, burosumab treatment was associated with an increase in LS-BMD and improvements in pain and physical activity, suggesting that this therapy may improve healthy life expectancy.
The adverse events associated with burosumab use, including nasopharyngitis, contusion, eczema, fatigue, and headache, are mild, but no patients with decompensated liver cirrhosis were included in previous clinical trials [10, 11], and therefore little is known about the suitability of burosumab for the treatment of patients with liver cirrhosis. Although the metabolism of antibody-based medication in patients with liver cirrhosis patients has not been described in detail, it has been reported that denosumab, a human monoclonal antibody that is used for the treatment of osteoporosis, can be safely used in patients with chronic liver disease, including liver cirrhosis [24]. In addition, a fully human monoclonal anti-tumor necrosis factor-α antibody that is used for the treatment of rheumatologic conditions was shown not to increase the risks of ascites, hepatic encephalopathy, or variceal bleeding in patients with compensated cirrhosis [25]. Although hepatic encephalopathy occurred twice in the present patient, it also occurred prior to burosumab treatment. The causes of these episodes were attributed to volume depletion or constipation. In addition, no laboratory signs of deteriorating liver function, including changes in albumin, bilirubin, or FIB-4 index, occurred during burosumab treatment. Therefore, burosumab treatment appears to be safe, even in patients with decompensated liver cirrhosis. In the future, as burosumab treatment of patients with TIO increases, the long-term clinical course, such as changes in FN-BMD and any adverse effects, including tumor progression, should be monitored.
In summary, we have reported for the first time the burosumab treatment of a patient with FGF23-related hypophosphatemic osteomalacia and decompensated liver cirrhosis, in whom the causative tumor was difficult to identify. Burosumab treatment is probably safe and could improve the bone metabolism and physical activity of patients with FGF23-related hypophosphatemic osteomalacia with decompensated liver cirrhosis.
NT: investigation, data curation, writing original draft, YI: conceptualization, review and editing of manuscript, YN, MK, TM, YS: investigation, data curation, TS, ME: supervision. All authors have read and approved the final version of the manuscript.
This article does not report the results of any studies of human participants or animals performed by any of the authors.
Informed consent was obtained from the patient for publication of this case report.
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
YI has received honoraria for serving as an advisory board member and for speaker fees from Kyowa Kirin International Plc. All other authors have no conflicts of interest to declare.