Abstract
Anti-thyroglobulin antibodies (TgAb) and/or anti-thyroid peroxidase antibodies (TPOAb) positivity at baseline is a risk marker for thyroid immune-related adverse events (thyroid-irAEs) in anti-programmed cell death-1 antibody (PD-1-Ab) treatment; however, it is unknown if TgAb and TPOAb titers are associated with clinical characteristics of thyroid-irAEs. Among 586 patients treated with PD-1-Ab at Nagoya University Hospital between 2 November 2015 and 30 September 2021, 57 patients developed thyroid-irAEs (thyrotoxicosis [n = 38]; hypothyroidism without prior thyrotoxicosis {isolated hypothyroidism} [n = 19]) in whom thyroid function, and TgAb and TPOAb titers were determined at baseline and at the onset. The changes in TgAb (median, 54.8 vs. 0.2 IU/mL; p = 0.002) and TPOAb titers (31.6 vs. 0 IU/mL; p = 0.032) from baseline to onset of developing thyroid-irAEs were greater in patients with thyrotoxicosis than patients with isolated hypothyroidism. Higher TgAb and TPOAb titers, and the TgAb titer at baseline were associated with an earlier onset of thyrotoxicosis and higher peak free thyroxine levels, respectively. Twenty-eight patients who developed hypothyroidism after thyrotoxicosis had higher TgAb (54.5 vs. 10.7 IU/mL; p = 0.011) and TPOAb titers at baseline (46.1 vs. 9.0 IU/mL; p < 0.001) and greater changes in TgAb (61.7 vs. 7.8 IU/mL; p = 0.025) and TPOAb titers (52.8 vs. –0.8 IU/mL; p < 0.001) than patients who did not develop hypothyroidism. The TgAb titer at baseline and changes in the TgAb and TPOAb titers were greater in patients with thyrotoxicosis than patients with isolated hypothyroidism, suggesting that the magnitude of the thyroid autoimmune response reflects the clinical types of thyroid-irAEs.
IMMUNE CHECKPOINT INHIBITORS (ICIs), including anti-programmed cell death-1 antibody (PD-1-Ab), have been approved for several types of malignancies; however, ICIs are known to cause immune-related adverse events (irAEs) in various organs, including the lungs, skin, gastrointestinal tract, liver, and endocrine glands [1, 2]. Endocrine irAEs include thyroid dysfunction, pituitary dysfunction, primary adrenal insufficiency, type 1 diabetes mellitus, and hypoparathyroidism [3].
Thyroid dysfunction, consisting of thyrotoxicosis and hypothyroidism, is one of the most frequent endocrine irAEs to occur in PD-1-Ab treatment [4, 5]. Most patients develop thyrotoxicosis due to destructive thyroiditis, but few develop thyrotoxicosis due to Graves’ disease [6, 7]. Our studies [8, 9], as well as other studies [10, 11], have shown that anti-thyroid antibodies (ATAs [anti-thyroglobulin antibodies {TgAb} and/or anti-thyroid peroxidase antibodies {TPOAb}]) positivity at baseline is a risk marker for thyroid-irAEs. Most patients usually develop thyrotoxicosis 2–6 weeks after the administration of PD-1-Ab [3], and our prospective study reported that 75% (9 of 12) of patients with destructive thyroiditis subsequently developed hypothyroidism [8]. In contrast, a substantial percentage of patients develop hypothyroidism without overt thyrotoxicosis (isolated hypothyroidism). The onset (in days) of isolated hypothyroidism is generally considered to be later than thyrotoxicosis. Thus, while there are clearly different types of thyroid dysfunction induced by ICIs, it is unknown if there is a difference in TgAb and TPOAb titers between patients with thyrotoxicosis and patients with isolated hypothyroidism, and if TgAb and TPOAb titers are associated with the subsequent development of hypothyroidism after thyrotoxicosis.
In this study we examined the association of TgAb and TPOAb titers with the clinical types of thyroid-irAEs (thyrotoxicosis and isolated hypothyroidism), the number of days to onset of thyroid-irAEs, and the risk of hypothyroidism following thyrotoxicosis.
Materials and Methods
Patients
To clarify the clinical features of endocrine irAEs, we conducted a prospective study analyzing irAEs in patients treated with ICIs at Nagoya University Hospital between 2 November 2015 and 30 September 2021 (UMIN000019024). During the study period, 586 patients were treated with PD-1-Ab (nivolumab, n = 347; pembrolizumab, n = 239). Among them, all patients (n = 57) who developed thyroid-irAEs induced by PD-1-Ab were included in this study. The patient characteristics are shown in Table 1. We have previously reported the nivolumab and pembrolizumab treatment regimens [9, 12]. Nivolumab or pembrolizumab treatments were continued until disease progression, death, occurrence of unacceptable severe adverse events, or if the patient withdrew consent for treatment. Patients treated with a combination of a PD-1-Ab and another ICI, patients with a history of thyroid disease (including Graves’ disease and hypothyroidism treated with levothyroxine replacement therapy), and patients with thyroid tumors who underwent a thyroidectomy at the time of enrollment were excluded from this study. Written informed consent was obtained from all patients. This study was approved by the Ethical Committee of Nagoya University Hospital.
Table 1
Patient characteristics
|
Total (n = 57) |
Type of thyroid-irAE |
p value |
| Thyrotoxicosis (n = 38) |
Isolated hypothyroidism (n = 19) |
| Age, years |
65.9 ± 10.8 |
64.6 ± 11.7 |
68.4 ± 8.4 |
0.383 |
| Gender, n |
| Male |
31 (54.4%) |
19 (50.0%) |
12 (63.2%) |
0.347 |
| Female |
26 (45.6%) |
19 (50.0%) |
7 (36.8%) |
|
| Tumor type, n |
| MM |
15 (26.3%) |
13 (34.2%) |
2 (10.5%) |
0.068 |
| NSCLC |
13 (22.8%) |
10 (26.3%) |
3 (15.8%) |
|
| HN |
12 (21.1%) |
6 (15.8%) |
6 (31.6%) |
|
| RCC |
6 (10.5%) |
5 (13.2%) |
1 (5.3%) |
|
| Gastric cancer |
4 (7.0%) |
1 (2.6%) |
3 (15.8%) |
|
| Esophageal cancer |
2 (3.5%) |
0 (0.0%) |
2 (10.5%) |
|
| Mesothelioma |
2 (3.5%) |
1 (2.6%) |
1 (5.3%) |
|
| Pancreatic carcinoma |
1 (1.8%) |
1 (2.6%) |
0 (0.0%) |
|
| Urothelial cell carcinoma |
1 (1.8%) |
1 (2.6%) |
0 (0.0%) |
|
| Gastroesophageal junction cancer |
1 (1.8%) |
0 (0.0%) |
1 (5.3%) |
|
| Duration of follow-up, days |
455 ± 341 |
442 ± 308 |
481 ± 338 |
0.526 |
| Time to onset of thyroid-irAEs after initiation of PD-1-Ab treatment, days |
72 ± 76 |
41 ± 19 |
134 ± 104 |
<0.001 |
| History of immunotherapy, n |
1 (1.8%) |
1 (2.6%) |
0 (0.0%) |
1.000 |
| ATAs positivity at baseline, n |
| Positive for TgAb |
25 (43.9%) |
19 (50.0%) |
6 (31.6%) |
0.186 |
| Positive for TPOAb |
27 (47.3%) |
18 (47.4%) |
9 (52.9%) |
1.000 |
| Negative for TgAb and TPOAb |
21 (36.8%) |
14 (36.8%) |
7 (36.8%) |
1.000 |
| Baseline TSH level, μIU/mL |
1.78 (1.16–3.42) |
1.44 (0.96–2.39) |
3.41 (1.68–4.55) |
0.001 |
| Baseline FT4 level, ng/dL |
0.98 (0.89–1.08) |
0.98 (0.90–1.08) |
1.00 (0.86–1.06) |
0.930 |
Values are expressed as the mean ± SD, n (%), or median (interquartile range).
Abbreviations: ATAs, anti-thyroid antibodies; FT4, free thyroxine; irAE, immune-related adverse event; HN, head and neck cancer; MM, malignant melanoma; NSCLC, non-small cell lung carcinoma; PD-1-Ab, anti-programmed cell death-1 antibody; RCC, renal cell carcinoma; TSH, thyroid stimulating hormone; TgAb, thyroglobulin antibody; TPOAb, anti-thyroid peroxidase antibody
Serum levels of free T3 (FT3), free T4 (FT4), and thyroid stimulating hormone (TSH) were determined at baseline and every 6 weeks for 24 weeks after the first administration of PD-1-Ab in our prospective study to assess thyroid-irAEs. Thyroid function was further evaluated thereafter if clinically needed until the follow-up clinic evaluations ended. TgAb and TPOAb levels were assessed at baseline and at the onset of thyroid-irAEs. To analyze the ATA titer changes over time, TgAb and TPOAb titers were measured in the available stored samples collected 6 and 12 weeks after PD-1-Ab administration in the patients who developed thyroid-irAEs and in the patients who were positive ATAs at baseline but did not develop thyroid-irAEs. If ATAs were measured more than once during the period from the initiation of PD-1-Ab treatment to the onset of thyroid-irAEs, the value measured closest to the onset date was used as the value before the onset. The serum levels of FT3, FT4, TSH, TgAb, TPOAb, and TSH receptor antibody (TRAb) were measured as described previously [9]. The normal TPOAb, TgAb, and TRAb values were <16 IU/mL, <28 IU/mL, and <2.0 IU/mL, respectively. Thyroid-irAEs were defined according to the clinical guidelines of the Japan Endocrine Society for endocrine irAEs [3] in the current study. Destructive thyroiditis was defined as a decreased TSH level, elevated FT3 and/or FT4 levels, and TRAb negativity. Hypothyroidism was defined as an increased TSH level and decreased FT4 level. Because the measurable ranges of TgAb and TPOAb were 10.0–4,000 IU/mL and 9.0–600 IU/mL, respectively, the TgAb, TPOAb, and TPOAb levels <10.0 IU/mL, <9.0 IU/mL, and >600 IU/mL were assigned 10.0 IU/mL, 9.0 IU/mL, and 600 IU/mL, respectively.
Statistical analysis
Patient characteristics treated as continuous variables (age and follow-up period) are expressed as means ± standard deviation. Nominal variables (tumor type, gender, history of prior immunotherapy, and ATA positivity at baseline and at the onset of thyroid-irAEs) were compared using Fisher’s exact test. Spearman’s rank correlation coefficient (rs) was determined to assess the correlation between two variables. The Mann-Whitney U-test was used to compare two individual groups or paired samples between two groups. The Wilcoxon non-parametric rank-sum test corrected by the Bonferroni method was used to analyze TgAb and TPOAb titer time course changes after PD-1-Ab treatment. To determine the cut-off value of TgAb and TPOAb titers to identify patients who developed subsequent hypothyroidism after thyrotoxicosis, receiver operating characteristic (ROC) curve analysis was performed. All statistical tests were two-sided, and significance was defined as a p value <0.05. Analyses were performed using JMP Pro® (16.1.0; SAS Institute, Inc., Cary, NC, USA) and IBM SPSS Statistics 28 (IBM, Armonk, NY, USA).
Results
Patient characteristics
Fifty-seven patients who developed thyroid-irAEs induced by PD-1-Ab were analyzed in the current study (thyrotoxicosis due to destructive thyroiditis in 38 patients [66.7%] and isolated hypothyroidism in 19 patients [33.3%]; Table 1). Thirty-one patients (54.4%) were male and 26 (45.6%) were female, with a mean age of 65.9 ± 10.8 years. The number of patients who were TgAb- and TPOAb-positive at baseline was 25 (43.9%) and 27 (47.3%), respectively. Twenty-one patients who developed thyroid-irAEs were neither TgAb- nor TPOAb-positive at baseline. Among the 21 patients, 8 and 4 were TgAb- and TPOAb-positive at the time of onset, respectively. Eleven patients remained ATA-negative at the time of thyroid-irAEs onset; ATA status was not measured in one patient. The time to onset of thyrotoxicosis was significantly shorter than the time to onset of isolated hypothyroidism. The TSH levels at baseline were significantly higher in patients who developed isolated hypothyroidism than in patients who developed thyrotoxicosis (Table 1 and Supplementary Fig. 1). There were no significant differences in other clinical demographic data (age, gender, tumor type, duration of follow-up evaluations, history of prior immunotherapy, the prevalence of ATA positivity at baseline, and the baseline FT4 level) between patients who developed thyrotoxicosis and patients who developed isolated hypothyroidism (Table 1).
TgAb and TPOAb titers in each type of thyroid-irAEs
TgAb and TPOAb titers were significantly increased at the time of onset compared to those at baseline in the thyrotoxicosis group, respectively, but not in the isolated hypothyroidism group (Table 2). Although the ATAs time course changes during PD-1-Ab treatment could not be measured due to early onset in the thyrotoxicosis group, TgAb and TPOAb titers from the initiation of PD-1-Ab treatment to the onset of isolated hypothyroidism were measured in 13 of 19 patients. The median TgAb titer was significantly increased before the time of onset (median = 8 weeks) compared to baseline (median = 15.1 [interquartile range, 14.3–44.4] vs. 10.0 [10.0–20.5] IU/mL; p = 0.013), even though the value was within the normal range (<28 IU/mL). In contrast, no changes were observed in TPOAb titers over time.
Table 2
Changes in TgAb and TPOAb titers from baseline to onset in each type of thyroid-irAE
|
n |
At baseline |
At onset |
| TgAb (IU/mL) |
| Thyrotoxicosis |
38 |
28.3 (10.9–233.3) |
94.5* (58.0–433.2) |
| Isolated hypothyroidism |
18 |
10.1 (10.0–43.2) |
11.6 (10.0–99.25) |
| TPOAb (IU/mL) |
| Thyrotoxicosis |
38 |
14.8 (9.7–87.1) |
52.8* (9.9–173.8) |
| Isolated hypothyroidism |
18 |
14.7 (10.0–23.5) |
13.0 (9.0–61.6) |
Values are expressed as the median (interquartile range). “n” represents the number of patients. * p < 0.001 (vs. baseline)
Abbreviations: irAE, immune-related adverse event; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody
The ΔTgAb and ΔTPOAb, calculated by the subtraction of the titer at baseline from the titer at the onset of thyroid-irAEs, were significantly greater in patients with thyrotoxicosis than patients with isolated hypothyroidism (median ΔTgAb, 54.8 [interquartile range, 8.0–126.8] vs. 0.2 [0–7.8] IU/mL; p = 0.002 and median ΔTPOAb, 31.6 [0–81.2] vs. 0 [–1.9–18.0] IU/mL; p = 0.032, respectively; Fig. 1A and 1B). In addition, the TgAb level, but not the TPOAb level, was significantly greater in patients with thyrotoxicosis than patients with isolated hypothyroidism at baseline (p = 0.044) as well as at the time of thyroid-irAEs onset (p = 0.005, respectively; Fig. 1C–1F).
TgAb and TPOAb titer time course changes during PD-1-Ab treatment in patients who were positive ATAs at baseline but did not develop thyroid-irAEs
Among the 586 patients in this study, 17 and 56 patients were positive for TgAb and TPOAb at baseline, respectively, but did not develop thyroid-irAEs. Of the TgAb- and TPOAb-positive patients, 10 and 41 had measurements (6 and 12 weeks after the initiation of PD-1-Ab treatment), respectively. No change in TgAb and TPOAb titers was detected over time compared to baseline (Supplementary Table 1).
TgAb and TPOAb titers at baseline had a negative correlation with the number of days to the onset of thyroid-irAEs from the first administration of PD-1-Ab
The number of days to onset of thyrotoxicosis from the first administration of PD-1-Ab was negatively correlated with the TgAb (r = –0.484, p = 0.002, Fig. 2A) and TPOAb levels at baseline (r = –0.543, p < 0.001, Fig. 2B). The number of days to the onset of isolated hypothyroidism from the first administration of PD-1-Ab was negatively correlated with the TgAb level at baseline (r = –0.618, p = 0.005, Fig. 2C), but not with the TPOAb level at baseline (r = –0.274, p = 0.256, Fig. 2D).
TgAb titers at baseline were positively correlated with the highest FT4 levels in patients with thyrotoxicosis
The highest FT4 levels in patients with thyrotoxicosis were positively correlated with TgAb titers at baseline (r = 0.394, p = 0.014; Fig. 3A), but not with TPOAb titers at baseline (r = 0.307, p = 0.061; Fig. 3B). In contrast, the highest FT3 levels in patients with thyrotoxicosis were not correlated with TgAb titers at baseline (r = 0.257, p = 0.120; Fig. 3C) or TPOAb titers at baseline (r = 0.221, p = 0.183; Fig. 3D).
TgAb and TPOAb titers in thyrotoxicosis with and without subsequent hypothyroidism
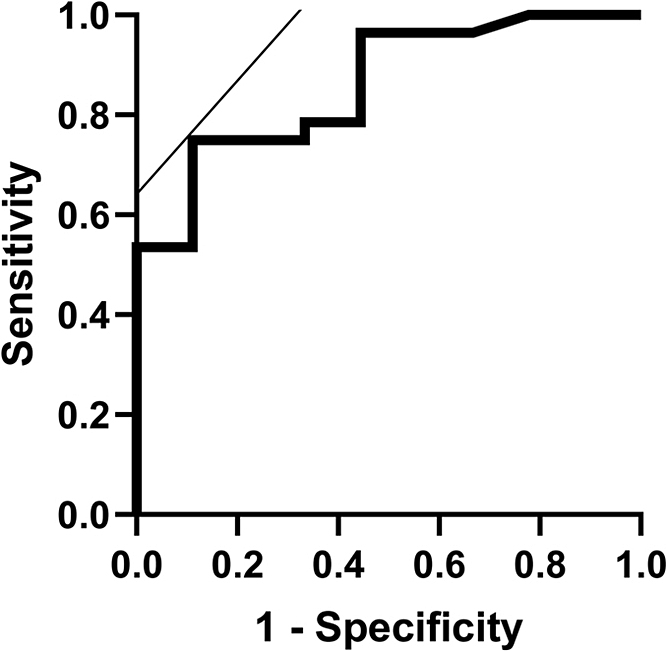
Of the 38 patients who developed thyrotoxicosis, the thyroid function outcome after thyrotoxicosis was unknown in one patient because the patient was transferred to another hospital. Among the remaining 37 patients with thyrotoxicosis, 28 (75.7%) subsequently developed hypothyroidism (Table 3). ΔTgAb (61.7 [12.6–179.2] vs. 7.8 [3.1–44.5] IU/mL; p = 0.025) and ΔTPOAb (52.8 [12.2–107.1] vs. –0.8 [–1.3–0] IU/mL; p < 0.001) were significantly greater in patients who subsequently developed hypothyroidism than in patients who did not develop hypothyroidism (Fig. 4A and 4B). In addition, patients who developed hypothyroidism after thyrotoxicosis had higher TgAb and TPOAb titers not only at baseline (TgAb, 54.5 [16.5–321.0] vs. 10.7 [10.0–18.9] IU/mL; p = 0.011 and TPOAb, 46.1 [11.3–131.4] vs. 9.0 [8.5–11.2] IU/mL, p < 0.001), but also at the time of thyroid dysfunction onset (TgAb, 172.0 [68.7–517] vs. 18.2 [13.1–63.4] IU/mL; p < 0.001 and TPOAb, 133.3 [21.1–199.3] vs. 9.0 [8.0–19.5] IU/mL; p < 0.001) than patients who did not develop hypothyroidism after thyrotoxicosis (Fig. 4C–4F). ROC curve analyses revealed that the TgAb cut-off value at the time of thyroid-irAE onset to identify patients who developed subsequent hypothyroidism was 70.5 IU/mL (AUC, 0.86; sensitivity, 75%; specificity, 89%; Supplementary Fig. 2). The TPOAb cut-off value at the time of onset was 13.7 IU/mL (AUC, 0.91; sensitivity, 82%; specificity, 100%), which was within the normal range. Among the patients who developed thyrotoxicosis, the number of days to the onset of thyrotoxicosis was significantly shorter in patients who developed subsequent hypothyroidism after thyrotoxicosis than patients who did not develop hypothyroidism after thyrotoxicosis (33 ± 13 vs. 64 ± 17 days; p < 0.001; Table 3). There was no difference in other clinical background demographic data (age, gender, tumor type, follow-up period, history of prior immunotherapy, baseline TSH level, and baseline and highest FT4 level) between the patients who developed subsequent hypothyroidism after thyrotoxicosis compared with patients who did not develop hypothyroidism after thyrotoxicosis (Table 3).
Table 3
Characteristics of patients who developed thyrotoxicosis induced by PD-1-Ab
|
Total |
Outcome of thyroid function after thyrotoxicosis |
p value |
| Without subsequent hypothyroidism |
With subsequent hypothyroidism |
|
(n = 37) |
(n = 9) |
(n = 28) |
|
| Age, years |
65.3 ± 11.1 |
60.9 ± 8.7 |
66.8 ± 11.4 |
0.178 |
| Gender, n |
| Male |
19 (51.4%) |
5 (55.6%) |
14 (50.0%) |
1.000 |
| Female |
18 (48.6%) |
4 (44.4%) |
14 (50.0%) |
|
| Tumor type, n |
| MM |
13 (35.1%) |
2 (22.2%) |
11 (39.3%) |
0.275 |
| NSCLC |
10 (27.0%) |
3 (33.3%) |
7 (25.0%) |
|
| HN |
6 (16.2%) |
1 (11.1%) |
5 (17.9%) |
|
| RCC |
5 (13.5%) |
1 (11.1%) |
4 (14.3%) |
|
| Mesothelioma |
1 (2.7%) |
1 (11.1%) |
0 (0.0%) |
|
| Pancreatic carcinoma |
1 (2.7%) |
0 (0.0%) |
1 (3.6%) |
|
| Urothelial cell carcinoma |
1 (2.7%) |
1 (11.1%) |
0 (0.0%) |
|
| Duration of follow-up, days |
442 ± 308 |
368 ± 216 |
479 ± 326 |
0.566 |
| Time to onset of thyrotoxicosis, days |
41 ± 20 |
64 ± 17 |
33 ± 13 |
<0.001 |
| History of immunotherapy, n |
1 (2.7%) |
0 (0.0%) |
1 (3.6%) |
1.000 |
| Baseline TSH level, μIU/mL |
1.38 (0.95–2.42) |
1.66 (1.02–2.01) |
1.34 (0.94–2.47) |
1.000 |
| Baseline FT4 level, ng/dL |
0.98 (0.90–1.08) |
1.05 (0.92–1.10) |
0.97 (0.89–1.07) |
0.416 |
| Highest FT4 level, ng/dL |
2.10 (1.67–2.53) |
2.07 (1.53–2.15) |
2.19 (1.70–2.69) |
0.130 |
Values are expressed as the mean ± SD, n (%), or median (interquartile range).
Abbreviations: FT4, free thyroxine; HN, head and neck cancer; MM, malignant melanoma; NSCLC, non-small cell lung carcinoma; PD-1-Ab, anti-programmed cell death-1 antibody; RCC, renal cell carcinoma; TSH, thyroid stimulating hormone; TgAb, anti-thyroglobulin antibody; TPOAb, anti-thyroid peroxidase antibody

Discussion
In this study we demonstrated that ATAs titers were increased from baseline to onset of thyroid-irAEs in patients who developed thyrotoxicosis, changes in ATAs titers during the development of thyroid-irAEs induced by PD-1-Ab were greater in patients with thyrotoxicosis than patients with isolated hypothyroidism, higher ATA titers at baseline were associated with earlier onset and severity of thyrotoxicosis, and changes in ATA titers were greater in patients who developed hypothyroidism after thyrotoxicosis than patients who did not develop hypothyroidism after thyrotoxicosis.
It has been reported that higher TPOAb titers are associated with more severe lymphocytic infiltration of the thyroid gland [13] and increased parenchymal heterogeneity as demonstrated by thyroid ultrasonography in patients with Hashimoto’s thyroiditis [14], indicating destruction of thyroid follicular architecture by lymphocytic infiltration. Another study reported the association between higher anti-thyroglobulin hemagglutination and anti-thyroid microsomal hemagglutination antibody titers and the incidence of hypothyroidism in patients with Hashimoto’s thyroiditis [15], suggesting that thyroid autoantibody titers reflect the severity of inflammation within the thyroid gland. Thus, increased titers at the time of thyroid-irAEs onset and greater changes in TgAb and TPOAb titers among patients with thyrotoxicosis, as shown in the current study, suggest that thyrotoxicosis induced by PD-1-Ab is caused by more severe inflammation in the thyroid gland compared to isolated hypothyroidism. In addition, this study showed that the TgAb titers in the isolated hypothyroidism group were within the normal range and the TgAb and TPOAb titers did not change over time in the patients who were positive for ATAs at baseline but did not develop thyroid-irAEs, suggesting a mild immune reaction in these groups. This possibility may also explain the reason why the changes in TgAb and TPOAb titers were greater in patients who developed subsequent hypothyroidism after thyrotoxicosis than patients who did not develop hypothyroidism after thyrotoxicosis. Another possibility is that the increase in TgAb and TPOAb titers reflects the exposed amount of thyroid antigens due to destruction. In either possibility, changes in ATA titers were shown to be associated with clinical types of thyroid-irAEs in this study. In addition, the cause of thyroid dysfunction in patients with ATA seroconversion at the time of onset (n = 10) can be considered an immune abnormality; however, it is unclear if this was the case in ATA-negative patients (n = 11).
In addition to the difference in ATA titer changes, this study showed that TgAb titers, but not TPOAb titers, were higher at baseline and at the time of thyroid-irAEs onset in patients with thyrotoxicosis than patients with isolated hypothyroidism. Because it has been reported that CD4+ T cells specific to thyroglobulin are essential in the development of thyrotoxicosis induced by PD-1-Ab in a mouse model [16], the immune response to thyroglobulin may have a predominant role in the development of thyrotoxicosis; however, given that the changes in TPOAb titers were also significantly different between patients with thyrotoxicosis and isolated hypothyroidism, further studies are needed to clarify the contribution of the immune response to thyroid peroxidase in the development of thyrotoxicosis induced by PD-1-Ab.
Several studies have reported that elevated TSH levels are a risk factor for thyroid-irAEs induced by ICIs [17-19]. It has also been shown that TSH levels at baseline are significantly higher in patients who developed overt hypothyroidism induced by PD-1-Ab or anti-programmed cell death ligand 1 antibody (PD-L1-Ab) than patients who developed subclinical hypothyroidism or patients who did not develop thyroid dysfunction [20], suggesting that higher TSH levels at baseline, even within the normal range, become a risk factor for overt thyroid-irAEs. In the current study TSH levels at baseline were significantly higher in patients who developed isolated hypothyroidism than in patients who developed thyrotoxicosis. Because TSH levels reflect thyroid hormone levels [21] and are also associated with future development of hypothyroidism [22], it is possible that patients who have relatively low thyroid function (patients with high or high normal TSH levels) at baseline are prone to develop isolated hypothyroidism because the amount of thyroid hormone leaked by destruction of thyroid follicles is small. It is also possible that the isolated hypothyroidism group had higher TSH levels than the other groups because TSH levels at baseline in the thyrotoxicosis group were within the normal range in most cases. It would be interesting to clarify the difference in TSH levels between patients who develop thyrotoxicosis and those who do not develop thyroid-irAEs in a future study.
This study also clarified the association between TgAb and TPOAb titers at baseline with the early onset of thyrotoxicosis induced by PD-1-Ab. Although a retrospective study showed that absolute lymphocyte counts >820/μL in peripheral blood 2 weeks after nivolumab administration is associated with an increased risk of early onset of any irAEs in patients with advanced non-small-cell lung cancer [23], it remains unknown if this parameter can be applied to thyroid-irAEs. It was reported that ATAs titers are positively correlated with antibody-mediated cell cytotoxicity [24] and the severity of infiltration with inflammatory cells, such as lymphocytes into the thyroid gland [25-27]. In addition, a case report showed that the lymphocytes count was increased at the onset of postpartum thyroiditis, a type of destructive thyroiditis [28]. Therefore, patients with higher TgAb and TPOAb titers at baseline may develop thyrotoxicosis in an early phase via a mechanism of stronger humoral and/or cellular immune responses after initiation of PD-1-Ab treatment. Although there is a study that suggested other thyroid autoantibodies, except for TPOAb, have a role in antibody-mediated cell cytotoxicity [29], further studies are needed to clarify the involvement of humoral immune responses in the development of thyroid-irAEs.
There was a correlation between TgAb (p = 0.014) titers with the highest FT4 levels in patients with thyrotoxicosis. Because thyrotoxicosis can be caused by leakage of thyroid hormone from destroyed thyroid follicles at the onset of painless thyroiditis in patients with Hashimoto’s thyroiditis [30], TgAb titers at baseline may predict the severity of thyrotoxicosis induced by PD-1-Ab. In contrast, there was no correlation between the highest free triiodothyronine levels and ATAs titers in this study, which may be explained as follows: 1) the increase in FT4 levels relative to triiodothyronine levels is higher in patients with destructive thyroiditis [31]; and 2) triiodothyronine levels may be suppressed in cancer patients with malnutrition and/or poor general condition [32].
It is important to know which patients are at risk for developing subsequent hypothyroidism after developing thyrotoxicosis induced by PD-1-Ab. Based on the findings in this study, a TgAb titer ≥ 70.5 IU/mL at the onset of thyrotoxicosis may be useful in predicting subsequent hypothyroidism after thyrotoxicosis. In a retrospective study that analyzed patients treated with PD-1-Ab, PD-L1-Ab, or PD-1-Ab plus anti-cytotoxic T-lymphocyte antigen 4 antibody (CTLA-4-Ab), Inaba et al. [33] reported that TgAb titers at baseline and at the onset of thyroid dysfunction were significantly higher in patients who required continuous levothyroxine treatment (n = 15) than patients who did not require continuous levothyroxine treatment (n = 10), although 6 of the 10 patients who were not treated with levothyroxine exhibited subclinical thyroid dysfunction [subclinical thyrotoxicosis (n = 1) and subclinical hypothyroidism (n = 5)] [33]. Another study that analyzed 37 patients who developed overt thyrotoxicosis during PD-1-Ab, PD-L1-Ab, or PD-1-Ab plus CTLA-4-Ab treatment, reported that the incidence of hypothyroidism following overt thyrotoxicosis was higher in patients with TgAb positivity, but not in patients with TPOAb positivity, at baseline compared to patients who were TgAb- and TPOAb-negative [34]. As shown herein, among patients who developed overt thyrotoxicosis, greater changes in TgAb and TPOAb titers and greater TgAb and TPOAb titers at baseline may predict thyroid function outcome after thyrotoxicosis. Although there was no difference in the highest FT4 levels between patients who did and did not develop hypothyroidism after thyrotoxicosis in the current study, it has been reported that the maximum FT4 levels are higher in patients who develop thyroid-irAEs requiring continuous replacement therapy after thyrotoxicosis than in patients who develop transient thyroid-irAEs [33], suggesting a greater amount of thyroid hormone leaks out after severe thyroid destruction due to inflammation.
Several studies also reported that the development of thyroid-irAEs is associated with longer overall survival (OS) [35]. Furthermore, another study reported that patients who develop thyroid-irAEs requiring continuous replacement therapy have a longer OS than patients who develop transient thyroid-irAEs, although patients with several types of malignancy were included [33]. Because it is preferred to analyze OS in each malignancy, a corollary study is needed to clarify the association between OS with different types of thyroid-irAEs (e.g., thyrotoxicosis vs. isolated hypothyroidism or thyrotoxicosis without subsequent hypothyroidism vs. thyrotoxicosis with subsequent hypothyroidism).
There were some limitations in this study. First, the total number of patients was relatively small; however, this study exclusively and consecutively analyzed the characteristics of patients with thyroid-irAEs induced by PD-1-Ab. Further studies are warranted to apply the findings from this study to patients treated with other ICIs. Second, there were some patients who had TgAb and TPOAb titers outside detectable ranges. Third, the highest FT4 levels might be missed, even though thyroid function was evaluated every 6 weeks in this study.
In conclusion, TgAb titers at baseline and changes in TgAb and TPOAb titers were greater in patients with thyrotoxicosis than patients with isolated hypothyroidism, suggesting that the magnitude of the thyroid autoimmune response reflects clinical types of thyroid-irAEs.
Acknowledgements
The authors would like to thank all physicians who collaborated with us in this prospective study (UMIN000019024).
Disclosure Statement
S.I. received personal fees from Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb, and MSD K.K. unrelated to this study. M.A. received a research grant from Kyowa Kirin Co. Ltd. unrelated to the submitted work. H.A. received grants from Ono Pharmaceutical Co., Ltd. and MSD K.K., and personal fees from Ono Pharmaceutical Co., Ltd. and MSD K.K. unrelated to this study. The remaining authors have nothing to disclose.
S.I. and H.S. are members of the Endocrine Journal Editorial Board.
Supplementary Table 1
Time course TgAb and TPOAb titer changes during PD-1-Ab treatment in patients who were positive for ATAs at baseline but did not develop thyroid-irAEs
|
n |
at baseline |
6 w |
12 w |
| TgAb (IU/mL) |
10 |
83.4 |
82.8 |
101.4 |
| (33.9–147.8) |
(46.0–301.8) |
(33.6–215.6) |
| TPOAb (IU/mL) |
41 |
20.9 |
18.1 |
17.0 |
| (17.1–27.5) |
(12.8–33.1) |
(11.4–32.6) |
Values are expressed as the median (interquartile range). “n” represents the number of patients.
Abbreviations: ATAs, anti-thyroid antibodies; irAEs, immune-related adverse events; PD-1-Ab, anti-programmed cell death-1 antibody; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; 6 w, 6 weeks after PD-1-Ab initiation; 12 w, 12 weeks after PD-1-Ab initiation
References
- 1 Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, et al. (2021) Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 9: e002435.
- 2 Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, et al. (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36: 1714–1768.
- 3 Arima H, Iwama S, Inaba H, Ariyasu H, Makita N, et al. (2019) Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan Endocrine Society. Endocr J 66: 581–586.
- 4 Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, et al. (2019) Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev 40: 17–65.
- 5 Gonzalez-Rodriguez E, Rodriguez-Abreu D, Spanish Group for Cancer Immuno-Biotherapy (GETICA) (2016) Immune checkpoint inhibitors: review and management of endocrine adverse events. Oncologist 21: 804–816.
- 6 Iwama S, Kobayashi T, Yasuda Y, Arima H (2022) Immune checkpoint inhibitor-related thyroid dysfunction. Best Pract Res Clin Endocrinol Metab 36: 101660.
- 7 Iwama S, Kobayashi T, Arima H (2021) Clinical characteristics, management, and potential biomarkers of endocrine dysfunction induced by immune checkpoint inhibitors. Endocrinol Metab (Seoul) 36: 312–321.
- 8 Okada N, Iwama S, Okuji T, Kobayashi T, Yasuda Y, et al. (2020) Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer 122: 771–777.
- 9 Kobayashi T, Iwama S, Yasuda Y, Okada N, Tsunekawa T, et al. (2018) Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: a prospective study. J Endocr Soc 2: 241–251.
- 10 Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, et al. (2018) Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci 109: 3583–3590.
- 11 Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, et al. (2020) Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci 111: 1468–1477.
- 12 Iwama S, Kobayashi T, Yasuda Y, Okuji T, Ito M, et al. (2022) Increased risk of thyroid dysfunction by PD-1 and CTLA-4 blockade in patients without thyroid autoantibodies at baseline. J Clin Endocrinol Metab 107: E1620–E1630.
- 13 Yoshida H, Amino N, Yagawa K, Uemura K, Satoh M, et al. (1978) Association of serum anti-thyroid antibodies with lymphocytic infiltration of thyroid-fland-sturies of 70 autopsied cases. J Clin Endocrinol Metab 46: 859–862.
- 14 Wakita Y, Nagasaki T, Nagata Y, ImanishiY, Yamada S, et al. (2013) Thyroid heterogeneity, as indicated by the CV of ultrasonographic intensities, correlates with anti-thyroid peroxidase antibodies in euthyroid Hashimoto’s thyroiditis. Thyroid Res 6: 5.
- 15 Hasegawa M, Iino S, Ito K, Hamada N (1990) The clinical course of Hashimoto’s thyroiditis during a 5 year observation period in relation to the change in thyroid function and the titers of the antithyroid antibody. Nihon Naibunpi Gakkai Zasshi 66: 207–217 (In Japanese).
- 16 Yasuda Y, Iwama S, Sugiyama D, Okuji T, Kobayashi T, et al. (2021) CD4(+) T cells are essential for the development of destructive thyroiditis induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Sci Transl Med 13: eabb7495.
- 17 Luongo C, Morra R, Gambale C, Porcelli T, Sessa F, et al. (2021) Higher baseline TSH levels predict early hypothyroidism during cancer immunotherapy. J Endocrinol Invest 44: 1927–1933.
- 18 Brilli L, Danielli R, Campanile M, Secchi C, Ciuoli C, et al. (2021) Baseline serum TSH levels predict the absence of thyroid dysfunction in cancer patients treated with immunotherapy. J Endocrinol Invest 44: 1719–1726.
- 19 Pollack RM, Kagan M, Lotem M, Dresner-Pollak R (2019) Baseline TSH level is associated with risk of anti-PD-1-induced thyroid dysfuinction. Endocr Pract 25: 824–829.
- 20 Yoon JH, Hong AR, Kim HK, Kang HC (2021) Characteristics of immune-related thyroid adverse events in patients treated with PD-1/PD-L1 inhibitors. Endocrinol Metab (Seoul) 36: 413–423.
- 21 Sawin CT, Castelli WP, Hershman JM, McNamara P, Bacharach P (1985) The aging thyroid—thyroid-deficiency in the Framingham study. Arch Intern Med 145: 1386–1388.
- 22 Aminorroaya A, Meamar R, Amini M, Feizi A, Nasri M, et al. (2017) The TSH levels and risk of hypothyroidism: results from a population based prospective cohort study in an Iranian adult’s population. Eur J Intern Med 41: 55–61.
- 23 Egami S, Kawazoe H, Hashimoto H, Uozumi R, Arami T, et al. (2021) Absolute lymphocyte count predicts immune-related adverse events in patients with non-small-cell lung cancer treated with nivolumab monotherapy: a multicenter retrospective study. Front Oncol 11: 618570.
- 24 Calder EA, Penhale WJ, McLeman D, Barnes EW, Irvine WJ (1973) Lymphocyte-dependent antibody-mediated cytotoxicity in Hashimoto thyroiditis. Clin Exp Immunol 14: 153–158.
- 25 Chiovato L, Bassi P, Santini F, Mammoli C, Lapi P, et al. (1993) Antibodies producing complement-mediated thyroid cytotoxicity in patients with atrophic or goitrous autoimmune-thyroiditis. J Clin Endocrinol Metab 77: 1700–1705.
- 26 Prummel MF, Wiersinga WM (2005) Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pract Res Clin Endocrinol Metab 19: 1–15.
- 27 Rebuffat SA, Nguyen B, Robert B, Castex F, Peraldi-Roux S (2008) Antithyroperoxidase antibody-dependent cytotoxicity in autoimmune thyroid disease. J Clin Endocrinol Metab 93: 929–934.
- 28 Mizuno S, Inaba H, Kobayashi K, Kubo K, Ito S, et al. (2021) A case of postpartum thyroiditis following SARS-CoV-2 infection. Endocr J 68: 371–374.
- 29 Metcalfe RA, Oh YS, Stroud C, Arnold K, Weetman AP (1997) Analysis of antibody-dependent cell-mediated cytotoxicity in autoimmune thyroid disease. Autoimmunity 25: 65–72.
- 30 Kravets I (2016) Hyperthyroidism: diagnosis and treatment. Am Fam Physician 93: 363–370.
- 31 Noh JY, Momotani N, Fukada S, Ito K, Miyauchi A, et al. (2005) Ratio of serum free triiodothyronine to free thyroxine in Graves’ hyperthyroidism and thyrotoxicosis caused by painless thyroiditis. Endocr J 52: 537–542.
- 32 Chopra IJ, Hershman JM, Pardridge WM, Nicoloff JT (1983) Thyroid-function in nonthyroidal illnesses. Ann Intern Med 98: 946–957.
- 33 Inaba H, Ariyasu H, Iwakura H, Kurimoto C, Takeshima K, et al. (2021) Distinct clinical features and prognosis between persistent and temporary thyroid dysfunctions by immune-checkpoint inhibitors. Endocr J 68: 231–241.
- 34 Muir CA, Wood CCG, Clifton-Bligh RJ, Long GV, Scolyer RA, et al. (2022) Association of antithyroid antibodies in checkpoint inhibitor-associated thyroid immune-related adverse events. J Clin Endocrinol Metab 107: E1843–E1849.
- 35 Kobayashi T, Iwama S, Yasuda Y, Okada N, Okuji T, et al. (2020) Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: a prospective study. J Immunother Cancer 8: e000779.
 https://orcid.org/0000-0001-5894-5945
https://orcid.org/0000-0001-5894-5945
 https://orcid.org/0000-0002-3281-0337
https://orcid.org/0000-0002-3281-0337
 https://orcid.org/0000-0003-3151-1287
https://orcid.org/0000-0003-3151-1287
 https://orcid.org/0000-0003-2037-0095
https://orcid.org/0000-0003-2037-0095
 https://orcid.org/0000-0003-0674-2956
https://orcid.org/0000-0003-0674-2956
 https://orcid.org/0000-0002-8589-9937
https://orcid.org/0000-0002-8589-9937
 https://orcid.org/0000-0002-1479-2251
https://orcid.org/0000-0002-1479-2251
 https://orcid.org/0000-0001-6289-1249
https://orcid.org/0000-0001-6289-1249
 https://orcid.org/0000-0002-4353-231X
https://orcid.org/0000-0002-4353-231X
 https://orcid.org/0000-0003-1924-7639
https://orcid.org/0000-0003-1924-7639
 https://orcid.org/0000-0002-8546-1636
https://orcid.org/0000-0002-8546-1636
 https://orcid.org/0000-0003-3746-1997
https://orcid.org/0000-0003-3746-1997