2014 Volume 20 Issue 2 Pages 175-182
2014 Volume 20 Issue 2 Pages 175-182
The acyl migration in the production of 1,3-diacylglycerol (1,3-DAG) by immobilized lipozyme RM IM-catalyzed esterification reaction in a solvent-free system was investigated, particularly including the acyl migration of 1,3-DAG to 1,2-diacylglycerol (1,2-DAG), which affected by some of the system's parameters (substrate molar ratio, enzyme load and reaction temperature). The results showed that all parameters had strong positive influences on acyl migration. With the increasing substrate molar ratio of oleic acid to glycerol from 1:1 to 3:1, the acyl migration showed a decreasing-increasing pattern. However, the degree of acyl migration increased gradually concomitant with the increasing of enzyme load (2 – 10 wt% of substrates) and reaction temperature (45 – 75°C). The conditions for the highest yields of 1,3-DAG and the relatively low levels of acyl migration were optimized as follows: temperature 65°C, oleic acid/glycerol molar ratio 2:1, enzyme load 6%, reaction time 2 h, 0.01 MPa vacuum, 200 rpm stirring speed.
Diacylglycerol (DAG), has two congurations, namely, 1,3-DAG and 1,2(2,3)-DAG, which are known to be used as additives or carriers in the food, medicine, cosmetic industry, and so on (Fureby et al., 1997; Kaewthong et al., 2005). It is reported that 1,3-DAG oil has benecial effects on suppressing the accumulation of body fat and preventing the increase of bodyweight (Meng et al., 2004; Reyes et al., 2008; Lo et al., 2008; Yanai et al., 2008; Morita et al., 2009). However, because of the minor content of 1,3-DAG in the natural form, the synthesis of 1,3-DAG becomes more signicant. So, more and more attentions have been paid to 1,3-DAG production in recent years.
In the preparation of 1,3-DAG, several chemoenzymatic and biotechnological methods are available (Guanti et al., 2004; Villeneuve et al., 2000). It seems that high yield of 1,3-DAG cannot be obtained by direct chemical methods because this methods lack positional selectivity. The enzymatic synthesis of 1,3-DAG has been considered more effective due to its milder and simpler reaction conditions, higher selectivity, greener process and safe products. In general, the preparation of 1,3-DAG enzymatically by direct esterication, glycerolysis, interesterication, partial hydrolysis, or the combination of partial hydrolysis and esterication (Blasi et al., 2007; Cheong et al., 2007; Weber et al., 2004; Yang et al., 2004; Liu et al., 2011; Palla et al., 2012). Comparing with other methods, when the direct esterication used, the fatty acid and glycerol can fast synthesis 1,3-DAG, especially, when the reactions are carried out in a solvent-free system.
Lipozyme RM IM is well known as one of the lipases with a strict 1,3-positional specificity, which was used to catalyze esterification of glycerol with oleic acids for 1,3-DAG production in the solvent-free system. Acyl migration should occur during the 1,3-DAG production process, which was not conducive to the product yield and purity. However, most of the studies which discussed the acyl migration problem during the enzymatic production of specific-structured lipid were focused on the triglyceride and fatty acid (Xu et al., 1998; Mu et al., 2001). Few reports discussed the relationship between 1,3-DAG and 1,2-DAG (acyl migration) in production of 1,3-DAG system. Therefore, in this paper, the acyl migration phenomenon was explored and demonstrated, based on which different factors, including molar ratio of the reactants, enzyme load and temperature, which may influence the acyl migration and incorporation of oleic acids into glycerol were studied systematically.
Materials Lipozyme RM IM, a commercial sn-1,3 specic lipase, in which Rhizomucor miehei lipase was immobilized on a microporous ion-exchange resin (water content 2.7%, measured by the Karl Fischer moisture meter), was purchased from Novozymes A/S (Bagsvaerd, Denmark). The standards of 1-monoolein, 1,3-diolein, 1,2-diolein, triolein (>99.0%) and chromatographic grade oleic acid were purchased from Sigma-Aldrich (Shanghai, China). Glycerol from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China) was of analytical grade. All other reagents and solvents were of analytical or chromatographic grade also purchased from Sinopharm Chemical Reagent Co. Ltd. to suit analytical requirements.
Esterification reaction The lipase-catalyzed esterification reaction was performed in a 100 mL round bottom flask, thermostated to the desired operating temperature, and stirred by a magnetic stirrer (200 rpm). The compositions of the reaction mixtures were shown as follows: the require amount of oleic acid, glycerol, and a certain amount of Lipozyme RM IM. The reaction temperature was ranged between 45 – 75°C, substrate oleic acid/glycerol molar ratio was from 1:1 to 3:1, and enzyme load was 2 – 10% (wt% of substrates). The reaction was carried out at the normal pressure (4 Å molecular sieves used as the water removal agent) and 0.01 MPa vacuum, respectively. During the esterification reaction, the conversion of oleic acid, 1,3-DAG and 1,2-DAG contents were analyzed.
Determination of esterification efficiency The upper lipid layer from the reaction mixture after centrifugation was titrated with 0.1 mol/L KOH solution to determine the amount of unconverted fatty acids in terms of acid value (AV). The esterication efciency was dened as the consumed oleic acid amount during the reaction, using the formula:
 |
where AV1 and AV2 were the AV of the initial substrates and products after a period of reaction, respectively. All analyses were performed in triplicate, and the mean values were reported.
Analysis of the reaction mixture During the enzymatic reaction, 0.25 mL samples were withdrawn from the upper lipid layer in reaction vessel and dissolved in 5 mL hexane. Then the samples were centrifuged at 10, 000 × g for 10 min in order to remove impurities in the sample, and subsequently were determined by HPLC system equipped with an evaporative light scattering detector (ELSD). The stationary and mobile phases were a silica column (5 µm, 250 mm × 4.6 mm) and a gradient elution program was achieved by mobile phase A (n-hexane/isopropanol = 99:1, v/v) and B (n-hexane/isopropanol/acetic acid = 1:1:0.01, v/v/v). The gradient was operated as follows: 0 – 10 min, 100% A; 10 – 14 min, 80% A and 20% B; 14 – 15 min, 70% A and 30% B; 15 – 20 min, 100%A. The flow rate was xed at 0.5 mL/min. The column temperature and drift pipe temperature were controlled at 35 and 70°C, respectively. Injection volume of 5 µL was kept constant for all samples during analysis. The compositions of acylglycerols were calculated as weight percentage of the samples analyzed using calibration curves of standards. All reactions were run in triplicate, and the mean values were plotted. Significance was evaluated at 5% (P < 0.05). Although no standard errors are shown in Figures 1 to 5, the standard deviations in the results varied between 0.001 and 0.954 for all of the experiments conducted.

Effects of water removal on the esterification synthesis of 1,3-DAG. Reaction conditions: temperature, 65°C; oleic acid/glycerol molar ratio, 2:1; enzyme load, 6 wt% of substrates; 200 rpm stirring speed. Reaction performed with water removal by evaporation under 0.01 MPa vacuum (▴ and ▵ represented the conversion of oleic acid and 1,3-DAG content, respectively), reaction at the normal pressure in a closed vessel with water removal using 4 Å molecular sieves (▪ and □ represented the conversion of oleic acid and 1,3-DAG content, respectively).

Effect of the substrate molar ratio on the acyl migration of 1,3-DAG to 1,2-DAG. Reaction conditions: temperature, 65°C; enzyme load, 6 wt% of substrates; 0.01 MPa vacuum; 200 rpm stirring speed. The time course of 1,3-DAG content base on the substrate ratio of 1:1 (♦); 1.5:1 (▪); 2:1 (▴); 2.5:1 (*) and 3:1(•), respectively; the time course of 1,2-DAG content base on the substrate ratio of 1:1 (⋄); 1.5:1 (□); 2:1 (▵); 2.5:1 (×) and 3:1 (○), respectively.
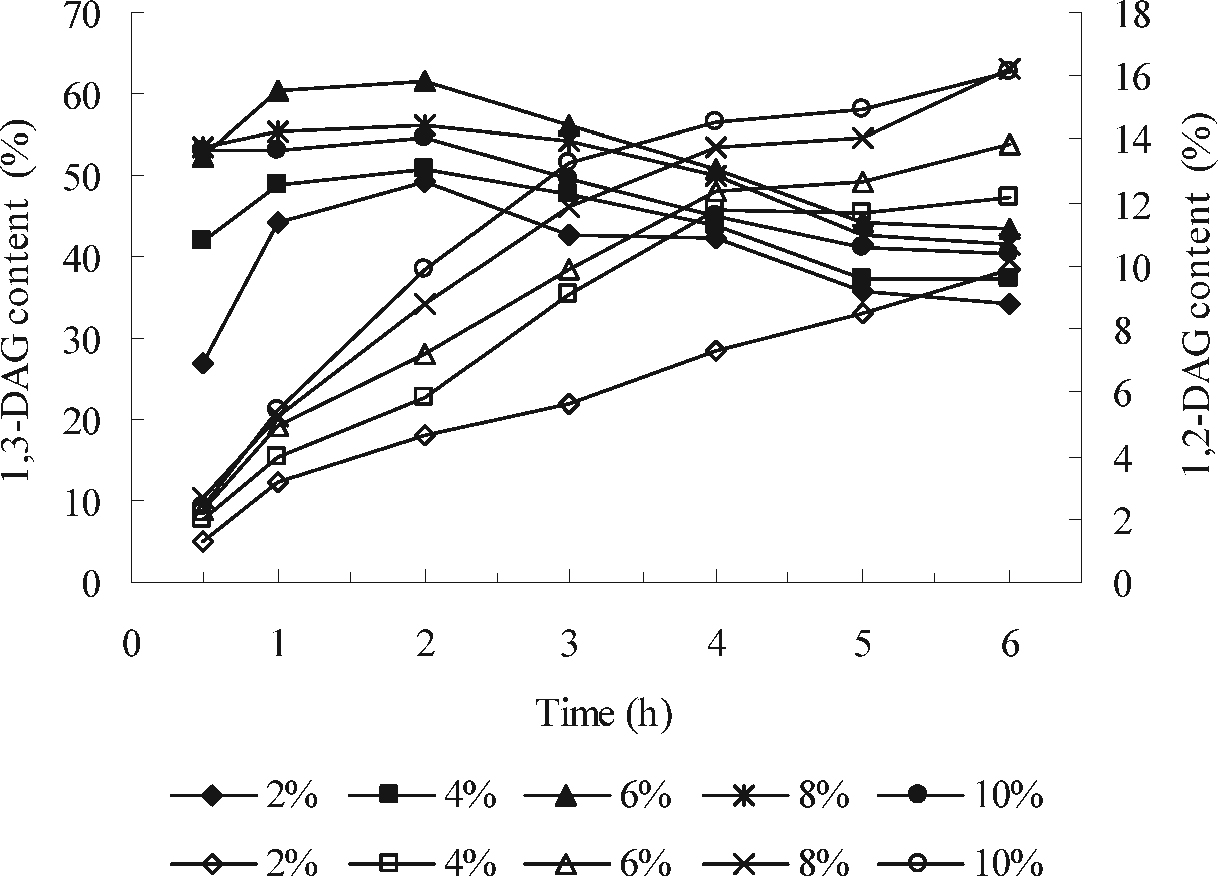
Effect of enzyme load on the acyl migration of 1,3-DAG to 1,2-DAG. Reaction conditions: temperature, 65°C; oleic acid/glycerol molar ratio, 2:1; 0.01 MPa vacuum; 200 rpm stirring speed. The time course of 1,3-DAG content base on the enzyme load (wt% of substrates) of 2% (♦); 4% (▪); 6% (▴); 8% (□*) and 10% (•), respectively; the time course of 1,2-DAG content base on the enzyme load of 2% (⋄); 4% (□); 6% (▵); 8% (×) and 10% (○), respectively.

Effect of reaction temperature of on the acyl migration of 1,3-DAG to 1,2-DAG. Reaction conditions: oleic acid/glycerol molar ratio, 2:1; enzyme load, 6 wt% of substrates; 0.01 MPa vacuum; 200 rpm stirring speed. The time course of 1,3-DAG content base on the reaction temperature of 45°C (♦); 55°C (▪); 65°C (▴) and 75°C(*), respectively; the time course of 1,2-DAG content base on the reaction temperature of 45°C (⋄); 55°C; 65°C (▵) and 75°C (×), respectively.

Time course of esterication of glycerol with oleic acid. Reaction conditions: temperature, 65°C; oleic acid/glycerol molar ratio, 2:1; enzyme load, 6 wt% of substrates; 0.01 MPa vacuum; 200 rpm stirring speed. TAG (♦); FFA (free fatty acid, ▪); 1,3-DAG (▴); 1,2-DAG (▵); 1-MAG (×).
Selection of operation modes for the esterification reaction The synthesis of 1,3-DAG by direct esterification is based on the 1,3-positional specificity of immobilized lipozyme RM IM in the solvent-free system. It is known that a small amount of water is necessary for the catalytic function of the lipase, in order to maintain enzyme structure and to achieve catalytic activity (Watanabe et al., 2003). If the water content is too high, the hydrolysis reaction will be favored, and yields will decrease. The removal of extra water formed concomitantly in the esterification reaction is very important for shifting the reaction equilibrium toward high yields of 1,3-DAG. Therefore, it was necessary to study the effects of the dehydration way to the reaction.
As shown in Fig. 1, the equilibrium was reached after 5 h with only 55.3% conversion of the oleic acid for the reaction which was performed at the normal pressure in a closed magnetic stirrer reactor using 4 A molecular sieves as the water absorbing agent. Water, the most volatile component in this reaction system, was removed efficiently by 0.01 MPa vacuum, and the equilibrium was reached after 3 h at 87.7% conversion of the oleic acid. The maximum yields of 1,3-DAG was 45.8% and 62.6% were achieved when the esterification reaction conducted 2h dehydrated by 4 Å molecular sieves and 0.01 MPa vacuum, respectively. Maybe the water formed in the initial stage of the reaction helps the enzyme reach maximal activity. Compared to the reaction in normal pressure dehydrated by 4 Å molecular sieves, more 1,3-DAG content and high conversion of the oleic acid were detected in the reactions in 0.01 MPa vacuum. Similar results with other water removing methods for the enzymatic synthesis of symmetrical 1,3-DAG by direct esterication of glycerol in a solvent-free system had also been reported (Rosu et al., 1999). For the rest of the experiments, 0.01 MPa vacuum was used for the water removal.
Effect of substrate molar ratio on the acyl migration The effect of the molar ratio of oleic acid to glycerol on acyl migration is shown in Fig. 2. The 1,3-DAG content showed an increasing-decreasing pattern with the increasing substrate molar ratio of oleic acid to glycerol. The 1,3-DAG content was increased when the substrate molar ratio of oleic acid to glycerol changed from 1:1 to 2:1, and followed, which decreased accompanied by the increasing of substrate molar ratio of oleic acid to glycerol from 2:1 to 3:1. Obviously, the highest 1,3-DAG content was obtained at the substrate ratio of 2:1. On the contrary, the 1,2-DAG content showed a decreasing-increasing pattern with the increasing substrate molar ratio of oleic acid to glycerol from 1:1 to 3:1, and the minimized acyl migration (namely, the minimum content of 1,2-DAG) was obtained at the substrate molar ratio of 2:1. The results obtained above were accordances with expected data, namely, when the theoretical stoichiometric molar ratio of oleic acid to glycerol of 2:1, the 1,3-DAG production reached the maximum. If the content of oleic acid is too high, the utilization rate of substrates is not high. If the glycerol content is too high, not only to increase the viscosity of the system, but also is not conducive to the reaction; the polarity of system increases, the lipase is easily deposited on the bottom of the reactor with the glycerol together, which is difficult mixing with the oleic acid efficiently, reducing the reaction rate and conversion rate of the esterification process.
When the molar ratio of oleic acid to glycerol was 2:1, the reactions proceeded for several hours, the maximum 1,3-DAG content (61.8%) was obtained at the reaction of 2 h and then the content of 1,3-DAG decreased while the degree of acyl migration (1,2-DAG content) increased gradually (from 2.2 to 11.6%) in the whole reaction period. Almost a balance between 1,3-DAG and 1,2-DAG was reached after the reaction of 5 h.
Effect of enzyme load on the acyl migration The effect of the enzyme load on the reaction was presented in Fig. 3. It was obvious that an increase in enzyme load from 2 to 6% (wt% of substrates) resulted in a considerable increase in the content of 1,3-DAG (from 49.3 to 61.7%) at the reaction of 2 h, while the 1,2-DAG content increased only from 4.7 to 7.2%. To future increase the enzyme load to 10%, the content of 1,3-DAG reduced to 54.7% while the 1,2-DAG content increased from 7.2 to 9.9%. The decrease in 1,3-DAG content when large amounts of enzyme were employed may be due to the biocatalyst agglomeration and possible diffusion problems. Similar results that excess enzyme in the reaction decrease the efficiency of biocatalyst in other enzymatic reactions had also been reported (Watanabe et al., 2001; Yang et al., 2005).
Similar trends of 1,3-DAG content was detected for the reaction conducted during 6 h with different amounts of enzyme loaded. The maximum 1,3-DAG content was obtained at 6% enzyme load (wt% of substrates) when the reactions proceeded for 2 h. Under this quantity of enzyme load, the content of 1,2-DAG increased gradually from 2.2 to 13.8% in the whole reaction period. Similar results were reported to support of Lipozyme RM IM-catalyzed acyl migration (Fureby, 1995).
Effect of reaction temperature on the acyl migration The effect of reaction temperature on acyl migration of lipozyme RM IM-catalyzed esterification in a solvent-free system is shown in Fig. 4. Under different reaction temperatures, the content changes of 1,3-DAG and 1,2-DAG along with the reaction time were investigated. The content curves of effects of reaction temperature on 1,3-DAG and 1,2-DAG were similar to those effects of the enzyme load. The content of 1,3-DAG increased when the reaction temperature was changed from 45 to 65°C, however, when the temperature surpassed 65°C, the opposite effect was obtained. When the temperature was 65°C, the 1,3-DAG content increased rapidly in the first 1 h, and in the next 1 h, there was a slow but steady increased of 1,3-DAG, the maximum 1,3-DAG content was obtained at the 2h and then decreased. The 1,2-DAG increased smoothly from 2.2 to 13.8% over the whole reaction period when the temperature was 65°C. Similar trends of 1,3-DAG and 1,2-DAG contents were detected for other reaction temperatures.
During the whole reaction period, the 1,3-DAG content reached the maximum at 65°C, while the 1,2-DAG content increased along with the increasing of temperature from 45 to 75°C. This phenomenon may be due to an increase in temperature can reduce mixture viscosity, enhance mutual solubility and improve the diffusion process of substrates (Duan et al., 2010). However, too high temperature will lead to higher lipase deactivation rate.
The trends of product compositions under optimal conditions Fig. 5 shows typical experimental time courses for 1,3-DAG synthesis using the immobilized lipozyme RM IM in a solvent-free system. As the reaction process progressed, the content of oleic acid decreased with time increased until reaction reaches equilibrium, while the TAG and 1,2-DAG content increased until reaction reached equilibrium in the whole reaction period. And the content of 1,3-DAG and 1-monacylglycerol (1-MAG) almost simultaneously increased, and then decreased with reaction time increased until reaction reached equilibrium. The initial accumulation of 1,3-DAG indicated a positional preference (specicity) of the intracellular lipase over 1(3)-position of the glyceride and partial glyceride (Li et al., 2010a). While the accumulated 1,3-DAG gradually turned into 1,2-DAG, which indicated the occurring of acyl migration during the reaction process.
In this reaction, 2-monacylglycerol (2-MAG) was not detected in the first four reaction hours, and the 2-MAG amout was still very small even in the later two hours. Therefore, the content of 2-MAG got from the synthesis of fatty acid and glycerol but not from the acyl migration was assumed to be negligible since an enzyme with a high 1, 3-selectivity was used. Acyl migration from 1-MAG to 2-MAG was not considered since the 2-MAG content for 1-MAG/2-MAG was ten times lower than those for the rest of the reactions. So we only considered the acyl migration of 1,3-DAG to 1,2-DAG.
Acyl migration process analysis during the esterification reaction From the perspective of the mechanism of enzyme-catalyzed esterification reaction, the lipase contains a nucleophilic serine residue composed with the triplet structure form of “serine-histidine-aspartic acid” (Brzozowski et al., 1991). The area of connection substrate and active site is covered by a lid of α-helix when the lipase in non-active status. When the lipase absorbed on the oil-water interface under the induction of hydrophobic interactions of oil phase, the lid is opened and active site exposed to strengthen the hydrophobicity in the area of around the slit and hole of connected substrate (Al-Zuhair et al., 2007). In the esterication reaction process of glycerol with oleic acid, the hydrophobic long chain oleic acid substrate into slits, and was contact with active center triplet structure at the bottom of the slit. The hydroxyl oxygen of serine of the active center of lipase had nucleophilic, and the carbonyl oxygen of substrate oleic acid had positive charge, which prompted the nucleophilic reaction and formed enzyme-oleic acid acyl compound, and then it esterified with the 1,3-position of glycerol to form 1-MAG and water, subsequently to form 1,3-DAG and water.
Fig. 6 shows the reaction scheme of esterfication of glycerol with oleic acid using Lipozyme RM IM as the catalyst, which was proposed by Lortie et al. (1993) on the basis of model for triolein synthesis. Under the role of 1,3-regiospecific lipase, 1-MAG formed firstly, and then, which continued to react with oleic acid to form 1,3-DAG, and acyl migration occurred concomitantly to form 2-MAG. The resulting 1,3-DAG also occurred acyl migration to form 1,2-DAG, followed by the formation TAG. Namely, 2-MAG and 1,2-DAG which appeared in the reaction product, caused by the acyl migration of 1-MAG and 1,3-DAG, respectively. Acyl migration not only increases the incidence of by-products, makes the reaction system complicated, but also increases the difficulty of subsequent product separation, so it is necessary to analyze the acyl migration phenomenon.

Reaction scheme of esterification of glycerol with oleic acid using Lipozyme RM IM as the catalyst
Acyl migration between 1,2-DAG and 1,3-DAG was similar to acyl migration between 1-MAG and 2-MAG. Therefore, the latter was detailed description. Acyl migration belongs bimolecular nucleophilic substitution(SN2) (Li et al., 2010b). Carbonyl carbon of carbon backbone of 2-MAG had positive charge as an electron-withdrawing effect of carbonyl oxygen, and nucleophilic primary hydroxyl oxygen attacked the carbonyl carbon to promote nucleophilic reaction. At this time,“hydroxyl-carbonyl carbon-acyl group”at an angle of 180°, so the lone pair electrons and σ antibonding orbitals of “carbonyl carbon-acyl” could achieve the maximum overlap. Then an unstable five-coordinate transition state formed, and made extranuclear electron orbit of carbonyl carbon change from sp2 into sp3. The hydrogen atom was easily detached from the hydroxy, and transferred to alkoxy of the acyl group of MAG to form 1-MAG (Fig. 7).

Acyl migration between MAG isomers
Lipozyme RM IM was used as the catalyst for synthesis of 1,3-DAG by a direct esterification of glycerol with oleic acids in a solvent-free system. The effects of substrate molar ratio, enzyme load and reaction temperature on the acyl migration were discussed, particularly including the acyl migration of 1,3-DAG to 1,2-DAG. The results showed that all parameters had strong positive influences on acyl migration, which showed a decreasing-increasing pattern with the increasing substrate molar ratio of oleic acid to glycerol from 1:1 to 3:1. With the increasing of enzyme load (2 – 10 wt% of substrates) and reaction temperature (45 – 75°C) in the esterification reaction, the degree of acyl migration increased gradually. The acyl migration can not be totally avoided in present systems, but can be reduced to a correspondingly low level. The optimal conditions selected for the highest content of 1,3-DAG and the relatively low levels of acyl migration were as follows: temperature 65°C, oleic acid/glycerol molar ratio 2:1, enzyme load 6 wt% of substrates, reaction time 2 h, 0.01 MPa vacuum, 200 rpm stirring speed. When the reaction continued at these conditions, the yields of 1,3-DAG decreased while 1,2-DAG increased with reaction time increased until the ratio of 1,3-DAG and 1,2-DAG kept constant and the reaction reached equilibrium. The results obtained in the present study should be helpful for researchers who are interested in regulating the acyl migration in the synthesis of structured lipid area.
Acknowledgement This research was supported by the National Natural Science Foundation of China (31201382), National Twelve-Five plan for Science & Technology Support (2011BAD02B04) and the program for New Century Excellent Talents in University of China (NCET-10-0457).