2014 年 20 巻 1 号 p. 115-120
2014 年 20 巻 1 号 p. 115-120
To improve the quality of fish (sea bream) served to elderly people who have difficulty in chewing and/or swallowing, the effects of various concentrations of protease (papain) on the palatability, and physicochemical and histological properties of minced sea bream (MSB) were examined. In papain-treated MSBs, an increase in the total free amino acid content and a significant decrease in meat toughness were observed with increasing concentrations of papain. Sensory evaluation revealed that the texture of the 0.2% and 0.3% papain-treated MSBs was more tender and easier to eat than 0.1% papain-treated MSBs. However, MSBs treated with 0.1% and 0.2% papain were tastier than those treated with 0.3% papain, with the latter treatment leading to the highest levels of bitter components (hypoxanthine and inosine) in the fish meat. Additionally, histological examination confirmed the complete disappearance of muscle cells and connective tissues in 0.3% papain-treated MSB. These results suggest that processing of MSB with a papain concentration of 0.2% is the most suitable for palatable consumption of this fish by the elderly.
For healthy people, the consumption, chewing, and swallowing of food are regarded as ordinary eating actions that can even be performed unconsciously. Elderly individuals, however, experience a deterioration of mastication and swallowing functions due to physical reasons such as loss of teeth, denture installation, and decreased functioning of the tongue (Mioche et al., 2004; Van der Bilt et al., 2006). Elderly people with poor eating functions are thus more sensitive to food texture, especially in terms of food ingestion and swallowing. Ordinary dietary choices are limited for the elderly; therefore, protein deficiency has become a nutritional problem in the elderly population (Kuzuya 2003; Smoliner et al., 2008; Funami 2011; Awad et al., 2012). Although meat and processed meat products are good sources of protein, the cooking process increases the toughness of such products, thus making them hard to chew, which is especially problematic for the elderly (Takahashi et al., 2003; Purwanti et al., 2010). We previously investigated the effects of protease (papain) treatment on the texture, palatability, and muscle tissues of minced chicken meat as a processing method targeting the elderly with poor eating functions, in order to improve their quality of life (QOL) (Matsuguma et al., 2013). Our study revealed that the tenderness and palatability of minced chicken meat are closely related to morphological changes in chicken meat tissues. A high concentration of papain affects the endomysium, resulting in a marked reduction in its palatability. Because the effect of papain treatment on muscle tissues appears to vary depending on the type of meat, we considered that it is useful to investigate the palatability of different types of meat that have been subjected to papain degradation. From the viewpoint of improving the QOL of the elderly, processing foods by papain treatment would also significantly improve the range of available meat choices for this specific population, as well as identify essential requirements for meat palatability.
This study focused on the quality of fish, which are another good source of protein. Fish offer a wider variety of proteins than animal meats because a wider range of fish species is available, depending on seasonal variations. Fish protein with its lower percentage of trans fats is also preferred to the less-healthy animal protein, and is equally palatable. Among the commonly consumed fish, white-fleshed varieties contain much lower amounts of histidine than red-fleshed fish do, leading to a lower likelihood of fish poisoning stemming from histamine production by bacteria such as Morganella morganii (Emborg and Dalgaard, 2008). Therefore, white-fleshed meat is preferred for the elderly (Afilal et al., 2006). However, because white-fleshed fish contain very high levels of the connective tissue protein collagen, they generally have a much tougher texture than red-fleshed fish, which poses problems for the elderly in terms of mastication.
In the present study, we attempted to soften minced sea bream (MSB) meat, which has a particularly tough quality among white-fleshed fish meats, by using various concentrations of papain. We then conducted a sensory evaluation of the papain-treated MSBs to investigate their food properties and palatability for consumption by the elderly. We also investigated physical and palatability factors by focusing on the changes of umami components in MSBs and the structural changes of MSB tissues after papain treatment.
Reagents Glutamic acid and ninhydrin used in this study were procured from Wako Pure Chemical Ind. (Osaka, Japan) and Kanto Chemical Co. (Tokyo, Japan), respectively. Papain was purchased from Nagase ChemteX Co. (Osaka, Japan). Hypoxanthine (Hx), inosine (HxR), disodium inosine 5′-monophosphate (IMP), and adenosine 5′-monophosphate monohydrate (AMP) were purchased from Wako Pure Chemical Ind. and used as a reference for the analysis of nucleic acid-related compounds. Other reagents and solvents used were of special grade or HPLC-grade.
Sample preparation
(1) Processing and papain treatment of sea bream meat
Sea bream meat was derived from the lateral muscle of sea bream (Pagrus major, approximately 1 kg body weight) farmed in Fukuoka, Japan. The meat was minced once using an electric meat chopper (3.2-mm-diameter mesh plate, MS-12; Nantsune, Osaka, Japan). Papain was added to the minced meat (150 g) at final concentrations of 0.1%, 0.2%, and 0.3% and mixed 20 times by hand. Approximately 30 g of each meat sample was placed in casings (200 × 300 mm, 90-mm film thickness; Kurilon Chemicals, Osaka, Japan) and packaged using a vacuum packaging machine (VP-105F; Nihon Dennetsu, Nagano, Japan), and then stored for 24 h at 0°C. After this storage period, the meat was heated in hot water at 95°C until the core temperature of the sample reached 85°C. This heated meat was used as the enzyme-treated MSB. As a control, minced meat that had not been subjected to papain treatment was also preserved and heated similarly.
(2) Freeze-drying
MSBs prepared using the above-described method were placed side by side without overlaps on an aluminum tray and frozen for 24 h at −30°C in a preliminary freezing test machine (NR-308FC; Panasonic, Osaka, Japan). Subsequently, the tray containing the frozen MSBs was placed in a vacuum dryer (280 × 300 mm internal dimensions, 300-L dehumidification capacity, Model FREEZVAC-1S2M; Tozai Trading, Tokyo, Japan) for 24 h under 10-Pa vacuum pressure, at a trap cooling temperature of −50°C. The freeze-dried MSBs were then lyophilized using a fiber mixer (MX-X103; Panasonic) and stored at −18°C prior to the analyses of total free amino acids and nucleic acid-related compounds.
Analyses and sensory evaluation
(1) Total free amino acid content
Total free amino acid content was determined according to the method described by Rosen (1957). Freeze-dried MSB (1 g) was refluxed 3 times in 75% ethanol (80°C, 30 min). The supernatant was collected and adjusted to 100 mL with 75% ethanol. Approximately 500 µL of 0.1 M acetate buffer (pH 5.0) and 500 µL of 2% ninhydrin solution were sequentially added to 1 mL of the diluted supernatant and allowed to react for 15 min at 100°C. The reaction solution was then cooled to room temperature, following which 5 mL of 50% isopropyl alcohol was added and the mixture stirred. Absorbance at 570 nm was then measured. Total free amino acid levels were expressed as glutamic acid equivalent per 100 g of MSB before freeze-drying (mg/100 g).
(2) Toughness
Toughness was measured using a tensipresser (TTP-50BX; Takemoto Denki, Osaka, Japan). Briefly, the MSB was compressed using a 20-mm-diameter plunger at a compression speed of 10 mm/s, clearance of 5 mm, and a measurement temperature of 20°C ± 2°C. Toughness was calculated from the resulting tension. Each sample was measured 3 times to calculate the mean and standard deviation of toughness.
(3) Sensory evaluation
Sensory evaluation was carried out by 9 female panelists (average age: 22.6 years) of the Faculty of Nutrition Science, Nakamura Gakuen University, Japan, who had been sufficiently explained in the evaluation procedure. During the evaluation, the 4 kinds of MSBs, namely, fish from the control and enzyme-treated (0.1%, 0.2%, and 0.3%) groups, were presented together and evaluated using a closed panel method at a room temperature of 22°C ± 2°C. The panelists used a 5-point rating scale (Pérez Elortondo et al., 2007), ranging from −2 to 2, with 0 representing the control group. The evaluation items were as follows: (1) toughness (tough/tender), (2) ease in crushing the meat with the tongue (difficult/easy), (3) ease in swallowing the meat (difficult/easy), and (4) palatability (dislike/like). The panelists evaluated a pair of test samples from left to right without repeating the procedure. The same panelists evaluated all the combinations and all orders.
(4) Nucleic acid-related compounds
Hx, HxR, IMP, and AMP levels were determined according to the method described by Veciana-Nogues et al., (1997) with minor modifications. Freeze-dried and powdered MSB (200 mg) samples, adjusted to a volume of 2 mL by adding 100 mM citrate buffer (pH 8.0), were filtered through a cellulose acetate membrane (0.45-µm pore size). The analysis was conducted using a Wakosil-II 5C18 column (4.5 mm I.D. × 150 mm; Wako Pure Chemical Ind.), with a mobile phase of 100 mM triethylammonium phosphate buffer (pH 6.8) and acetonitrile (at a ratio of 100:1 [v/v]), at a flow rate of 1.0 mL/min and column temperature of 30°C. The level of nucleic acid-related compounds was expressed as the amount per 100 g of MSB before freeze-drying (mg/100 g).
(5) Histological examination
For the histological examination, MSB prepared and enzyme-treated as described before, but without heat treatment, was used. Five-millimeter blocks were cut out from the MSB and fixed for 24 h in 4% formaldehyde (Wako Pure Chemical Ind.) adjusted with 0.1 M phosphate buffer (pH 7.4). The fixed samples were then washed with water, dehydrated with 70 - 100% ethanol in a stepwise manner, subjected to xylene penetration, and embedded in paraffin. Specimens were prepared as 6-µm slices using a microtome. After deparaffinization, the specimens were washed with water and stained with Mayer's hematoxylin (Muto Pure Chemicals, Tokyo, Japan) for 20 min and then washed with water. Next, the sections were stained with 1% eosin Y stain (Muto Pure Chemicals, Tokyo, Japan) for 5 min, followed by rinsing with water and dehydration using an ethanol series. The dehydrated products were then subjected to xylene penetration. The specimens were finally mounted and examined under an optical microscope (BH-2; Olympus Co., Tokyo, Japan).
Statistical analyses The results are expressed as the mean ± standard deviation. Multiple comparisons based on Tukey's method were used for statistical analysis of the results. The differences in the results were considered significant when the probability value was less than 0.05 (P < 0.05).
Effects of papain treatment on amino acid levels of minced sea breams Amino acid levels in the MSB samples were examined to investigate the effects of papain (0.1%, 0.2%, and 0.3%) on proteolysis of the MSB. Figure 1 shows the total amino acid levels in MSBs before (control group) and after papain treatment. The total amino acid level in MSBs preserved for 24 h at 0°C after addition of the enzyme was significantly increased compared with the control (P < 0.01). In addition, the levels increased significantly with increase in the enzyme concentration up to 0.3% (P < 0.01). These results indicate that papain proteolysis progresses in sea bream meat during the 24-h preservation, even when the enzymatic treatment temperature is 0°C.

Effects of various papain concentrations on the total amino acid contents of fish meat.
Different letters indicate significantly differences (P < 0.01).
Measurement of toughness of minced sea bream using a tensipresser The effect of papain treatment (0.1%, 0.2%, and 0.3%) on the toughness of MSB was measured using a tensipresser (Fig. 2). Compared with the control, the toughness of the meat was decreased in all enzyme-treated MSBs, with 0.2% and 0.3% enzyme treatments showing a more significant decrease in the meat toughness than 0.1% papain treatment (P < 0.05). The results indicate that the toughness of MSB is markedly reduced after treatment with 0.1% papain.
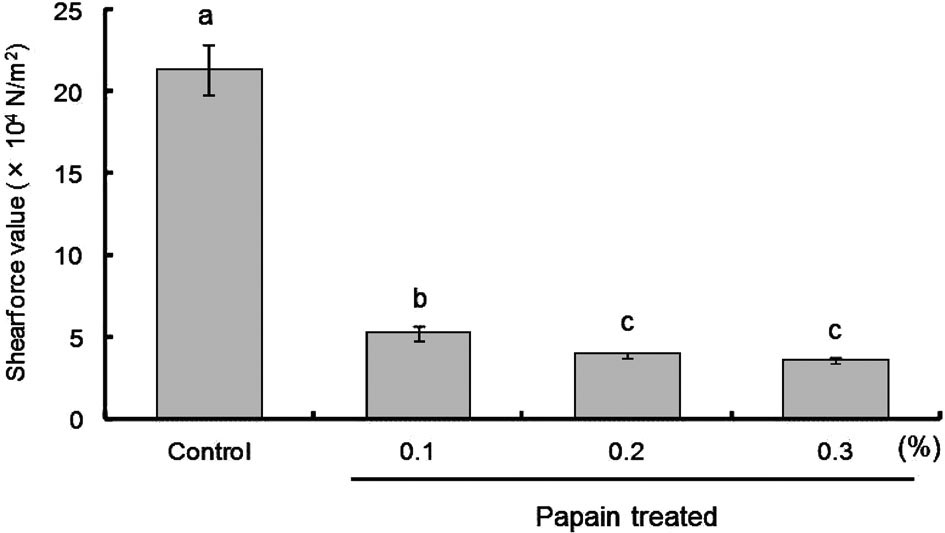
Shear force value of fish meat treated with papain.
Different letters indicate significant differences (P < 0.05).
Sensory evaluation of papain-treated sea bream meat Figure 3 shows the results of the sensory evaluation of MSBs treated with papain (0.1%, 0.2%, and 0.3%). In accord with the tensipresser results described above, enzyme-treated MSBs had a higher level of tenderness than the control (tenderness rating: 0). In addition, 0.2% and 0.3% enzyme-treated MSBs showed a significantly higher level of tenderness than the 0.1% enzyme-treated MSB (P < 0.05). These results indicate that sufficient sensorial tenderness can be achieved by treatment with 0.2% and 0.3% papain.
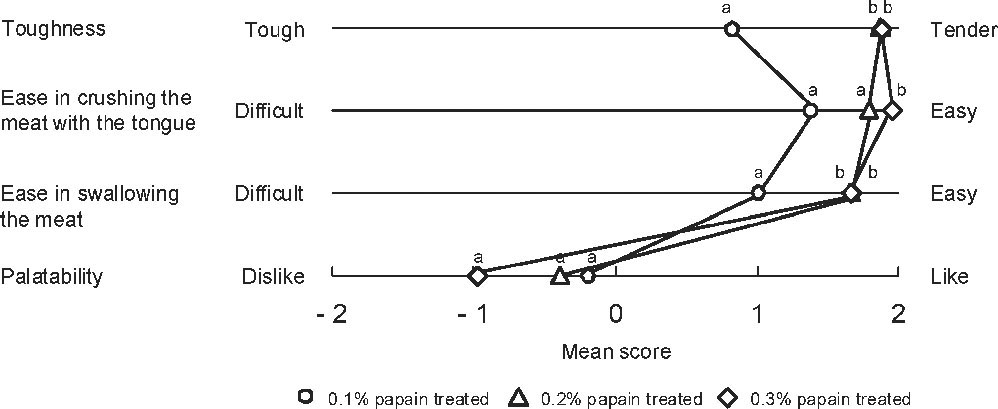
Effects of various papain concentrations on the texture and palatability of fish meat.
Nine panel members rated 4 parameters of fish meat treated with papain.
Furthermore, enzyme-treated MSBs could be more easily crushed with the tongue. Treatment of MSB with 0.3% papain resulted in a significantly better meat breakdown than that achieved upon treatment with 0.1% and 0.2% concentrations (P < 0.05). Similarly, MSBs that had been treated with 0.2% and 0.3% papain could be more easily swallowed than the meat treated with 0.1% enzyme (P < 0.05). These results indicate that the toughness, ease in crushing with the tongue, and ease in swallowing of enzyme-treated MSBs are strongly related to each other, and relatively better results are obtained when 0.2% and 0.3% papain are used. However, in terms of palatability, the MSB treated with 0.3% papain was less preferred, although no significant differences were observed among the 3 enzyme concentrations used. Because the palatability of processed foods is related to their high elasticity and increased binding property (Chen, 2009), it is probable that softening of the MSB by the enzymatic treatment would decrease the meat's palatability. Although the palatability of the 0.2% and 0.3% enzyme-treated MSBs showed relative differences in terms of sensory evaluation, the tenderness of these samples was similar. Therefore, the palatability of enzyme-treated MSBs may not only be based on toughness but also on differences in other taste components.
Effects of papain treatment on the level of nucleic acid-related compounds in minced sea breams Table 1 shows the levels of nucleic acid-related compounds (Hx, HxR, IMP, and AMP) in MSBs treated with papain (0.1%, 0.2%, and 0.3%) and those untreated (control). The level of Hx increased with increasing papain concentration, with the most significant difference observed between the 0.3% enzyme-treated MSB and the control (P < 0.01).
| Test compound | Purine nucleotides (mg/100 g) | |||
|---|---|---|---|---|
| Hx | HxR | IMP | AMP | |
| Control | 28.3 ± 0.7a | 485.2 ± 9.8 a | 8.7 ± 0.3 a | 21.7 ± 0.7 a |
| 0.1% Papain treated | 31.3 ± 2.2 ab | 501.6 ± 23.4 ab | 8.5 ± 0.5 a | 12.8 ± 0.9 b |
| 0.2% Papain treated | 31.7 ± 1.2 ab | 488.4 ± 14.3 ac | 8.2 ± 0.3 a | 12.2 ± 0.4 b |
| 0.3% Papain treated | 33.3 ± 0.4 b | 542.1 ± 3.4 b | 8.8 ± 0.2 a | 8.1 ± 0.1 c |
Hx : hypoxanthine; HxR : inosine; IMP : inosine 5′-monophosphate; AMP : adenosine 5′-monophosphate.
Different letters indicate significant differences (P < 0.01).
For HxR, however, only the 0.3% enzyme-treated MSB showed a significantly higher level (P < 0.01) than the control, whereas 0.1% and 0.2% enzyme-treated MSBs showed no significant differences compared to the control. In contrast, the IMP levels were 8.2 - 8.8 mg/100 g in all treated and non-treated samples, indicating no significant variations as a result of enzyme treatment. The AMP levels, however, decreased with increasing papain concentrations and were significantly lower than that of the control (P < 0.01), with the 0.3% enzyme-treated MSB showing a significantly lower level than the 0.1% and 0.2% enzyme-treated samples (P < 0.01). Animal muscle generally contains large amounts of ATP that are eventually depleted after the animal's death. Nucleic acid compounds related to umami (AMP and IMP) and bitterness (HxR and Hx) are produced as the ATP in meat degrades (Kuda et al., 2007). The production of these compounds is influenced by conditions such as meat processing and storage. Fish meat is more susceptible to the action of endogenous proteases than animal meat is (Yamanaka, 2002; Zhou and Li-Chan, 2009). In the present study, the level of nucleic acid-related compounds in MSBs was influenced by papain treatment, and production of bitter-tasting compounds was enhanced with increasing enzyme concentration. The fact that the 0.3% enzyme-treated MSBs showed much lower AMP and much higher Hx and HxR levels than MSBs treated with 0.1% and 0.2% enzyme concentrations might be one of the reasons for its low palatability sensory evaluation. These results indicate that preservation of MSB for 24 h at 0°C after the addition of 0.2% papain may provide the optimum quality and palatability of sea bream meat for the elderly.
Histological examination of papain-treated minced sea breams Figure 4 shows muscle tissues (muscle tissues, black; connective tissues, white) in MSBs before (control) and after enzymatic treatment (0.1%, 0.2%, and 0.3% papain). Edible fish generally consist of skeletal muscles, which are composed of bundles of muscle fibers that contain myofibrils. These bundles are known to assemble and extend linearly in the lateral muscles (Kaale and Eikevik, 2013). Several bent muscle portions were observed in the myofibrils of the control (A), which may be attributable to the physical rupturing of the muscle bundles during mincing. However, mincing alone was not sufficient in reducing the toughness of the sea bream meat because the muscle tissue was confirmed to have clear borders with the connective tissue. However, in 0.1% enzyme-treated MSB (B), the amount of bent muscle portions was greater than that observed in the control. In addition, we observed partial disappearance of the border between muscle tissues and connective tissues, which is likely attributable to the effects of papain on the degradation of collagen fibers. It is possible that the enzyme reaction had reduced the toughness of the sea bream meat, as supported by the tensipresser measurements and the sensory evaluation. In the 0.2% enzyme-treated MSB (C), the higher papain amount might have affected the progression of degradation of the collagen fibers and connective tissues, leading to partial disappearance of the muscle bundles that constituted the muscle tissues. Complete disappearance of connective tissues, together with the myofibrils and sarcoplasm that are usually intermingled at the colloidal state, was evident in the 0.3% enzyme-treated MSB (D). These results were highly consistent with the trend of increased free amino acids in samples treated with higher papain concentrations. In addition, as more muscle cells and surrounding connective tissues of the MSB are degraded, more components involved in umami and bitterness are released, thereby significantly affecting palatability (Goll et al., 1982; Shimomura, 1997). Therefore, the complete disappearance of muscle cells and connective tissues in the 0.3% enzyme-treated MSB would be concomitant with the release of more nucleic acid-related compounds associated with bitterness, thereby leading to the significant reduction in palatability observed in the sensory evaluation.

Optical micrographs of fish meat before and after papain treatment. (A) Before papain treatment; (B) after 0.1% papain treatment; (C) after 0.2% papain treatment; (D) after 0.3% papain treatment.
The preservation of MSB for 24 h at 0°C after treatment with 0.2% papain resulted in a higher level of tenderness, and it was tastier than the conventional MSB. These results may thus be adapted for the preparation of fish for the elderly, especially when it could minimize the production of nucleic acid-related compounds associated with bitterness and, in turn, preserve the connective tissues of the meat.
Papain affects the progression of proteolysis of MSB during preservation for 24 h at 0°C, thus influencing the meat's tenderness and toughness, while also affecting palatability depending on the enzyme concentration used. Taking into account the sensory evaluation results, treatment of MSBs with 0.2% papain proved to be the most suitable concentration for processing this fish for consumption by the elderly.