2024 年 71 巻 3 号 p. 245-252
2024 年 71 巻 3 号 p. 245-252
11Beta-hydroxysteroid dehydrogenase 1 (11β-HSD1) is a key enzyme involved in metabolic syndrome. Transcript-specific epigenetic regulation of the gene encoding 11β-HSD1 (HSD11B1) has been reported. We examined the mRNA level and methylation status of the HSD11B1 promoter region in the adipose tissue of patients with primary aldosteronism (PA). We compared 10 tissue specimens from patients with PA caused by aldosterone-producing adenoma (APA) with 8 adipose tissue specimens from patients with subclinical Cushing’s syndrome (SCS) caused by cortisol-producing adenomas, 4 tissue specimens from patients with Cushing’s adenoma (Cu), or 7 tissue specimens from patients with non-functioning adrenal adenoma (NFA). PA, SCS, and Cu were diagnosed according to the guideline of the Japan Endocrine Society. The mRNA level of HSD11B1 was quantified using real-time PCR. Isolated DNA was treated with bisulfite and amplified using primers specific to the human HSD11B1 promoter region. The glycohemoglobin level was significantly higher in patients with APA, SCS, or Cu than in those with NFA (p < 0.05). Blood pressure was significantly higher in patients with APA than in those with SCS, Cu, or NFA (p < 0.01). The HSD11B1 mRNA level was significantly increased in the adipose tissues of APA or SCS patients compared with Cu or NFA patients (p < 0.05). The methylation ratio was significantly lower in SCS patients than in APA, Cu, or NFA patients (p < 0.05). HSD11B1 expression is partly controlled by an epigenetic mechanism in human tissues. The pathophysiological role of epigenetic regulation of HSD11B1 expression in adipose tissue requires further study.
THE AVAILABILITY OF HUMAN CORTISOL to bind and activate the glucocorticoid receptor (GR) is controlled by 11Beta-hydroxysteroid dehydrogenase (11β-HSD). Type 1 11β-HSD (11β-HSD1) is highly expressed in adipose tissue, the liver and skeletal muscle and plays a central role in obesity, diabetes mellitus, and hypertension [1, 2]. Visceral adipose tissue is independently correlated with hypertension, insulin resistance, dyslipidemia, and ultimately, coronary heart disease [3, 4]. Mice overexpressing the gene encording 11β-HSD1 (HSD11B1), specifically in adipose tissue, develop visceral obesity, insulin resistance, dyslipidemia and hypertension [5]. Importantly, the level of circulating glucocorticoids (GCs) was not elevated in this model, suggesting that increased intracellular GC availability influences the observed phenotypes. Morgan et al. [6] reported that adipose-specific HSD11B1 knockout mice were protected from the induction of lipolysis in adipose tissue.
Epigenetic alterations, specifically DNA methylation, either due to natural variations within a population or as a response to external stimuli, frequently lead to alterations in the expression of genes of potential physiological relevance [7]. The role of methylation of the HSD11B1 promoter has been reported in the placenta and skeletal muscle [8, 9].
Reports of primary aldosteronism (PA) have increased in frequency, and PA accounts for 5–10% of the hypertensive population. Increased prevalences of diabetes mellitus and metabolic syndrome in patients with PA reported in the German Conn’s Registry and in the Japan Primary Aldosteronism Study (JPAS) [10, 11]. High levels of aldosterone in people without insulin resistance at baseline were found to predict the development of insulin resistance 10 years later [12]. Experimental and clinical evidence indicates that a high aldosterone level impairs glucose metabolism by inhibiting insulin secretion and increasing insulin resistance [13]. Urbanet et al. [14] reported no differences in HSD11B1 mRNA levels or inflammation factors in adipose tissues between aldosterone-producing adenoma (APA) and nonfunctioning adenoma (NFA). Wu et al. [15] demonstrated increased inflammation and fibrosis in peripheral adipose tissues in patients with APA.
Subclinical Cushing’s syndrome (SCS) is a common disease in patients with metabolic syndrome. SCS is associated with a greater prevalence of cardiovascular risk factors [16]. Sconfienza et al. [17] recently reported that SCS or possible SCS was more prevalent (11.7%) than Cushing’s adenoma (Cu) (3.1%) in functioning adrenal incidentalomas. The circulating cortisol level is not as high in SCS as in Cu. The complications of cardiovascular diseases are similar between SCS and Cu [18].
Mariniello et al. [19] reported that adipose tissue HSD11B1 mRNA was very high in obese subjects compared with control subjects. They found no differences in the expression of this gene between Cu patients and controls. Arai et al. [20] reported impaired 11β-HSD1 activity in a patient with SCS.
We evaluated the methylation status and expression of HSD11B1 in the adipose tissues of patients with APA in comparison with patients with SCS, Cu, or NFA.
Human tissue samples were obtained from Kanazawa University Hospital. Adrenal tumors and adjacent adipose tissues were collected after surgical removal. SCS, Cu, and APA were diagnosed according to the respective guidelines of the Japan Endocrine Society [21, 22]. The immunohistochemical diagnoses of SCS, Cu, and APA were conducted according to a previous report [23]. No patients with APA showed hypercortisolemia. NFAs were detected incidentally by computed tomography performed for unrelated reasons. Patients with clinical NFA had no signs or symptoms of hormone excess; and they had normal serum potassium and plasma cortisol levels suppressible by 1 mg dexamethasone. All samples were frozen in liquid nitrogen and stored at –80°C. Both DNA and RNA were isolated simultaneously from adjacent adipose tissues and used to analyze CpG methylation status and mRNA expression, respectively.
The plasma concentration of aldosterone (PAC) was estimated by radioimmunoassay (RIA) [24]. Plasma ACTH, serum cortisol, and cortisone concentrations were measured using a commercial ELISA kit. Plasma renin activity was measured using a commercial kit (Yamasa Corporation, Chohu, Japan).
The purpose of the study was explained to, and written informed consent was obtained from, all study participants. The use of human tissues was approved by the Institutional Review Board of Kanazawa University Graduate School of Medical Science.
Real-time reverse-transcription PCRReal-time reverse-transcription PCR was performed using the TaqMan Gene Expression Assay, for human HSD11B1 (Applied Biosystems, Life Technologies Japan Ltd. Tokyo). Real-time PCR for the internal controls was conducted using the SYBR Green method according to a previous report using specific primers (Table 1) [9]. PCR was conducted on triplicates of each cDNA sample and the no template controls.
Oligonucleotide DNA used in this study
| Real-time reverse transcriptase PCR | |||
| NAME | Forward primer (5' to 3') | Reverse primer (5' to 3') | Footnotes |
| HSD11B1 | aggaaagctcatgggaggactag | atggtgaatatcatcatgaaaaagattc | Human |
| 18S rRNA | gttggtggagcgatttgtctg | agggc agggacttaatcaacGC | Human |
| Bisulfite sequencing | |||
| HSD11B1P1 | aatggataagtttttagggtaattaggat | aaaaaataaaacccaaaccaaaaa | CpG1 to CpG3 |
| Pyrosequencing | |||
| NAME | Forward primer (5' to 3') | Reverse primer (5' to 3') (biotinylated) | Sequencing primer |
| HSD11B1P1 | gttttgtatagttatgagtttggttatttg | tcaaacaatactaaccaatttccctatc | attatgaaatttattatataggttg |
Genomic DNA (500 ng) was treated with bisulfite and amplified. The human HSD11B1 promoter region was amplified using specific primers according to a previous report (Table 1) [9]. The CpG sites within the HSD11B1 promoter region are shown in Fig. 1. Bisulfite sequencing was performed using the Methylation DNA Modification Kit (EPIGENTEK, NY, USA) with specific primers (Table 1). Eight PCR product clones generated from the DNA of each sample were picked to analyze the DNA methylation status, as we described previously [25]. Methylation analysis by pyrosequencing was also performed using PyroMarkGold Q96 Reagents and the PyroMarkQ24 pyrosequencing system (Qiagen, NJ, USA) [24].

Schema of CpG dinucleotides within the human HSD11B1 gene promoter. Genome coordinates on chromosome 1. Nucleotide numbers are relative to the transcription start site. Open circle denotes CpG dinucleotides. Closed square denotes glucocorticoid response element (GRE).
Microsomes were prepared from the fat pads of adrenal glands according to a method described elsewhere. The protein concentration of microsomes was determined using Bio-Rad Protein Assay solution with BSA as the standard, according to the manufacturer’s instructions (Hercules, CA, USA). 11β-HSD1 activity was determined by measuring the conversion of 3H-cortisone to 3H-cortisol in the incubation medium as described previously [26, 27].
Statistical analysisData are expressed as the mean ± SEM. Data were compared by two-way analysis of variance (ANOVA) or Friedman’s test, and when each test indicated significance, the protected Fisher’s least significance difference test or Scheffe’s F test was performed. Statistical significance was inferred for p < 0.05.
The clinical characteristics of the patients with APA, SCS, Cu or NFA are summarized in Table 2. Body mass index (BMI) was significantly lower in patients with APA than in those with SCS or NFA (p < 0.05). Waist circumference was significantly higher in patients with SCS or Cu than in those with APA or NFA (p < 0.05). APA patients showed significantly higher systolic and diastolic blood pressure compared with SCS or NFA patients (p < 0.01). Both SCS and Cu patients had significantly higher glycohemoglobin (HbA1c), serum triglyceride and LDL-cholesterol levels compared with APA or NFA patients (p < 0.01). The HbA1c level was significantly higher in the APA than NFA patients (p < 0.05). Table 2 shows the 11β-HSD1 activity in each experimental group. The HSD11B1 mRNA and enzyme activity levels in adipose tissue were significantly higher in SCS patients than in APA, Cu or NFAs patients (p < 0.01) (Fig. 2A). APA patients showed higher 11β-HSD1 activity and HSD11B1 mRNA levels compared with NFA patients (p < 0.05). The methylation ratio of CpG1 of the P1 promoter of HSD11B1 in adipose tissues was significantly lower in SCS than APA, Cu or NFA patients (p < 0.01) (Fig. 2B). Inverse correlation between CpG methylation and CYP11B2 mRNA levels was seen in the Supplementary Fig. 1. There was no significant difference in the methylation ratio between APA and NFA patients. The methylation ratio of C2 or C3 of the P1 promoter region did not differ among the four clinical groups (data not shown).
Clinical and hormonal characteristics of patients with SCS, APA, Cu and NFA.
| APA (n = 10) | SCS (n = 8) | Cu (n = 4) | NFA (n = 7) | p value | |
|---|---|---|---|---|---|
| Age (y.o) | 51 ± 3 | 49 ± 5* | 51 ± 4 | 55 ± 3 | *; p < 0.05 vs. NFA |
| Number of male | 6 | 3 | 2 | 4 | |
| BMI (kg/m2) | 23 ± 1* | 26 ± 1 | 25 ± 4 | 26 ± 2 | *; p < 0.05 vs. SCS, NFA |
| Waist C (cm) | 81 ± 4* | 95 ± 3 | 93 ± 6 | 94 ± 5 | *; p < 0.05 vs. NFA, SCS |
| SBP (mmHg) | 151 ± 3** | 139 ± 3* | 141 ± 4* | 127 ± 6 | *; p < 0.05 vs. NFA **; p < 0.01 vs. SCS, NFA |
| DBP (mmHg) | 94 ± 2* | 88 ± 4 | 89 ± 4 | 79 ± 5 | *; p < 0.05 vs. NFA |
| S-K (mEq/L) | 3.5 ± 0.2* | 3.8 ± 0.1 | 3.4 ± 0.4* | 4 ± 0.1 | *; p < 0.05 vs. NFA |
| S-Cr (mg/dL) | 0.8 ± 0.1 | 0.6 ± 0.04 | 0.8 ± 0.09 | 0.9 ± 0.2 | |
| FBS (mg/dL) | 100 ± 4 | 116 ± 9 | 159 ± 21* | 101 ± 5 | *; p < 0.05 vs. APA, NFA |
| HbA1c (%) | 5.9 ± 0.2 | 6.8 ± 0.1** | 7.4 ± 0.4** | 5.5 ± 0.2 | **; p < 0.01 vs. NFA, APA |
| Serum LDL-C (mg/dL) | 117 ± 7 | 138 ± 8* | 127 ± 16 | 98 ± 8 | *; p < 0.05 vs. APA, NFA |
| Serum TG (mg/dL) | 100 ± 11 | 153 ± 8* | 166 ± 36* | 118 ± 18 | *; p < 0.05 vs. APA |
| Serum HDL-C (mg/dL) | 65 ± 8 | 60 ± 4 | 59 ± 11 | 52 ± 5 | |
| PAC (pg/mL) | 218 ± 25# | 61 ± 12 | 64 ± 39 | 83 ± 18 | #; p < 0.001 vs. SCS, Cu, NFA |
| PRA (ng/mL·h) | 0.4 ± 0.1* | 1.2 ± 0.5 | 3.9 ± 2.1 | 1.2 ± 0.6 | *; p < 0.05 vs. SCS, Cu, NFA |
| Serum-E (ng/mL) | 1.9 ± 0.14 | 2.4 ± 0.13* | 6.7 ± 5** | 1.8 ± 0.05 | *; p < 0.05 vs. APA, NFA, Cu **; p < 0.01 vs. APA, NFA |
| Serum-F (μg/dL) | 11 ± 1 | 20 ± 2# | 35 ± 7#, * | 11 ± 1 | #; p < 0.001 vs. APA, NFA *; p < 0.05 vs. SCS |
| Serum-F after 1mg DST | 0.9 ± 0.1 | 12 ± 2# | 25 ± 10 | 1.2 ± 0.1 | #; p < 0.001 vs. APA, NFA |
| Plasma ACTH (pg/mL) | 20 ± 3 | 6 ± 2** | 3.9 ± 2.1** | 26 ± 6 | **; p < 0.01 vs. NFA, APA |
| 11β-HSD1 activity (%) | 20 ± 1* | 30 ± 3** | 16 ± 3 | 14 ± 0.7 | **; p < 0.01 vs. APA, NFA, Cu *; p < 0.05 vs. NFA |
SCS, subclinical Cushing’s syndrome; APA, aldosterone-producing adenoma; Cu, Cushing’s adenoma; NFA, non-functioning adenoma; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; Waist C, waist circumference; S-K, serum potassium; S-Cr, serum creatinine; FBS, fasting blood glucose; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; PAC, plasma aldosterone concentration; PRA, plasma renin activity; E, cortisone; F, cortisol; DST, dexamethasone suppression test; 11β-HSD1, 11β-hydroxysteroid dehydrogenase 1
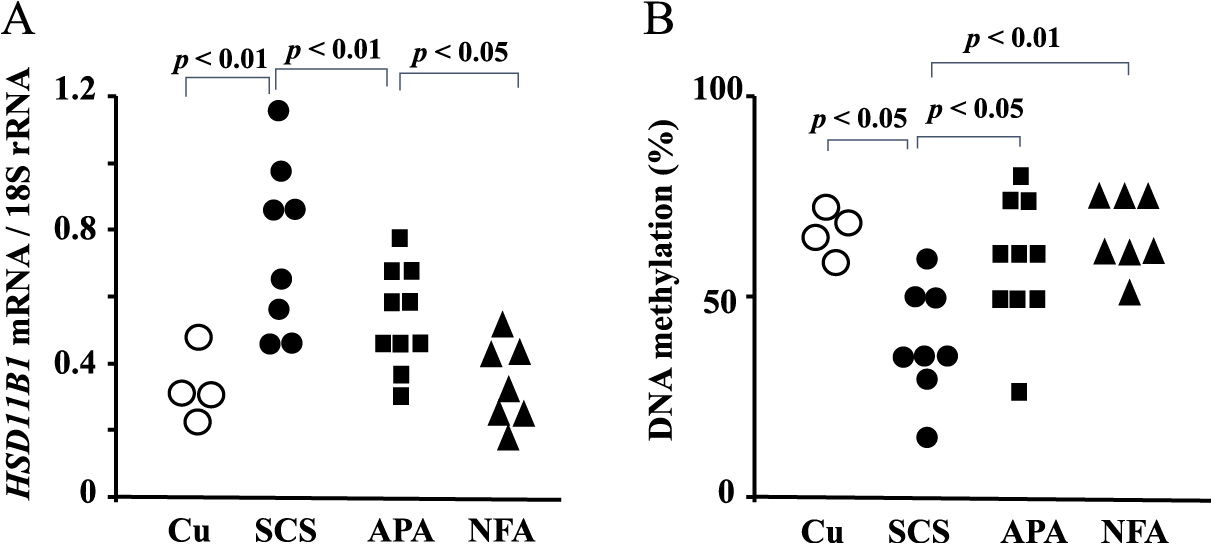
HSD11B1 mRNA levels (Fig. 2A) and methylation ratios (Fig. 2B) in patients with SCS, APA, Cu and NFA. The HSD11B1 mRNA level in adipose tissue was significantly higher in SCS patients than in APA, Cu or NFAs patients (p < 0.01). APA patients showed higher HSD11B1 mRNA level compared with NFA patients (p < 0.05). The methylation ratio of CpG1 of the P1 promoter of HSD11B1 in adipose tissues was significantly lower in SCS than APA , Cu (p < 0.05) or NFA patients (p < 0.01).
In this study, the HSD11B1 mRNA level was higher in the adipose tissues of APA and SCS patients than in NFA patients. The methylation ratio of HSD11B1 was low in SCS compared with APA or NFA adipose tissues. Metabolic abnormalities such as high HbA1c and LDL-cholesterol levels were seen in patients with APA as well as those with SCS.
A role of the local cortisol level in visceral adipogenesis has been implicated in both animal and human studies. Administration of GCs in mice induces metabolic syndrome, which is prevented in HSD11B1-knockout mice [28]. Tomlinson et al. [29] reported a patient with Cushing disease who was protected from the classic Cushing phenotype, owing to a functional defect in 11β-HSD1 activity, as evidenced by serum and urinary biomarker levels. These findings suggest that intracellular metabolism of GCs by 11β-HSD1, rather than circulating DCs is critical to the development of metabolic syndrome.
Patients with APA have a higher risk of cardiovascular events, renal injury, and metabolic disorders [30, 31]. PA is considered to dysregulate glucose homeostasis in multifactorial ways. Hypokalemia induced by hyperaldosteronism impairs insulin secretion [32]. In our study, the serum potassium level of APA patients was 3.5 mEq/L on average. Akehi et al. [11] recently reported a higher prevalence of diabetes in patients with PA (21.6%) compared with the general population (12.1%). In their study, among patients with PA, the prevalence of diabetes was not significantly associated with hypokalemia. Kwak et al. [33] found that hyperglycemia was more prevalent in PA patients, regardless of hypokalemia status, than in the controls. The authors suggested that the effect of aldosterone on glucose metabolism is independent of potassium status.
GC co-secretion is frequently found in PA and contributes to the associated metabolic risk. We reported that a high prevalence of diabetes in patients with PA was associated with subclinical hypercortisolism [11]. Gerards et al. [34] reported that type 2 diabetes mellitus was detected in 20% of PA patients with subclinical hypercortisolism. In their study, some patients with APA treated with adrenalectomy did not show a complete cure of glucose intolerance. In an in vitro study, the mineralocorticoid receptor antagonists (MRAs) spironolactone, eplerenone, and RU-28318 did not prevent the inhibition of insulin secretion by aldosterone, suggesting that its impact on insulin secretion was mediated via a mineralocorticoid receptor-independent mechanism [32].
In our study, 11β-HSD activity and gene expression in adipose tissues of were significantly increased in APAs than in NFAs. Wu et al. [15] reported that expression of genes related with fibrosis and adipogenesis in adipose tissue was higher in patients with APA than in patients with NFA. Human studies have shown that 11β-HSD1 expression in visceral adipose tissue is positively associated with visceral obesity with hypertension [35]. Japanese patients with PA are usually not obese. A large number of cohort studies of PA in Japan showed that the BMI is 24 on average [11, 30]. In our patients with APA, the BMI was significantly lower than those with NFAs. We reported that treatment with a mineralocorticoid receptor antagonist decreased the visceral fat area in patients with PA [36]. Shibayama et al. [37] reported that the visceral fat area is correlated with the plasma aldosterone level in patients with hyperaldosteronism. Although the effect of aldosterone on 11β-HSD1 in adipose tissue has not been elucidated, Hori et al. [38] showed that a MRA inhibits 11β-HSD1 expression in the left ventricle of rat with cardiac fibrosis. In APA, aldosterone may increase adipose 11β-HSD1 and tissue-specific activation of cortisol influences glucose metabolism.
MicroRNAs (miRNAs) are a class of non-coding small RNA molecules that regulate gene expression. Han et al. [39] reported that miRNA-340, -561 and -579 are potential regulator of HSD11B1 expression. Cocistre et al. [40] reported that several miRNAs in fat surrounding APA were significantly higher than in adjacent adipose tissue of NFA. Further studies are needed to clarify the pathophysiological roles for miRNAs in adipose tissues of APA.
SCS is a condition involving biochemical cortisol excess without the classical signs or symptoms of overt Cushing’s syndrome. The prevalence of SCS is suggested to be 1–10% in patients with diabetes mellitus. The available data suggest that SCS may be associated with not only diabetes mellitus but also obesity, hypertension and metabolic syndrome. Although an association between abdominal obesity and metabolic dysfunction has been established, the underlying mechanisms remain to be elucidated. The increased mRNA and enzyme activity levels of 11β-HSD1 in the adipose tissue of SCS may increase local GC availability and contribute to diabetes, hypertension, and dyslipidemia.
In our results, adipose 11β-HSD1 mRNA and enzyme activity levels did not differ between Cu and NFA, which concords with the findings of Mariniello et al. [19]. Espindola-Antunes et al. [41] also reported the absence of a correlation between HSD11B1 mRNA expression in visceral adipose tissue and the salivary free cortisol level in patients with Cu.
DNA methylation of the 5'-cytosine of CpG dinucleotides is a major epigenetic modification in eukaryotic genomes and is required for mammalian development. We reported that the hypomethylation status of CYP11B2 is negatively correlated with its mRNA level in APA [24]. CYP11B1 gene expression is also controlled by methylation in the adrenal gland [42]. Inder et al. [8] reported a negative correlation between changes in the methylation status and mRNA level of HSD11B1 in the skeletal muscle of diabetic patients. In our study, HSD11B1 methylation status was decreased and gene expression increased in the adipose tissue of SCS patients. These results indicate that HSD11B1 gene expression is related to its methylation level in the adipose tissue of SCS patients. Although the HSD11B1 mRNA level in adipose tissue was significantly elevated in APA compared with NFA patients, the methylation status did not differ. These results suggest a different regulatory mechanism of HSD11B1 expression between APA and SCS patients. Further studies are necessary to clarify the regulatory mechanism of HSD11B1 gene expression.
This study has the following limitations. The number of samples was small. APA patients were treated with MRAs before surgery and the effects of MRA on adipose 11β-HSD1 are unclear.
HSD11B1 expression is partly controlled by an epigenetic mechanism in human tissues. The mechanism of epigenetic regulation of HSD11B1 and its pathophysiology in adipose tissue require further study.
The authors declare no conflicts of interest.
Health and Labour Sciences Research Grant (20FC1020).

Correlation between DNA methylation ratio and HSD11B1 mRNA level in patients with subclinical Cushing’s syndrome and non-functioning adenoma.