2014 Volume 20 Issue 1 Pages 51-58
2014 Volume 20 Issue 1 Pages 51-58
Chlorella sp.-XJY isolated from the Ili River in Northern Xinjiang was cultivated under mixotrophic conditions. The maximum biomass and crude polysaccharide production significantly increased (2.06 g/L and 145.5 mg/L, respectively) under mixotrophic conditions than under photoautotrophic conditions. Dilute alkaline was employed to extract crude polysaccharides from Chlorella sp.-XJY. The effects of time, temperature, and pH value on extraction yield were investigated using response surface methodology. The optimal conditions were determined as follows: 3.2 h extraction time, 96.4°C extraction temperature, and pH 10.94. Analysis of variance showed that the contribution of the quadratic model was significant (R2 = 0.9808) for the responses. Under the optimum conditions, the extraction yield of crude polysaccharides was 10.56%, which matches well with the predicted yield of 10.72%.
Microalgae that can survive under different environmental stresses, are rich sources of biologically active substances (Singh et al., 2005; Gupta and Abu-Ghannam, 2011; Holdt and Kraan, 2011), widely used in cosmetics, food, and pharmaceuticals (Smit, 2004; Nishizawa et al., 2005). Polysaccharides, especially those sulfated from marine algae, possess important biological activities, including antioxidant (Song et al., 2010; Zhang et al., 2010), anticoagulant (Costa et al., 2012; Sugiura et al., 2012), antitumor (Jiao et al., 2009), antiviral (Lee et al., 2011; Yang et al., 2011), and immune-inflammatory (Sun et al., 2008).1
Microalgae for polysaccharide production have advantages over terrestrial plants and other polysaccharide sources because of their variety of species, short growth cycles, and ability to grow almost anywhere with the sunlight and some simple nutrients (Mata et al., 2010). Considerable progress has been made in the past decades toward optimization of polysaccharide extraction from herbal plants (e.g., Cordycepsmilitaris, Plantaoo asiatica L., and Ganoderma lucidum) (Song, Li & Liu, 2009; Huang et al., 2010) , macroalgae (e.g., Hizikia fusiformis and Gracilaria vermiculophylla) (Zhu et al., 2010), and a few microalgae.
The extraction of biologically active polysaccharides is the most important stage in the production of medicine and food from microalgae. Hot water technology is the most conventional extraction method for polysaccharides. However, this method has low extraction efficiency (Gan et al., 2010; Sun et al., 2010; Jiang et al., 2011; Ye et al., 2011). Generally, microwave- or ultrasonic-assisted techniques (Yongjiang et al., 2009; Patil et al., 2011) with similar or better extraction yields when compared with conventional extraction processes could extract compounds more selectively and quicker. However, extraction temperature and polysaccharide activities are difficult to control in the industrialization of ultrasonic or microwave extraction processes. Various types of alkali solution with different concentrations, time, and temperature conditions were used to extract polysaccharides. Compared with aqueous solutions, alkali solutions have better capability to extract plant acidic polysaccharides (Huang et al., 2010; Ai et al., 2012; Wang et al., 2012). The extraction process must be optimized using mathematical models to obtain a high extraction yield of acidic polysaccharides.
The culture method also has a great potential in increasing the biomass and polysaccharide production of microalgae. Kong et al., (2011) reported that the growth and carbohydrate content of microalgae under mixotrophic conditions could be significantly increased. However, little attention has been given to the extraction of crude polysaccharides from mixotrophic microalgae.
Response surface methodology (RSM) is an effective statistical technique for optimizing complex processes. RSM has been increasingly used to optimize the extraction conditions for organism polysaccharides (Dasu and Panda, 2000; Wu et al., 2007; Song et al., 2009; Zhu et al., 2010; Chen and Walker, 2011; Song et al., 2012; Sui et al., 2012). The main advantage of RSM is the reduction of the number of experimental trials needed to evaluate multiple variables and their interactions. Hence, RSM is less laborious and time consuming than other methods.
Despite of all the above-mentioned benefits, research on the application of dilute alkali extraction process in microalgae is limited. This study aims to evaluate the extraction of crude polysaccharides from a new microalga Chlorella sp.-XJY using dilute alkali solution and this microalga was isolated from the Ili River in Northern Xinjiang and cultured under mixotrophic conditions. An experimental design of RSM was proposed to optimize the effects of temperature, time, and pH value on the extraction yield of crude polysaccharides.
Microorganism and culture conditions The microalga in this experiment was separated and purified from the Ili River in Northern Xinjiang using the spread plate method. The microalga was preserved in the Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan. The microalga was preliminary identified as Chlorella sp.-XJY through observation of morphological characteristics, including cell size, shape, and chloroplast core protein number.
Samples of the microalga were cultivated in a basal BG11 medium (Rippka, Deruelles et al., 1979) at 25°C ± 2°C with continuous illumination of 4,000 lux. The cells were grown in 1 L conical flasks containing 500 mL medium supplemented with or without organic carbon sources for mixotrophic or photoautotrophic cultures, respectively. The culture medium, except for organic substrates, was sterilized in an autoclave at 121°C for 20 min. The three organic carbon sources glucose, glycerol and sodium acetate were autoclaved separately and added at the concentration indicated in the text. The optical density (OD540 nm) of the microalgal stock culture was adjusted to 0.19 ± 0.01. The culture was inoculated into the medium to reach 10% (v/v) concentration. The conical flasks were shaken by hand three times a day, and their positions were exchanged to ensure the homogeneous illumination. Each experiment was conducted in duplicate.
Biomass analysis Cell growth was monitored from the OD540 nm values and then correlated with the biomass determined directly. The samples were diluted by appropriate ratios to ensure that the measured OD540 nm values were within the range of 0.2 to 0.8, if applicable. When the change of culture suspension liquid's absorbance compared with the previous day is scarcely varied, we consider the cultivations reach the late logarithmic phase. Cultivations were continued until the cell growth reached the late logarithmic phase. In different culture conditions, the time that same microalga achieves the late logarithmic phase is different. When the glucose concentration in the culture medium were 0, 5, 10, 20, 50 g/L, the time of microalgal culture reached to the late logarithmic phase was 3, 6, 6, 5, 5 days, respectively. Not every cell could achieve the late logarithmic phase on the same day, and we studied the whole level of the culture suspension liquid.
Biomass usually at its maximum absorbance value reaches its maximum. Cultivations were continued until the cell growth reached early stationary phase. The culture medium was centrifuged at 5,000 rpm for 10 min, and the pellet was freeze dried. The dry weight of the microalgal biomass was determined gravimetrically and expressed as g/L.
Determination of crude polysaccharides Extractions were carried out in a conical flask placed in an incubator: 0.1 g of lyophilized microalgal powder was added to 10 ml of the glycine-sodium hydrooxide buffer (solid-liquid ratio: 1:100, w/v) at different pH in each flask. The pH's of the mixtures were adjusted with 0.1 M HCl/NaOH and subsequently incubated in an incubator at different incubation temperatures and times (Table 1). The concentration of polysaccharides in the extract was determined as glucose using the modified anthrone-sulfuric acid method (Yemm and Willis, 1954). A spectrophotometer was used to analyze polysaccharides in the extracted solution. The extraction yield of crude polysaccharides (%) was calculated using the following Eq. 1:
 |
| Variables | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| pH (X1) | 9 | 10 | 11 |
| Extraction temperature (X2) (°C) | 80 | 90 | 100 |
| Extraction time (X3) (h) | 2 | 3 | 4 |
where C is the concentration of crude polysaccharides calculated from the calibrated regression equation based on the absorbance at 620 nm, mg·mL−1; V is the diluted volume of extraction solution, mL; and W is the mass of cultured Chlorella sp.-XJY, mg.
Experimental design A Box-Behnken design (BBD) was applied to optimize statistically the extraction yield of crude polysaccharides from Chlorella sp.-XJY. According to previous single-factor experiments, the extraction time, temperature and pH value remarkably influenced the extraction yield of crude polysaccharides. The range of independent variables and their levels are presented in Table 1. In this table, the three factors were designated as X1, X2, and X3 with three levels, namely, 1, 0, and −1 for high, intermediate, and low values, respectively. The three test variables were coded according to the following Eq. 2:
 |
where xi is the coded value of the independent variable, Xi is the actual value of the independent variable, X0 is the actual value of the independent variable at the center point, and ΔX is the step change value of the independent variable. The experimental data were then fitted to the following second-order model Eq. 3:
 |
where Y is a dependent variable (extraction yield of crude polysaccharides); A0 is a constant; Ai, Aii, and Aij represent the coefficients of the linear, quadratic, and interactive terms, respectively; and Xi and Xj are the levels of the independent variables that represent the linear, quadratic, and cross-product effects of the X1, X2, and X3 factors of the response, respectively. The model was used to evaluate the effects of each independent variable on the response. The accuracy and general ability of the polynomial model could be evaluated by the coefficient of determination (R2).
The experimental design was analyzed and the predicted data were calculated using the Design-Expert software (v.7.1.6, Stat-Ease, Inc, Minneapolis, USA) to estimate the response of the independent variables. Subsequently, three additional experiments were conducted to verify the validity of the statistical experimental strategies.
Mixotrophic cultivation of Chlorella sp.-XJY The microalgae used for polysaccharide extraction were optimized not only by adding into different organic carbon sources but also by selecting the optimal concentration of the carbon source.
The effects of different organic carbon sources on the growth of Chlorella sp.-XJY were investigated to determine the utilization of carbon sources. As shown in Fig. 1., cultures supplemented with glucose (10 g/L), glycerol (10 g/L), or sodium acetate (10 g/L) attained higher biomass (2.0, 0.6, and1.0 g/L, respectively) than the control grown under photoautotrophic conditions. The maximum biomass obtained with glucose was more than twofold to threefold greater than that obtained using glycerol and sodium acetate. Therefore, glucose was considered as the best carbon source for the growth of Chlorella sp.-XJY.

Biomass of Chlorella sp.-XJY cultivated with 10g/L of different carbon sources, including glucose, glycerol, and sodium acetate. The medium without carbon source served as the control.
Different concentrations (from 5 g/L to 50 g/L) of glucose were added in basal BG11 medium to investigate the effects of organic carbon source concentration on the growth behavior, polysaccharide production, and productivity of Chlorella sp.-XJY. The laboratory findings (Fig. 2.) displayed that the biomass, polysaccharide production, and productivity were improved from the glucose concentration of 0 g/L to 10 g/L but decreased from 10 g/L to 50 g/L. The maximum of biomass, polysaccharide production, and productivity were 2.06 g/L, 145.5 mg/L, and 26.5 mg/(L·d), respectively, which were 9-, 13-, and 7-fold higher than those of the photoautotrophic control, respectively.

Biomass, polysaccharide production, and productivity with different initial glucose concentrations of Chlorella sp.-XJY. Polysaccharide production was estimated by multiplying the extraction yield of crude polysaccharides with the corresponding biomass concentration. The polysaccharide productivity was calculated by dividing the polysaccharide production with the number of cultivation days.
Kong et al. (2011) found that the biomass and carbohydrate production of C. vulgaris could be enhanced from 0.24 g/L and 10.83 mg/L under photoautotrophic condition to 4.24 g/L and 936.23 mg/L under the optimized mixotrophic medium components, respectively. The contents of carbohydrates and lipids increased as the content of proteins decreased in the cellular constituents.
This change coincided with the report (Orús et al., 1991) citing that a reduction was compensated by an increase in the contents of lipids and carbohydrates. In comparison, the biomass and polysaccharide production in our original mixotrophic medium reached 2.06 g/L and 145.5 mg/L, respectively.
Optimum conditions for the extraction of crude polysaccharides from Chlorella sp.-XJY with RSM
(1) Statistical analysis and the model fitting
BBD was presented to explore the optimal combination of time, temperature, and pH value for enhancing the extraction yield of crude polysaccharides from Chlorella sp.-XJY cultivated under mixotrophic conditions.
The experimental and predicted responses of the extraction yield of crude polysaccharides are provided in Table 2. The mathematical model generated from the experimental data was expressed by the following quadratic Eq. 4:
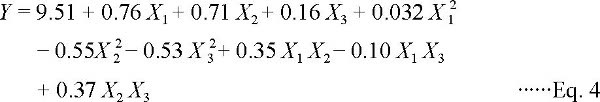 |
| Std. | Coded levels | variable | Extraction yield of crude polysaccharides (%) | ||
|---|---|---|---|---|---|
| A | B | C | Actual values | Predicted values | |
| 1 | −1 | −1 | 0 | 8.01 | 7.88 |
| 2 | 1 | −1 | 0 | 8.75 | 8.69 |
| 3 | −1 | 1 | 0 | 8.53 | 8.59 |
| 4 | 1 | 1 | 0 | 10.67 | 10.80 |
| 5 | −1 | 0 | −1 | 7.95 | 7.99 |
| 6 | 1 | 0 | −1 | 9.74 | 9.71 |
| 7 | −1 | 0 | 1 | 8.49 | 8.52 |
| 8 | 1 | 0 | 1 | 9.87 | 9.83 |
| 9 | 0 | −1 | −1 | 7.84 | 7.93 |
| 10 | 0 | 1 | −1 | 8.71 | 8.61 |
| 11 | 0 | −1 | 1 | 7.42 | 7.52 |
| 12 | 0 | 1 | 1 | 9.76 | 9.67 |
| 13 | 0 | 0 | 0 | 9.58 | 9.51 |
| 14 | 0 | 0 | 0 | 9.74 | 9.51 |
| 15 | 0 | 0 | 0 | 9.21 | 9.51 |
The significance of each coefficient was determined using the F-test and the p-value (Table 3). A smaller p-value signifies a more significant coefficient (Muralidhar et al., 2001). The p-value is important in understanding the pattern of mutual interactions between the variables, wherein values below 0.05 indicate that the test parameter is significant at the 5% level of significance. As shown in Table 3, the regression patterns of the linear term (A, B) and quadratic coefficients (B2, C2) were highly significant (p < 0.01), whereas the cross product coefficients (AB and BC) were significant (p < 0.05). Therefore, the pH (A), extraction temperature (B), and extraction time (C) largely affected the extraction yield of crude polysaccharides.
| Variables | Sum of squares | DF | Mean square | F value | p value |
|---|---|---|---|---|---|
| Model | 11.88 | 9 | 1.32 | 28.36 | 0.0009a |
| A | 4.58 | 1 | 4.58 | 98.32 | 0.0002a |
| B | 3.98 | 1 | 3.98 | 85.63 | 0.0002 a |
| C | 0.21 | 1 | 0.21 | 4.54 | 0.0863 |
| AB | 0.49 | 1 | 0.49 | 10.57 | 0.0226a |
| AC | 0.043 | 1 | 0.043 | 0.92 | 0.3824 |
| BC | 0.54 | 1 | 0.54 | 11.62 | 0.0191a |
| A2 | 3.69E-3 | 1 | 3.69E-3 | 0.079 | 0.7895 |
| B2 | 1.11 | 1 | 1.11 | 23.9 | 0.0045a |
| C2 | 1.03 | 1 | 1.03 | 22.07 | 0.0053a |
| Residual | 0.23 | 5 | 0.047 | - | - |
| Lack of Fit | 0.086 | 3 | 0.029 | 0.39 | 0.7768 |
| Pure Error | 0.15 | 2 | 0.074 | - | - |
| Cor Total | 12.11 | 14 | - | - | - |
a Means significance (Values of “Prob > F” less than 0.0500).
The model F-value of 28.36 ( p < 0.01) implied that the quadratic response surface model was highly significant. Moreover, the lack-of-fit F-statistic that was used to test the adequacy of the model indicated that the p-value (0.7768) for the extraction yield of crude polysaccharides was not significant. Abnormality was not observed in the diagnoses of residuals. The second-order polynomial regression model was in a good agreement with the experimental results (R2 = 0.9808). The experimental data fitted well with the quadratic model of ANOVA.
(2) Optimization of the procedure
The three-dimensional (3D) response surface and contour plots are the graphical representations of the regression function. The interactions between two test variables and relationships between responses and experimental levels of each variable are shown. The effects of temperature, time and pH value on the extraction yield of crude polysaccharides from Chlorella sp.-XJY and their interactions are graphically illustrated in Figs. 2 - 4. In the response surface and contour plots, the extraction yield of crude polysaccharides was acquired with two continuous variables, whereas another variable was a fixed constant at respective zero level (center value of the testing ranges).

Effect of temperature, pH, and their reciprocal interaction on the extraction yield of crude polysaccharides (extraction time is constant at 3 h) (a, 3D response surface; b, contour plots).

Effect of time, pH and their reciprocal interaction on the extraction yield of crude polysaccharides (extraction temperature is constant at 90 °C) (a, 3D response surface; b, contour plots).
The extract pH could significantly affect the extraction yield and activity of polysaccharides (Gan et al., 2010; Wang et al., 2012). The extraction yield of crude polysaccharides increased with increasing pH value and reached a maximum level at a particular pH value (Figs. 2 and 3). Excess acid or alkali may destroy the structure of polysaccharides, thereby, influencing their activities.
Ai et al. (2012) investigated the acute anti-inflammatory activity of water-soluble (WSP) and alkali-soluble (ASP, 0.05 mol/L NaOH) polysaccharides extracted from boat-fruited sterculia seeds. The bioactivity of ASP (4.15%) was much lower than that of WSP (17.84%). The result may be attributed to the effect of the destruction of the spatial structure on the functions of ASP extracted with alkali.
Extraction time is also an essential factor affecting the extraction efficiency and selectivity of the fluid (dilute alkali). This condition is possibly due to the time requirement of the exposure of soluble polysaccharides to the solvent, wherein the liquid can penetrate into the dried powdered material, dissolve the polysaccharides, and subsequently diffuse out from the material (Ye and Jiang, 2011). Different effects of extraction time on the extraction yield of crude polysaccharides are shown in Figs. 3 and 4. The results revealed that the extraction yield of crude polysaccharides in dilute alkali solution increased with prolonged extraction time but slightly decreased at higher levels. The optimal value for extraction time varied with different pH values and extraction temperatures.
The increase in the polysaccharide diffusion coefficient and the enhanced solubility of the polysaccharides in the extracting solvent at higher temperatures increased the release of polysaccharides from the dry power into the solution (Li et al., 2006). Temperature had a similar effect as time on the extraction yield of crude polysaccharides. The extraction yield achieved a maximum value with an increase in extraction temperature but slightly dropped thereafter (Figs. 2 and 4).

Effect of temperature, time and their reciprocal interaction on the extraction yield of crude polysaccharides (pH is constant at 10) (a, 3D response surface; b, contour plots).
Similarly studies on Phellinus igniarius (Guo et al., 2010), fruiting bodies of Pleurotus ostreatus (Sun et al., 2010), and Plantago asiatica L.(Ye and Jiang, 2011) found that the extraction yield of crude polysaccharides increases with increasing extraction temperature and time within a certain range but slightly reduces later from the optimum value. A similar phenomenon was found in the present study.
(3) Verification of results
Three dependent replicates were performed to validate that the optimal values of the extraction parameters were 3.19 h extraction time, 96.38°C extraction temperature, and pH 10.94. For convenience, the following parameters were used: 3.2 h extraction time, 96.4°C extraction temperature, and pH 10.94. The theoretical extraction yield of crude polysaccharides predicted under the indicated conditions was 10.72%.
The optimum extraction conditions were applied for the extraction yield of crude polysaccharides to verify the prediction from the model. The mean extraction yield for the crude polysaccharides was 10.56%, which corresponded well with the predicted value of the model equation (10.72%), confirming that the response model was adequate for the optimization.
In conclusion, the mixotrophic culture could dramatically improve the biomass, and polysaccharide production, and productivity of Chlorella sp.-XJY. The RSM is an effective tool for the optimization of crude polysaccharide extraction from Chlorella sp.-XJY. An optimal predicted extraction yield of crude polysaccharides of 10.72% was obtained by RSM with the following optimum conditions: 3.2 h extraction time, 96.4 °C extraction temperature, and pH 10.94. Under optimized conditions, the experimental extraction yield of 10.56% coincided closely with the predicted value of 10.72%.
Acknowledgments This project is financially supported by National Key Technologies R&D Program (NO. 2011BAD14B01).