Abstract
Maintenance of islet function after in vitro culture is crucial for both transplantation and research. Here we evaluated the effects of encapsulation in alginate fiber on the function of human islets which were distributed by the Alberta Islet Distribution Program. Encapsulated human islets from 15 deceased donors were cultured under 5.5 or 25 mM glucose conditions in vitro. The amounts of C-peptide and glucagon secreted from encapsulated islets into the culture media were measured periodically, and immunohistochemical studies were performed. Encapsulated islets maintained C-peptide and glucagon secretion for more than 75 days in 5 cases; in two cases, their secretion was also successfully detected even on day 180. α- and β-cell composition and β-cell survival in islets were unaltered in the fiber after 75 or 180 days of culture. The encapsulated islets cultured with 5.5 mM glucose, but not those with 25 mM glucose, exhibited glucose responsiveness of C-peptide secretion until day 180. We demonstrate that alginate encapsulation enabled human islets to maintain their viability and glucose responsiveness of C-peptide secretion after long-term in vitro culture, potentially for more than for 180 days.
THE USE OF HUMAN ISLETS becomes more important in studies of diabetes and transplantation, because there are many differences in morphology, functionality, and pathology between human islets and rodent islets [1]. Human islets for research are acquired from two sources. The first is a research-only human islet isolation center, and the second are specimens diverted to research use when isolated islets do not meet clinical transplant requirements. Although the latter are more likely of lower islet quality, these islets are very valuable and should be effectively used for research. The Alberta Islet Distribution Program (AIDP) provides human islets for international researchers when islet preparations do not meet the criteria for transplantation [2].
Human islet culture in vitro is also challenging because varied cellular damage accumulates during the shipping period; donor factors and other environmental conditions can also harm the tissues. Therefore, a culture method that allows human islets to retain their viability and functions as endocrine pancreas is required.
Alginate is a natural polysaccharide derived from brown seaweed; these derivatives are widely used as hydrocolloid. Strand et al. emphasized the important capsule properties of stability, permeability, and biocompatibility for encapsulated pancreatic islets and nominated alginate as a favorable material [3]. For alginate encapsulation, spherical beads are usually made by an electric bead generator. Onoe et al. reported that they made alginate fiber by using a microfluidic device with double-coaxial laminar flow and that these encapsulated islets reversed hyperglycemia after transplantation into the subrenal capsular space of diabetic mice [4]. We recently reported a simple method for encapsulating human iPS-derived islet-like spheroids into alginate fiber with a syringe and confirmed the normalization of blood glucose levels in diabetic mice after transplanting them into the intraperitoneal space [5]. Because these encapsulated human iPS-derived islet-like spheroids survived more than 6 weeks in vitro, we hypothesized that it should be possible to culture human islets in a similar way by encapsulating them in alginate fiber.
We decided to attempt long-term culture of human islets distributed from AIDP. We were able to maintain their function for 180 days by encapsulation in alginate fiber. To our knowledge, this is the longest culture of human islets in vitro; we believe that this culture method provides a novel tool for human islet research.
Materials and Methods
Human islets
Research on human islets was carried out at National Center for Global Health and Medicine (NCGM) and Gunma University according to their respective guidelines for Life Science Research Ethics and Safety. Human islets from 15 donors were obtained from AIDP. The details of these islets are outlined in Table 1. Human pancreas donated to the University of Alberta for clinical islet transplantation was used to prepare pancreatic islets according to the protocol approved by the Health Research Ethics Committee of the University of Alberta. Consent for research use was obtained from each donor family by organ donor organizations across Canada. When such isolated islets did not meet the criteria for clinical transplantation, they were air-transported to NCGM or Gunma University by FedEx. Depending on the local logistics, it took 2–6 days to transport islets from Canada to Japan.
Table 1
Characteristics of islet donors and conditions
| No. |
Islet
lot No. |
Age (yrs) |
Sex |
BMI |
Viability (%) |
Purity (%) |
Days for transportation |
Culture days |
Endogenous C-peptide (pM/1000IEQ) |
Endogenous glucagon (pM/1000IEQ) |
SI |
| CA |
JP |
CA |
JP |
C-peptide |
glucagon |
| #1 |
H-2405 |
47 |
M |
31.5 |
95 |
90 |
35 |
25 |
5 |
14 |
128,223 |
8,378 |
1.17 |
0.38 |
| #2 |
H-2373 |
59 |
M |
25.6 |
98 |
80 |
38 |
10 |
5 |
14 |
73,813 |
7,396 |
1.44 |
0.53 |
| #3 |
H-2388 |
39 |
M |
35.1 |
84 |
80 |
39 |
10 |
4 |
14 |
128,067 |
53,675 |
1.09 |
0.85 |
| #4 |
H-2422 |
20 |
M |
23.4 |
99 |
90 |
34 |
10 |
6 |
28 |
239,470 |
46,609 |
1.19 |
0.91 |
| #5 |
H-2411 |
47 |
F |
31.4 |
97 |
80 |
55 |
15 |
5 |
28 |
309,606 |
84,097 |
3.96 |
2.48 |
| #6 |
H-2446 |
19 |
F |
29.3 |
95 |
90 |
35 |
<10 |
2 |
28 |
609,525 |
ND |
0.96 |
ND |
| #7 |
H-2435 |
40 |
F |
35.5 |
90 |
90 |
30 |
10 |
4 |
42 |
368,868 |
54,309 |
1.14 |
0.89 |
| #8 |
H-2451 |
31 |
M |
22.4 |
82 |
80 |
40 |
10 |
3 |
42 |
539,412 |
124,613 |
1.17 |
0.70 |
| #9 |
H-2428 |
67 |
M |
31.1 |
93 |
90 |
55 |
30 |
6 |
42 |
419,179 |
92,515 |
1.13 |
1.04 |
| #10 |
H-2397 |
54 |
M |
28.7 |
90 |
90 |
40 |
10 |
4 |
75 |
90,589 |
13,551 |
0.77 |
0.70 |
| #11 |
H-2464 |
66 |
M |
25.7 |
79 |
90 |
78 |
40 |
4 |
75 |
279,504 |
94,653 |
4.84 |
0.17 |
| #12 |
H-2418 |
47 |
M |
29.7 |
96 |
90 |
55 |
25 |
5 |
120 |
514,740 |
84,033 |
3.54 |
0.89 |
| #13 |
H-2363 |
29 |
M |
20.8 |
89 |
75 |
40 |
35 |
4 |
180 |
795,421 |
ND |
2.06 |
ND |
| #14 |
H-2385 |
26 |
M |
24.4 |
80 |
90 |
55 |
40 |
3 |
180 |
758,858 |
42,893 |
1.19 |
1.22 |
| #15 |
H-2471 |
31 |
M |
20.8 |
90 |
ND |
40 |
ND |
3 |
3 |
ND |
ND |
ND |
ND |
CA; data in Canada, JP; data in Japan, SI; stimulation index, ND; no data available.
Upon arrival at NCGM, islets were incubated with fluorescein diacetate (FDA) /propidium iodide (PI) to check their viability and stained with Dithizone to evaluate their purity. Islets were observed by phase-contrast microscopy or fluorescent microscopy and photographed with a charge-coupled device camera (DP71; Olympus, Japan). Because human islets from donors are heterogeneous in size and morphology, Islet Equivalent (IEQ, 1 IEQ = an islet of diameter 150 μm) after dithizone staining is generally used to estimate the number of islets. In this study, we used 1,000 IEQ of human islets for each experiment. The IEQs in this study were calculated by mathematical compensation for islet diameters before shipping at AIDP in Canada. Then glucose stimulated C-peptide and glucagon secretion was assayed. Additionally, to examine the endogenous amounts of insulin and glucagon, each medium was replaced by 1.0 mL of acid ethanol (0.15 M HCl in 25% ethanol) and left at 5°C overnight. Their supernatants were then collected. The amounts of C-peptide and glucagon in the supernatants were measured using Human Ultrasensitive C-peptide ELISA or Human C-peptide ELISA and glucagon ELISA kits (Mercodia, Sweden).
Encapsulation into alginate fiber
Each aliquot of 1 × 103 IEQ of human islets was centrifuged and then washed in saline. Next, 0.25 mL of alginate (ALG100: a kind gift from Mochida Pharmaceutical Company, Japan) was added to the islets and injected into 10 mL of 0.1 M barium chloride, resulting in fiber formation. The fiber was immersed in this solution for 5 min at room temperature for cross-linking (Fig. 1). Then it was washed three times with saline and put in 2 mL culture medium. In vitro culture was started immediately.
Culture media
Fiber encapsulated human islets (islets fibers) were cultured in High Glucose D-MEM (25 mM glucose with L-glutamine, phenol red and sodium pyruvate, FUJIFILM Wako, Japan) or Low Glucose D-MEM (5.5 mM glucose with L-glutamine, phenol red and sodium pyruvate, FUJIFILM Wako, Japan) supplemented with 2% Fetal Bovine Serum (FBS), 1% Bovine Serum Albumin (BSA), 100 units/mL penicillin streptomycin at 37°C in 95% air and 5% CO2. Intact human islets (1 × 103 IEQ) were also cultured on 12-well petri plates with the same media. The media were changed twice a week.
In some experiments, CMRL1066 (with L-glutamine, without glucose, GMEP®, Japan) and RPMI1640 No Glucose (FUJIFILM Wako, Japan) supplemented with 2% or 10% FBS, 1% BSA, and penicillin streptomycin were used. Glucose concentration was adjusted to 5.5 mM by the addition of D (+)-glucose.
Measurement of hormones in the culture supernatant
Naked or islets fibers (1 × 103 IEQ each) were cultured in 12-well plates in 2 mL of low glucose DMEM (5.5 mM) or high glucose DMEM (25 mM) supplemented with 2% FBS. Each supernatant was sampled 24 hours after the medium change, and the amounts of C-peptide and glucagon in the supernatants were measured using Human Ultrasensitive C-peptide ELISA or Human C-peptide ELISA and glucagon ELISA kits.
Glucose stimulated C-peptide and glucagon secretion
Naked or islets fibers (1 × 103 IEQ each) were washed once with 1 mL of low glucose DMEM (G2: 2 mM) supplemented with 10 mM HEPES 0.1%BSA and were pre-incubated at 37°C for 30 min in the same medium. The islet medium was replaced by fresh medium, and they were incubated for another 30 min. Next, the medium was changed to high glucose DMEM (G20: 20 mM) supplemented with 10 mM HEPES and 0.1%BSA, and islets were incubated for another 30 min. All supernatants were collected in the same way and were added to the same volume of protease inhibitor cocktail solution (cOmpleteTM, Roche Sigma Aldrich, Germany). A stimulation index (SI) was calculated by dividing the concentration of C-peptide or glucagon secreted during high glucose exposure (20 mM) by that secreted during low glucose exposure (2 mM).
Immunohistochemistry
Islets fibers were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 3 μm sections. The primary antibodies were rat anti-C-peptide (1:200, DSHB, University of Iowa, USA) and rabbit anti-Glucagon (1:300, PA5-83352, ThermoFisher Scientific, USA). Secondary antibodies, either Alexa 594-conjugated goat anti-rat IgG or Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:400; ThermoFisher Scientific, USA) were used with DAPI (ThermoFisher Scientific, USA). TUNEL staining was performed using the ApopTag In Situ Detection Kit (Merck KGaA, Germany) with anti-insulin antibody (1:1,000, ab181547, Abcam, UK).
Mitochondrial respiration in islets
Mitochondrial respiration in islets was measured according to previously reported methods [6]. Briefly, naked islets or islets fibers were incubated in Final Wash/Culture Medium (99-785-CV, Corning Inc., USA) for 72 h. XF96 cell culture microplates (Agilent Technologies Inc., USA) were coated with Poly-L-lysine (Sigma Aldrich Co. LLC, USA) and filled with XF RPMI Medium (Agilent Technologies Inc., USA). The XF RPMI Medium was supplemented with 5.6 mmol/L glucose, 1 mmol/L pyruvate, and 2 mmol/L L-glutamine, all of which were purchased from Nacalai Tesque, Japan. The oxygen consumption rate (OCR) was measured using a Seahorse XF96 Analyzer (AgilentTechnologies Inc., USA).
Statistical analyses
At least three independent wells each for fiber and naked human islets were prepared. Due to the limited availability of islets from each donor, one well was used for each analysis timepoint. For comparisons of discrete data sets, unpaired Student’s t-test was used for parametric samples and Mann-Whitney U test for nonparametric samples. Data are expressed as the mean ± SEM. p < 0.05 was considered statistically significant.
Results
Selection of cells and culture medium
Immediately on arrival, we evaluated the cell viability by FDA/PI staining and the purity of human islets by dithizone staining. Glucose stimulated C-peptide secretion and the hormone content in islets were also measured on the same day or the next day. In some cases, the islets were cultured as they were on the petri dish, and, in the rest of them, an aliquot of 1 × 103 IEQ was mixed with alginate. Encapsulated fiber formation was achieved in the barium chloride solution; then each fiber was cultured in a 12-well plate (Fig. 1A).
The list of human islets used in this study is summarized in Table 1. As shown, the purity of all islets reduced after transportation (JP), but the reduction rate differed among samples. However, their viability was not much affected by their long trip from Canada via USA to Japan. A variety of shapes and sizes of islets was observed through phase-contrast microscopy. The staining patterns of dithizone also differed among samples. Representative images are shown in Fig. 1B. Obviously, the islets in case #6 (less than 10%) exhibit poor dithizone staining compared with those in cases #14 and #15 (35% and 40%).
Initially, we investigated C-peptide and glucagon secretion into medium from islets fibers using CMRL1066, RPMI1640, or DMEM with 2% or 10% FBS. DMEM with 2%FBS proved suitable for detecting the C-peptide and glucagon secretion in response to glucose after 6 weeks of culture (Supplemental Fig. 1). Therefore, we decided to use this medium for the culture of encapsulated human islets.
Comparison of naked human islets and human islets fibers during in vitro culture
Next, we evaluated the β-cell survival in human islets after alginate encapsulation by TUNEL staining for intact islets (naked) or islets fibers (fiber). There were no differences in the proportions of insulin- and TUNEL-positive apoptotic β-cells between naked and islets fibers at the beginning (0 hour) or after 72 hours of cultivation (Fig. 2A). No aspect of mitochondrial respiration in human islets, including mitochondrial basal respiration, maximal respiration, ATP production, and proton leak, was affected by encapsulation into the fiber at 72 hours (Fig. 2B). Although the ratio of β-cell apoptosis seemed to be increased in naked islets compared with islets fibers after 14 days, statistical analysis revealed no significant difference between them (Fig. 2C).
C-peptide and glucagon secretion from naked and islets fibers over 21 days
To compare endocrine function between naked and islets fibers, we periodically measured the amount of secreted hormone in the culture media 24 hours after medium changes. We confirmed in advance that bovine C-peptide did not cross-react with human C-peptide in our ELISA kits. Therefore, we were able to accurately monitor the amount of C-peptide secreted from human islets. As shown in Fig. 3, the amount of C-peptide secreted from naked islets cultured in low glucose conditions continued decreasing and reached almost undetectable levels within 3 weeks. On the other hand, C-peptide secretion from islets fibers from all 3 donors was maintained until 3 weeks (about 5,000 pM), albeit with a trend toward decreases in total amount of secretion (Fig. 3 left). Glucagon levels in supernatant also became undetectable in naked islets within 3 weeks, as did C-peptide. Although glucagon levels in media of islets fibers varied widely among donors during the first 2 weeks, glucagon secretion was still observed after 3 weeks in all 3 donors (Fig. 3 right).

Next, we examined the impact of long-term culture on human islets fibers in media containing 5.5 mM or 25 mM glucose (1 × 103 IEQ). To monitor their endocrine function, the amounts of C-peptide and glucagon secreted into culture media 24 hours after the medium change were periodically measured by ELISA assay. We evaluated glucose-stimulated C-peptide secretion at day 14 to determine whether islets could be maintained in culture or not. We stopped culturing islets in 4 cases, because they didn’t secrete enough C-peptide (less than 100 pM) at that time (Table 1, #1–4). We succeeded in culturing fibers for a long time in 5 cases (#10–14). In the case of #13, we stopped culturing islets in 25 mM glucose medium (high glucose medium) at 42 days for technical reasons but continued culturing islets in the 5.5 mM glucose medium (low glucose medium) until 180 days (Fig. 4C).
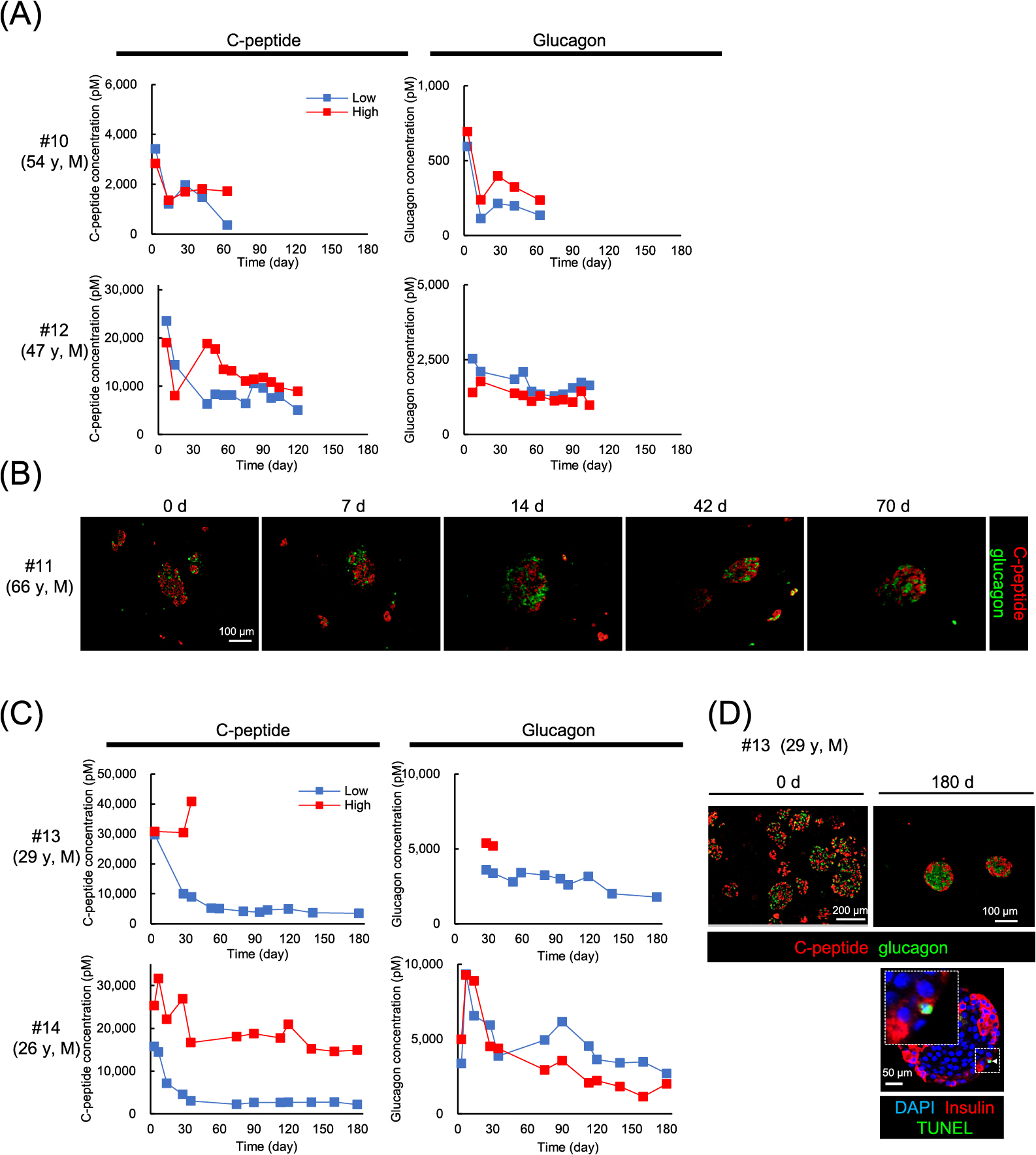
As shown in Fig. 4A and C, the concentration of C-peptide in the low glucose medium decreased until 30 days and remained constant thereafter (#12: –5,000 pM, #13, 14: –3,000 pM). Islets fibers in the high glucose medium exhibited higher levels of C-peptide than those in the low glucose medium after 6 weeks (#12: –10,000 pM, #14: –15,000 pM). Although glucagon was also constantly secreted under both conditions, there were no significant differences between levels in the high glucose and the low glucose medium (Fig. 4A, C).
In case #11, islets fibers were periodically sampled (day 0, 7, 14, 42, 70) and immunohistochemically stained. As shown in Fig. 4B, spherical structure was maintained, and both C-peptide positive cells and glucagon positive cells were detected in all samples up to 70 days. In case #13, similar results of immunohistochemical staining were obtained after 180 days culture. Of note, the proportion of TUNEL-positive apoptotic β-cells was less than 1%, even after 180 days culture (Fig. 4D).
Glucose concentration in culture media influenced responsiveness to glucose in islets fibers
We also investigated the effects of glucose concentration on glucose responsiveness of hormone secretion in islets fiber (#13 and #14) at days 14, 42, 67 (or 75), 101(or 120), and 180. The islets fibers cultured in the low glucose medium demonstrated marked increases in C-peptide secretion in response to 20 mM glucose at each timepoint, even at day 180. However, that responsiveness disappeared in the islets fibers cultured in the high glucose medium as early as 14 days after starting culture. (Fig. 5, left). The stimulation index of C-peptide for the islets fibers in low glucose medium group was significantly higher (8.14–20.50) than that in high glucose medium group (0.71–0.83) (Table 2). Suppression of glucagon secretion by high glucose was retained until day 180 in both groups, but the total amount of secreted glucagon tended to gradually decrease (Fig. 5, right). The SI of glucagon did not significantly differ between the low glucose medium group and the high glucose medium group. These results indicate that islets fibers in the low glucose medium preserved glucose responsiveness of β-cells for a long time, whereas those in the high glucose medium lost it within 14 days after starting culture.

Table 2
List of stimulation indices for C-peptide and glucagon
| Culture time |
Stimulation Index (mean ± SEM) |
| C-peptide |
Glucagon |
| Low glucose medium |
High glucose medium |
Low glucose medium |
High glucose medium |
| 14d |
8.14 ± 1.73 * |
0.71 ± 0.04 |
0.55 ± 0.05 |
0.64 ± 0.07 |
| 42d |
14.71 ± 2.89 * |
0.83 ± 0.04 |
0.66 ± 0.07 |
0.83 ± 0.08 |
| 67–75d |
20.50 ± 2.99 * |
0.77 ± 0.06 |
0.60 ± 0.03 |
0.84 ± 0.09 |
| 180d |
11.12 ± 3.69 |
0.72 ± 0.06 |
0.60 ± 0.11 |
0.68 ± 0.00 |
Discussion
In this study, we demonstrate that human islets can be cultured until 180 days and that their endocrine function is preserved in vitro by encapsulating them into alginate fiber. To the best of our knowledge, this is the longest verified culture period for human islets, even though they were originally isolated for clinical transplantation but distributed for research use because they did not meet the transplantation criteria. In fact, the dithizone positive rate for human islets on the arrival day was 40% or less in all samples tested (Table 1). Despite these unfavorable conditions, we succeeded in culturing 5 samples of human islets for more than 75 days. We intentionally stopped culturing the islets fibers at 75 days for #10 and #11, at 120 days for #12, and at 180 days for #13 and #14, not because they were exhausted, but for the purposes of histological analysis. Based on the data that showed stable secretion of C-peptide and glucagon even at day 180, it should be possible to continue culture much longer while maintaining function.
One of the most important points for successful long-term culture of human islets in this study was use of alginate as an encapsulation device. Alginate encapsulation was originally developed for islet transplantation to prevent attacks from immune cells [7]. In vitro, porcine islets seeded on an acellular collagen matrix and encapsulated in alginate were able to achieve long-term culture [8]. Human islets encapsulated in alginate sheets remained both viable and functional, with a stimulation index above 1.5, after 8 weeks of culture [9]. Qi et al. reported the protective effect of encapsulating human islets in inhomogeneous alginate-Ca2+/Ba2+ microcapsules before exposure to long shipments [10]. Human islets can be shipped safely for long distances without compromising viability or function after alginate encapsulation [11]. These reports are consistent with our results that alginate may protect against physical damage and provide a stable environment for islet cells. Alginate microencapsulation of human islets does not increase susceptibility to acute hypoxia [12]. In line with these reports, our results indicate alginate encapsulation doesn’t cause β-cell apoptosis when compared to naked islets. We hypothesized that there must be a significant difference in the ratio of TUNEL-positive cells between naked and islets fibers based on the data of Fig. 2C. In fact, when we analyzed the data by t-test, we verified a significant difference with a p-value of 0.011 between them. However, the difference was not significant by Mann-Whitney U test. We decided to utilize the Mann-Whitney U test as a non-parametric test because the number of samples was limited, and the data were not regarded as parametric. Therefore, we concluded that there was no statistically significant difference. The results of unchanged mitochondrial function in encapsulated human islets also supports the retention of islet cellular functions in the alginate fiber. In this study, central necrosis of human islets did not tend to increase within the fiber. Detailed analysis of central necrosis should be considered when assessing the viability of human islets in the fiber in future studies. Because the gap junction is also important for the maintenance of insulin secretion, further analysis to examine the effects of alginate-encapsulation on the expression of intercellular adhesion molecules in islets is warranted.
In terms of hormone secretion, there was a big difference between naked and islets fibers at 21 days. We couldn’t detect C-peptide or glucagon secretion in the media of naked islets after 21days, but we did detect certain amounts of these hormones in the media of islets fibers. One possible reason is that most of the naked islets collapsed within 21 days (data not shown).
We used DMEM as the culture medium for these human islets in the fiber. After isolation of human or porcine islets, CMRL1066, RPMI1640 supplemented with 10% FBS [8, 13] , or 15% human serum [10] is commonly used for their culture. Because we usually use DMEM during differentiation of human iPS-derived islets [14], we applied DMEM to culture human islets for future comparison with iPS-derived islets. When compared to CMRL1066 or RPMI1640, DMEM (5.5 mM glucose) with 2% FBS proved the most suitable combination for the detection of C-peptide and glucagon in culture media for encapsulated islets during 6 weeks in vitro culture in this study (Supplemental Fig. 1). With this medium, hormone secreted from naked islets was barely detected before 3 weeks (Fig. 3). Although the addition of FBS has been reported to increase viability and decrease apoptosis [15], our data indicate that 2% FBS is more appropriate for detecting C-peptide and glucagon by ELISA. Further study is needed to select the most compatible culture media and supplements for the culture of human islets fibers.
We found that the amount of C-peptide and glucagon secreted from human islets fibers gradually decreased until 5–6 weeks, after which those levels were maintained in both high and low glucose conditions. Repeated measurements of hormone levels in the culture media might be useful not only for assessing viability but also for evaluating hormone secretion capacity in encapsulated human islets. Intriguingly, human islets encapsulated in the fiber kept producing significant amounts of C-peptide and glucagon even under supraphysiologic high glucose conditions (25 mM) up to 180 days. However, the responsiveness to glucose was blunted by the chronic high glucose condition, although it was maintained under 5.5 mM glucose throughout the experiments (Fig. 5).
Mourad et al. reported that encapsulated adult porcine islets failed to respond to glucose stimulation by the 18th week of culture because of cell exhaustion [8]. We had expected that β-cells in high glucose medium group might exhaust due to the chronic high glucose environment. However, we didn’t observe any signs of exhaustion; the secretion levels of C-peptide were stable until 180 days. Eizirik et al. reported that seven-day exposure of human islets to 28 mM glucose impaired glucose responsiveness and that the rates of glucose oxidation, proinsulin biosynthesis, and total protein biosynthesis were decreased in those islets [16]. A recent report also demonstrated that glucose responsiveness of human islets was markedly attenuated by culturing under 20 mM glucose for 1–3 weeks compared with those under 5 mM glucose conditions [13] . Reducing glucokinase activity may be one of the reasons for the loss of glucose responsiveness, because glucokinase is a “glucose sensor” and regulates the rate of insulin secretion [17]. Metabolic changes and associated mitochondrial overactivation may also relate to the loss of glucose responsiveness, because they have been suggested to be early adaptations to glucose stress [18]. Taken together, glucose concentration is clearly an important factor in preserving glucose responsiveness for the culture of human islets in vitro.
Considering both our results and the baseline characteristics of islets shown in Table 1, several factors appear to influence the quality of the long-term culture of human islets. Purity of the islets on arrival had the greatest impact on the function of β-cells; the purity of successfully long-term cultured islets from 4 donors (#11–#14) exceeded 25% respectively. Although duration of air transportation varied from 2 to 6 days due to the local logistics, cell viability was maintained in all samples, indicating that no fatal damage was evoked by those differences. Body mass index (BMI) of donors may influence the long-term culture of human islets; islets from two donors (#1 and #9) with BMI >31 could not be cultured more than 14 or 42 days, even though their purity exceeded 25%. Islets from 3 donors (#12–#14) showed higher endogenous C-peptide contents, and those from 4 donors (#11–#14) exhibited higher stimulation indices (SI) of C-peptide. Because purity, endogenous contents of C-peptide, and SI are candidate functional indicators of β-cells, the overall score of those 3 factors may reflect their long-term viability. The only exception was donor #10, whose islet cells survived for a long time in vitro despite their low scores for these three factors. Factors influencing functionality of human islets are still in question [19]. It continues to be difficult to predict capability of long-term culture in fiber from the characteristics of distributed islets. This issue warrants further investigation of other factors, including age, gender, cause of death, warm and cold ischemia time, and drug use in terms of long-term culture of encapsulated islets.
We also confirmed that it is possible to extract RNA from encapsulated islet samples to assess mRNA expression (data not shown). In addition, Rodriguez et al. reported islet recovery from alginate microcapsule is feasible by using common chelators to depolymerize alginate polymers [20]. Thus, our new culture method for human islets can pave the way for elucidating their biological function in vitro by culturing them for long periods.
Acknowledgments
The authors are grateful to Dr. Barbara Lee Smith Pierce (University of Maryland University College) for her professional editorial work in the preparation of this article. This work was supported by The Grant of National Center for Global Health and Medicine (21A1017) to HO. JS acknowledges support from the Japan IDDM network, Manpei Suzuki Diabetes Foundation, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, Naito Foundation, Astellas Foundation for Research on Metabolic Disorders, Daiichi Sankyo Foundation of Life Science, and Japan Diabetes Society Carrier Development Award supported by Sanofi.
Disclosure
Approval of the research protocol
Human pancreas donated to the University of Alberta for clinical islet transplantation was used to prepare pancreatic islets according to the protocol approved by the Health Research Ethics Committee of the University of Alberta.
Protocol for the research project has been approved at National Center for Global Health and Medicine (G-000802-04 and 004447) and Gunma University (HS2020-174) according to the guidelines of the Life Science Research Ethics and Safety.
Informed consent
Informed consent for research use was obtained from donor families by organ donor organizations across Canada.
Approval date of registry and the registration No. of the study
NCGM (2021/3/5 : G-000802-04 and 2022/4/8 : 004447), Gunma University (2020/11/27 : HS2020-174).
Conflict of interest
Alginate was provided by the Mochida Pharmaceutical Company.
Jun Shirakawa is a member of Endocrine Journal’s Editorial Board.
References
- 1 Shirakawa J (2021) Translational research on human pancreatic β-cell mass expansion for the treatment of diabetes. Diabetol Int 12: 349–355.
- 2 Kin T, O’Gorman D, Schroeder A, Onderka C, Richer B, et al. (2011) Human islet distribution program for basic research at a single center. Transplant Proc 43: 3195–3197.
- 3 Strand BL, Coron AE, Skjak-Braek G (2017) Current and Future Perspectives on Alginate Encapsulated Pancreatic Islet. Stem Cells Transl Med 6: 1053–1058.
- 4 Onoe H, Okitsu T, Itou A, Kato-Negishi M, Gojo R, et al. (2013) Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat Mater 12: 584–590.
- 5 Fukuda S, Yabe SG, Nishida J, Takeda F, Nashiro K, et al. (2019) The intraperitoneal space is more favorable than the subcutaneous one for transplanting alginate fiber containing iPS-derived islet-like cells. Regen Ther 11: 65–72.
- 6 Inoue R, Tsuno T, Togashi Y, Okuyama T, Sato A, et al. (2022) Uncoupling protein 2 and aldolase B impact insulin release by modulating mitochondrial function and Ca(2+) release from the ER. iScience 25: 104603.
- 7 Basta G, Montanucci P, Calafiore R (2021) Microencapsulation of cells and molecular therapy of type 1 diabetes mellitus: the actual state and future perspectives between promise and progress. J Diabetes Investig 12: 301–309.
- 8 Mourad NI, Gianello P (2019) Long-term culture and in vitro maturation of macroencapsulated adult and neonatal porcine islets. Xenotransplantation 26: e12461.
- 9 Lamb M, Storrs R, Li S, Liang O, Laugenour K, et al. (2011) Function and viability of human islets encapsulated in alginate sheets: in vitro and in vivo culture. Transplant Proc 43: 3265–3266.
- 10 Qi M, Strand BL, Mørch Y, Lacík I, Wang Y, et al. (2008) Encapsulation of human islets in novel inhomogeneous alginate-ca2+/ba2+ microbeads: in vitro and in vivo function. Artif Cells Blood Substit Immobil Biotechnol 36: 403–420.
- 11 Vaithilingam V, Barbaro B, Oberholzer J, Tuch BE (2011) Functional capacity of human islets after long-distance shipment and encapsulation. Pancreas 40: 247–252.
- 12 Hals IK, Rokstad AM, Strand BL, Oberholzer J, Grill V (2013) Alginate microencapsulation of human islets does not increase susceptibility to acute hypoxia. J Diabetes Res 2013: 374925.
- 13 Tariq M, de Souza AH, Bensellam M, Chae H, Jaffredo M, et al. (2023) Prolonged culture of human pancreatic islets under glucotoxic conditions changes their acute beta cell calcium and insulin secretion glucose response curves from sigmoid to bell-shaped. Diabetologia 66: 709–723.
- 14 Yabe SG, Fukuda S, Nishida J, Takeda F, Nashiro K, et al. (2019) Induction of functional islet-like cells from human iPS cells by suspension culture. Regen Ther 10: 69–76.
- 15 Lemos NE, de Almeida Brondani L, Dieter C, Rheinheimer J, Bouças AP, et al. (2017) Use of additives, scaffolds and extracellular matrix components for improvement of human pancreatic islet outcomes in vitro: a systematic review. Islets 9: 73–86.
- 16 Eizirik DL, Korbutt GS, Hellerström C (1992) Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest 90: 1263–1268.
- 17 Whitticar NB, Nunemaker CS (2020) Reducing glucokinase activity to enhance insulin secretion: a counterintuitive theory to preserve cellular function and glucose homeostasis. Front Endocrinol (Lausanne) 11: 378.
- 18 Chareyron I, Christen S, Moco S, Valsesia A, Lassueur S, et al. (2020) Augmented mitochondrial energy metabolism is an early response to chronic glucose stress in human pancreatic beta cells. Diabetologia 63: 2628–2640.
- 19 Hart NJ, Powers AC (2019) Use of human islets to understand islet biology and diabetes: progress, challenges and suggestions. Diabetologia 62: 212–222.
- 20 Rodriguez S, Lau H, Corrales N, Heng J, Lee S, et al. (2020) Characterization of chelator-mediated recovery of pancreatic islets from barium-stabilized alginate microcapsules. Xenotransplantation 27: e12554.
 https://orcid.org/0000-0001-6938-7993
https://orcid.org/0000-0001-6938-7993







