2014 Volume 20 Issue 2 Pages 189-199
2014 Volume 20 Issue 2 Pages 189-199
Hydrodistillation was carried out from fresh leaves, air-dried leaves in shade, sun-dried leaves, frozen leaves and salted leaves. It was observed that air-dried in shade was the optimal choice for prolonging the pre-processing storage with the advantage of low cost and simplicity. Nine different macroporous resins (DM-21, LS-305, LS-305A, HPD-100, D001CC, XAD-16, XAD-7HP, FPC3500 and SK1B) were evaluated for the adsorption properties of perillaldehyde from hydrodistillation condensate of Perilla frutescens (L.) Britton var. crispa f. viridis leaves. The influences of phase contact time, initial perillaldehyde concentration and ethanol concentration were studied by the static adsorption/desorption method. The adsorption isotherm data were fitted well to the Freundlich model. The kinetic data were modeled using the pseudo-first order, pseudo-second order and intraparticle diffusion equations. The experimental data were well described by the pseudo-second order kinetic model. Column studies were also carried out to investigate the breakthrough and elution behavior at three different flow rates. Continuous column adsorption-regeneration cycles indicated negligible capacity loss of LS-305 during operation.
Perilla frutescens (L.) Britton var. crispa f. viridis is a member of the Lamiaceae family. There are both green-leafed and purple-leafed varieties, which are generally recognized as separate species by botanists. Perilla frutescens (L.) Britton var. crispa f. viridis is the green-leafed variety of Perilla frutescens of Chinese origin. The Chinese and Japanese call the green type Baisu and aojiso, respectively (Huang et al., 2011; Yamazaki et al., 1997). Its fresh leaves are used as vegetables in China and commonly used for seasoning pickles or as garnish for raw fish dishes or wrapping with roasted meats in Japan and Korea (Huang et al., 2011). Perilla raw material and phytopreparations are used for the treatment of poisonings with seafood and allergic reactions, digestive problems, asthmatic cough, and also in relieving or laxative preparations with antimicrobial, immunostimulating, antioxidant, and antitumor activities (Shin et al., 2000; Honda et al., 1986; Makino et al., 2001; Kang et al., 1992; Ueda et al., 2002). Perillaldehyde, the major volatile extracts in Perilla leaves (Honda et al., 1986; Ito et al., 1999), is widely used in food, flavor, perfumery, organic synthesis and pharmaceutical industry (Acto et al., 1970; Sun et al., 1998).
The analysis of volatiles from Perilla frutescens were investigated extensively (Huang et al., 2011; Baser et al., 2003; Bumblauskiene et al., 2009; Ito et al., 2002; Seo and Baek, 2009). Various methods exist for the extraction of volatile components, such as hydrodistillation or steam distillation (Huang et al., 2011; D'Antuono et al., 2002; Povilaitytoee and V enskutonis, 2000; Kong et al., 1999; Pickett et al., 1975), organic solvent extraction (Richter, 1996; Ortega-Heras and Gonz¡lez-SanJosé, 2002), and supercritical fluid extraction (Chan and Ismail, 2009; Nyam et al., 2010). Hydrodistillation or steam distillation is a conventional method used to extract essential oils from aromatic plants, it can be used in industry and has no chemical pollution. However, during distillation, substantial amounts of water-soluble compounds (alcohols, ketones, aldehydes, etc.) present in the essential oil are dissolved in the distillation condensate water due to their finite solubilities in water (Machale et al., 1997; Bohra et al., 1994; Fleisher, 1991; Amin et al., 2001). The recovery of these compounds becomes very important due to economic or environmental reasons (Machale et al., 1997). Recovery of these valuable compounds can be completed either by solvent extraction or adsorption. The choice of recovery depends on the concentration of the compounds present in the condensate water. Adsorption is the mostly widely used process for condensates with concentrations less than 2000 ppm due to low solubilities (Machale et al., 1997; Amin et al., 2001). In recent years, macroporous resins have been increasingly viewed as an alternative for the separation and enrichment of effective components from many natural extracts, due to its simpler operation, higher efficiency, less yielding cost, more friendly to environmental protection and easier regeneration (Du et al., 2008; Fu et al., 2006; Ma et al., 2009; Sun et al., 2009). However, little information on the methods suitable for industrial application for recovery perillaldehyde from Perilla frutescens leaves is available. Perilladehyde was found to have finite solubility in water from our preliminary tests. Hydrodistillation or steam distillation as a single recovery method leads to yield losses in the distillation condensate water. Previously, we have studied the nine resins, XAD-4, XAD-7HP, HP-20, SP-207, X-5, NKA, NAK-II, AB-8 and DG-23 for the recovery of perillaldehyde from hydrodistillation condensate of Perilla frutescens (L.) Britton var. crispa f. viridis leaves (Chang et al., 2013). In the present study, nine advanced macroporous resins (DM-21, LS-305, LS-305A, HPD-100, D001CC, XAD-16, XAD-7HP, FPC3500, SK1B) selected according to the resin manufacturer's recommendation and in accordance with current good manufacturing practice by our cooperative production unit (Qindao Pengyuan Kanghua Natural Products Co., Ltd., China) were used to systematically investigate the adsorption and desorption of perillaldehyde from hydrodistillation extracts and to develop a simple and efficient process for the recovery of perillaldehyde with the optimal resin. We describe hydrodistillation as a pre-treatment method for the isolation of the volatile components and macroporous resin adsorption as the concentration method to obtain the product of high perillaldehyde content. The yields of perilladehyde extracted by ethanol and hydrodistillated from fresh leaves, air-dried leaves in shade, sun-dried leaves, frozen leaves and salted leaves of Perilla frutescens (L.) Britton var. crispa f. viridis were also compared.
Materials Perillaldehyde was purchased from Aldrich (Milwaukee, WI, USA, 99% purity). HPLC grade acetonitrile was obtained from Shanghai Kefeng Chemical Reagents Co., Ltd (Shanghai, China). All other chemicals used were of analytical grade and commercially available. Ultrapure water was obtained by Wahaha Group Co., Ltd (Hangzhou, China).
Fresh leaves of Perilla Frutescens (L.) Britton var. crispa f. viridis and crude perillaldehyde ethanol extract (Termed as Sample 1 below) were provided by Qingdao Pengyuan Kanghua Natural Products Co., Ltd (Qingdao, China).
Adsorbent resins The resin XAD-16, XAD-7HP and FPC3500 were purchased from Rohm & Haas, USA. SK1B was from Mitsubishi Chemical Industries, Japan. D001CC was purchased from Chemical Plant of Nankai University, China. LS-305 and LS-305A were from Shaanxi Lanshen Special Resin Co., Ltd, China. HPD-100 was from Anhui Sanxing Resin Technology Co., Ltd, China. DM-21 was from Shandong Lukang Record Pharmaceuticals Co., Ltd, China. Table 1 shows the physical characteristics of the adsorbent resins as indicated by the manufactures.
| Resin | Surface area (m2/g) | Pore size (Å) | Polarity | Matrix |
|---|---|---|---|---|
| LS-305 | 900 – 1000 | 60 | apolar | SDVBb |
| DM21 | 1000 | 50 – 60 | apolar | Acrylic |
| LS-305A | 850 – 950 | 80 | apolar | SDVBb |
| XAD-16 | 800 | 150 | apolar | SDVBb |
| HPD-100 | 650 – 700 | 85 – 90 | apolar | SDVBb |
| XAD-7HP | 500 | 450 | semipolar | Acrylic |
| SK1B | weak cationic | SDVBb | ||
| FPC3500 | strong anionic | SDVBb | ||
| D001CC | strong cationic | SDVBb |
a information provided by the manufacturers.
b SDVB, styrene-divinylbenzene.
Pretreatment of resins Prior to use, the resins were leached with 95% ethanol for 24 h to ensure the wetting of the internal pores and washed several times with deionized water. Subsequently, the resins were soaked in 1 M NaOH for 5 h, and then washed thoroughly by deionized water. The resins were then soaked in 1 M HCl for 5 h. Finally, the resins were washed by deionized water thoroughly. Their surface moisture was removed by pressing gently between the folds of filter paper (Termed as filter paper dried resin, FPD) (Singh et al., 2008). The FPD resins were immediately packed in nylon-bags sealed with an impulse sealer, and kept in a stylofoam box until used. The dry-base weight of sorbent was measured by weighting the sorbent particles after drying for 12 h at 80°C. In all adsorption studies resin used was FPD resin. All the calculations are done on the basis of dry resin.
Analytical methods Perillaldehyde content was determined by the HPLC method using a Waters liquid chromatographic system with an ODS-C18 column (250 × 4.5 mm I.D., 5 µm particle). Acetonitrile-water (75:25 v/v) was used as a mobile phase with a flow-rate of 0.8 mL/min and at room temperature. The detection wavelength was 220 nm and the injection volume was 20 µL. The column temperature was maintained at 25°C. All the solutions were filtered through a 0.22 µm membrane filter prior to analysis. Triplicate injections were made for each concentration.
Preparation of Sample solutions Sample 1, crude perillaldehyde ethanol extract, was provided by Qingdao Pengyuan Kanghua Natural Products Co., Ltd (Qingdao, China).
Sample 2 (2a, 2b), Sample 1 (1500 mL) was added into a 2 L round-bottom flask subjected to hydrodistillation under ambient pressure and vacuum pressure to yield Sample 2a and Sample 2b, respectively. To ambient pressure distillation process, the round-bottom flask was heated with a jacket heater (Model KDM, Juancheng County Yongxing Instrument Factory of Shandong Province, China) set to the temperature range of 100 – 120°C. To vacuum pressure distillation, the round-bottom flask was heated by thermostated water bath at 65°C. Distillate was collected for each distillation until complete exhaustion.
Sample 3 (3a1, 3a2, 3b1, 3b2, 3c, 3d). In order to prolong the processing period, part fresh leaves were sun-dried, air-dried in shade for 2 and 4 days, respectively, and stored in airtight polyethylene bags until use. Part leaves were stored frozen at −20°C. Part leaves were salted.
Salting procedure was performed as follows: 800 g fresh leaves of Perilla Frutescens (L.) Britton var. crispa f. viridis and 160 g salt were mixed in a large pan and transferred into a clean sterilized 1.5 L glass jars with screw-cap and stored at ambient temperatures between 20 and 25°C.
Hydrodistillation experiment was carried out as follows: In a 2 L round-bottom flask, 150 g of fresh leaves , 68 g of sun-dried leaves or 70 g of air-dried leaves in shade (both corresponding to 500 g fresh leaves) , 450 g of frozen leaves, a glass jar of salted leaves along with the liquid leaving the leaves (corresponding to 800 g fresh leaves), was mixed with 1.5 L of distilled water, respectively, followed by hydrodistillation under ambient pressure, obtaining Sample 3a2, 3b1, 3b2, 3c and 3d, respectively. Sample 3a1 was prepared in the similar condition to Sample 3a2 except under vacuum pressure.
Prior to distillation, dried leaves were subjected to 4 h static maceration at ambient temperature in distilled water followed by hydrodistillation.
Preliminary choice of the resins The preliminary choice of the resins was evaluated by their adsorption capacities and desorption ratios. Batch adsorption and desorption runs were carried out at room temperature, natural pH, 100 rpm for 24 h in an orbital shaker (Model HY-4A, Changzhou Putian Instrument Co., Ltd, Jintan, China). In adsorption study, pre-weighed amount of FPD resin (equal to 0.25 g dry resin) and 20 mL of Sample 1, 100 mL of Sample 2b and Sample 3, respectively, with the initial perillaldehyde concentration of 318.3, 324.7 and 327.6 mg/L, respectively, were added into each flask with a lid.
After the attachment of adsorption equilibrium, the adsorbed resins were filtered through a 200-mesh screen, washed with deionized water to remove unbound material. This was followed by a 20 mL of 80% ethanol aqueous solution for Sample 1, Sample 2 and Sample 3, respectively, to desorb perillaldehyde.
The adsorption capacity of perillaldehyde on resin, qe (mg/g), was calculated according to:
 |
The desorption ratio of perillaldehyde on resin, D (%), was calculated according to:
 |
where Co (mg/L) and Ce (mg/L) are the initial and equilibrium liquid-phase concentrations, respectively, V (L) is the volume of solution and W (g) is the weight of dry resins. Cd (mg/L) and Vd (L) are the concentration of perillaldehyde in the desorption solution and volume, respectively.
Adsorption isotherm experiments Adsorption equilibrium data were obtained by introducing pre-weighed amount of five selected FPD resins, DM-21, LS-305, LS-305A, XAD-16 and HPD-100, (equal to 0.25 g dry resin) into 100 mL Sample 3 solution at different initial concentrations of perillaldehyde (304.0, 768.8, 1157.0, 1503.0, 2246.4, 3011.5, 3779.6 mg/L), shaking at 100 rpm at 25°C for 24 h.
Desorption experiments Pre-weighed amount of five selected FPD resins, DM-21, LS-305, LS-305A, XAD-16 and HPD-100 (equal to 0.25 g dry resin) was added into 100 mL Sample 3 solution at the initial concentration 1503.0 mg/L under periodic stirring at 25°C for 24 h. The adsorbed resins were filtered through a 200-mesh screen, washed with deionized water. This was followed by a 20 mL of different ethanol solution (50, 60, 70, 80, 95, 100%) to desorb perillaldehyde, shaking at 100 rpm at 25°C for 24 h.
Static adsorption and desorption kinetics The adsorption kinetics of the selected five macroporous resins were studied by contacting 100 mL Sample 3 solution (initial concentration 1503.0 mg/L) with pre-weighed amount of hydrated resins (equal to 0.25 g dry resin) on a shaker (100 rpm) at 25°C. The concentration of perillaldehyde in the adsorption solution was determined at different time until equilibration.
After the attachment of adsorption equilibrium, the adsorbed resins was filtered through a 200-mesh screen, then washed with deionized water, 80 mL of absolute ethanol aqueous solution was added into each flask with a lid. The flasks were shaken on a shaker (100 rpm) at 25°C. The concentration of perillaldehyde in the desorption solution was determined at different time.
Dynamic adsorption and elution experiments The separation properties of the three selected resins, DM-21, LS-305 and LS-305A, were evaluated by the dynamic tests.
Dynamic adsorption tests were carried out as follows: the hydrated test adsorbent was packed into a glass column (30 cm × 1 cm) with a bed volume (BV) of 12 mL. The Sample 3 with concentration of 873.6 mg/L was introduced downward into the column at a flow rate of 1, 3, and 5 BV/h, respectively, and then the adsorbent column was rinsed by 3 BV of deionized water.
Breakthrough adsorption capacity and the saturation capacity were calculated based on the amount of perillaldehyde adsorbed when the concentration of the exit from the column reached 5% and 95% of the inlet solution concentration, respectively. The adsorption capacity q (mg/mL resin) was calculated with Eq. (3) (Li et al., 2002; Cai et al., 2005; Zeng et al., 2009):
 |
where Ci (mg/L) is the concentration of initial solution, Ca (mg/L) is the average concentration of the effluent, Vs (L) is the volume of sample solution, Vw (mL) is the volume of wet resin.
Dynamic desorption test was performed as follows: the adsorbate-laden column was desorbed by absolute ethanol at rate of 1, 2 and 3 BV/h, respectively.
Statistical analysis All the experiments were performed in triplicate and data are showed as means values ± S.D. Statistical analysis was done using one-way analysis of variance (ANOVA), and the mean values were considered significantly different when p < 0.05.
Comparison of different preparation methods for Sample 3 solutions (3a1, 3a2, 3b1, 3b2, 3c, 3d) From preliminary experiments, the appropriate ratios of leaves to water were established when the yield of perillaldehyde was not affected by the ratio of leaves to water in which the minimized ratio was determined as the appropriate one.
With respect to the evaporating pressure, highly significant difference (p < 0.01) was observed between vacuum pressure (3a1) and ambient pressure (3a2), which could be due to the high volatility of perilladehyde that about 77.5% perilladehyde was sucked away to the vacuum pump.
Regarding the drying methods, the significant decrease (p < 0.05) was noted by sun-drying (3b1, 40% loss) compared to air-drying in shade (3b2), which is probably as a result of the higher temperature under sun-drying process.
Concerning the preparation procedures by air-drying in shade, freezing and salting, the yields of perilladehyde corresponding to Samples 3b2, 3c and 3d do not differ significantly compared to Sample 3a2 from the fresh leaves. Among them, Sample 3b2 from air-dried leaves in shade was slightly low but without significant difference, which is especially important for prolonging the pre-processing storage with the advantage of low cost and simplicity. As shown in Table 2, the appropriate ratios of leaves to water are high for freezing and salting methods, this is because after salting and thawing the frozen leaves, the volume occupied by the leaves decreased significantly. A high ratio of leaves to water is associated with enhanced processing capacity. However, these methods are of some obvious limitations, such as strong corrosion of salt to stainless steel container and environmentally unfriendly for salting method, high storage expense for freezing method. These limitations have restricted the application of the two techniques to large-scale industrial preparation.
| Sample | Sample preparation procedure | Appropriate ratio of leaves/water (w/v) | Yield of perillaldehydef (mg/g) |
|---|---|---|---|
| 3a1 | Fresh leaves vp | 1:10 | 0.193 ± 0.006* |
| 3a2 | Fresh leaves ap | 1:10 | 0.859 ± 0.029 |
| 3b1 | Sun-dried leaves ap | 1:20 | 0.495 ± 0.012* |
| 3b2 | Air-dried leaves in shade ap | 1:20 | 0.832 ± 0.025 |
| 3c | Frozen leaves ap | 1:2 | 0.906 ± 0.018 |
| 3d | Salted leaves ap | 1:1 | 0.899 ± 0.032 |
f based on fresh leaves.
vp hydrodistillation under vacuum pressure.
ap hydrodistillation under ambient pressure.
* statistically significant at level p < 0.05.
Preliminary choice of the resins Nine macroporous resins with different physical properties were tested through static adsorption, and the adsorption capacity (qe) and desorption ratio (D) were used to evaluate their adsorbent efficiency. Table 3 shows their adsorption properties from Sample 1, Sample 2 and Sample 3. Generally speaking, the adsorption capacity of adsorbent from aqueous solution is dominated by many factors, such as specific surface area, micropore structure and adsorbent polarity (Zeng et al., 2009). With respect to the adsorption of Sample 1 in Table 3, the hydrophobicity of the surface and its hydrophobic interactions with the adsorbate lead to good interaction with apolar perillaldehyde and increase its adsorption capacity. The surface of XAD-7HP resin contains semipolar groups, SK1B, FPC3500 and D001CC are ion-exchangers, their polarity unmatching between the resins and perillaldehyde may account for the decrease in the adsorption capacities. To other apolar resins, the amount of perillaldehyde adsorbed seems to be proportional to the adsorbent surface area. Similar results have been reported by other authors (Amin et al., 2001; Deosarkar and Pangarka, 2004). HPD-100, XAD-16, LS-305A, LS-305 and DM-21 have both relatively higher adsorption capacities and higher desorption ratio, with no significant differences, as compared with other semipolar and ion-exchange resins, which might indicate the weak interaction between solute and the adsorbent material may well account for their easy desorption characteristics. The HPLC chromatograms of the Sample 1 before adsorption and after desorption with LS-305 resin were shown in Fig. 1 (a, b). Through contrast of chromatograms, it could be seen that a large amount of hydrophobic impurities in Sample 1 were adsorbed and eluted simultaneously with perillaldehyde. Similar results were found by other resins used in this study (Figures not shown). Thus, the puritiy of perillaldehyde in the products obtained from them were quite low. Sample 2 was obtained by hydrodistillation from Sample 1. The HPLC chromatogram (Fig. 1c) of Sample 2 showed that most impurities in the Sample 1 were removed by hydrodistillation. Sample 3 was obtained by hydrodistillation from leaves, exhibiting the similar HPLC chromatogram to Sample 2 (Figure not shown). However, the adsorption capacities of the examined resins from Sample 3 increased to 4 – 10 folds compared to that from Sample 2. This is because about 30% ethanol was present in the aqueous phase of Sample 2, which is usually used as desorption solvent and unfavorable for the adsorption of perillaldehyde onto resins. Accordingly, Sample 3 may be choice as the most preferable sample comparing with Sample 1 and Sample 2.
| Resin | Sample 1a | Sample 2b | Sample 3c | |||
|---|---|---|---|---|---|---|
| qe (mg/g) | D (%) | qe (mg/g) | D (%) | qe (mg/g) | D (%) | |
| DM21 | 3.42 ± 0.11 | 67.8 ± 2.2 | 27.84 ± 0.76 | 95.8 ± 2.1 | 129.35 ± 3.71 | 94.3 ± 2.8 |
| LS-305 | 3.04 ± 0.08 | 72.7 ± 2.0 | 26.42 ± 0.86 | 96.0 ± 2.6 | 128.47 ± 3.74 | 99.5 ± 2.6 |
| LS-305A | 2.86 ± 0.06 | 72.8 ± 1.9 | 22.40 ± 0.64 | 98.9 ± 3.5 | 125.42 ± 3.53 | 98.2 ± 2.9 |
| XAD-16 | 2.36 ± 0.04 | 76.3 ± 2.5 | 23.30 ± 0.36 | 97.8 ± 2.5 | 122.36 ± 3.07 | 99.1 ± 3.2 |
| HPD-100 | 2.16 ± 0.04 | 77.5 ± 2.2 | 22.86 ± 0.46 | 97.2 ± 2.4 | 110.23 ± 3.85 | 98.8 ± 2.6 |
| XAD-7HP | 1.56 ± 0.04 | 78.2 ± 2.3 | 13.86 ± 0.30 | 90.6 ± 3.3 | 84.71 ± 2.24 | 91.2 ± 2.3 |
| D001CC | 0.78 ± 0.02 | 34.5 ± 0.7 | 7.46 ± 0.22 | 31.0 ± 0.9 | 70.79 ± 2.15 | 37.5 ± 1.1 |
| FPC3500 | 0.73 ± 0.02 | 27.9 ± 0.5 | 6.13 ± 0.20 | 27.7 ± 0.3 | 64.26 ± 1.83 | 27.2 ± 0.9 |
| SK1B | 0.65 ± 0.02 | 25.3 ± 0.6 | 5.65 ± 0.16 | 24.8 ± 0.9 | 62.78 ± 1.71 | 28.8 ± 0.8 |
a Sample 1: crude perillaldehyde ethanol extract.
b Sample 2: hydrodisllation condense from Sample 1 containing about 30% ethanol.
c Sample 3: hydrodisllation condense from Perilla frutescens leaves.

HPLC chromatograms of (a) Sample 1, crude perillaldehyde ethanol extract, (b) desorptive solution from LS-305 adsorbed Sample 1, (c) Sample 2, split out of Sample 1.
Considering the small adsorption capacities and difficult desorption characteristics of SK1B, FPC3500, D001CC and XAD-7HP, these four resins were inadaptable for adsorbents of perillaldehyde. Therefore, DM-21, LS-305, LS-305A, XAD-16 and HPD-100 were applied for the following study.
Static adsorption isotherms Considering the high volatility and thermal instability of perillaldehyde (Huang et al., 2011; Povilaitytoee and Venskutonis, 2000), adsorption isotherms of perillaldehyde onto DM-21, LS-305, LS-305A, XAD-16 and HPD-100 resins were performed at room temperature (25°C). Experimental equilibrium data were fitted to the Langmuir and Freundlich isotherm models to compare adsorption of resins (Mauro et al., 2002). These isotherms are represented mathematically as:
Langmuir equation:
 |
Freundlich equation:
 |
where qe (mg/g) is the mass of perillaldehyde adsorbed per mass of adsorbent, Ce (mg/L) is the concentration of perillaldehyde at equilibrium, qmax (mg/g) and KL (L/mg) are the Langmuir constants related to the maximum capacity and energy of adsorption, respectively, KF (L1/n mg1-1/n g−1) and 1/n are the Freundlich constants related to the adsorption capacity and intensity, respectively.
Fig. 2 shows the adsorption isotherms of perillaldehyde on the five resins. The isotherm constants and correlation coefficients were calculated and listed in Table 4. By comparing the correlation coefficients r2, it can be deduced that the experimental equilibrium adsorption data are well described by the Freundlich equation compared to Langmuir model in the concentration ranges studied. This suggests the multilayer adsorption of perillaldehyde on the surface of the resins.
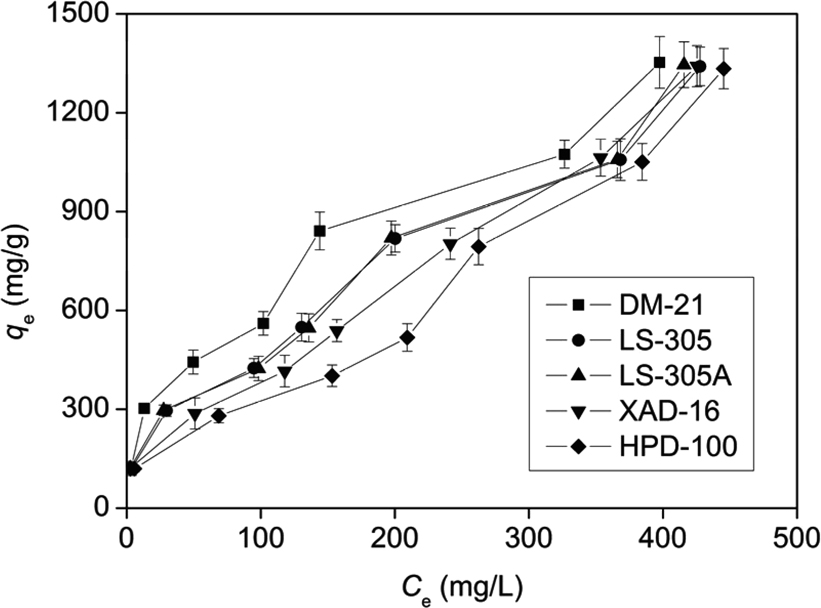
Equilibrium adsorption isotherms for perillaldehyde on five resins ((▪) DM-21, (•) LS-305, (▴) LS-305A, (▾) XAD-16 and (♦) HPD-100) shaking at 100 rpm at T = 25°C.
| Resins | Langmuir equation | Freundlich equation | ||||
|---|---|---|---|---|---|---|
| KL (L/mg) | qmax (mg/g) | r2 | KF (L1/n mg1-1/n g−1) | 1/n | r2 | |
| DM-21 | 5.33 × 10−2 | 813.93 | 0.9756 | 75.02 | 0.469 | 0.9796 |
| LS-305 | 5.61 × 10−2 | 687.52 | 0.9378 | 56.99 | 0.491 | 0.9717 |
| LS-305A | 8.63 × 10−2 | 651.04 | 0.9245 | 68.96 | 0.454 | 0.9566 |
| XAD-16 | 9.13 × 10−2 | 611.17 | 0.8976 | 62.89 | 0.455 | 0.9180 |
| HPD-100 | 3.96 × 10−2 | 633.87 | 0.9047 | 38.55 | 0.528 | 0.9060 |
In general, in the Freundlich Eq. (5), the adsorption can easily carry out when the 1/n value is between 0.1 and 0.5, but not, if 1/n value is between 0.5 and 1. Above 1, it is nearly impossible (Tan et al., 2008). According to the results in Table 4, the 1/n values were all between 0.1 and 0.5 except for HPD-100, indicating that the adsorption of perillaldehyde on the DM-21, LS-305, LS-305A, XAD-16 resins can be considered favorable and can take place easily.
The maximum adsorption capacities, qmax (obtained from Langmuir plots) are in the following orders: DM-21 > LS-305 > LS-305A > XAD-16 > HPD-100. Among them, DM-21 has the largest adsorption capacity in comparison with other four resins, this suggests that DM-21 was more favorable to the recovery of perillaldehyde from aqueous solutions.
Static desorption Ethanol is the preferable desorbent for macroporous resin because it can be easily removed from the solution and recycled and has low cost and no toxicity to the samples (Ma et al., 2009; Fan and Xu, 2008; Geng et al., 2009; Lin et al., 2012). The desorption ratios of perillaldehyde adsorbed on DM-21, LS-305, LS-305A, XAD-16 and HPD-100 in different concentrations of ethanol solution are presented in Fig. 3. At a range of ethanol concentration from 50% to 95%, the desorption ratios increased with the increase of ethanol concentration. When the ethanol concentration surpassed 95%, the desorption ratios did not differ significantly. From our preliminary test, we found that in the followed concentration stage, perillaldehyde-containing ethanol may be partially miscible with the aqueous phase forming an emulsion at the later concentration stage, which is difficult to separate from each other, thus resulting in the loss of perillaldehyde and a difficultly in determining perillaldehyde concentrations in samples. Therefore, absolute ethanol was selected as the desorption solution.

Effect of ethanol concentrations on desorption ratio of the five resins ((▪) DM-21, (•) LS-305, (▴) LS-305A, (▾) XAD-16 and (♦) HPD-100) shaking at 100 rpm at T = 25°C.
Static adsorption and desorption kinetics In order to investigate the mechanism of adsorption and rate controlling steps, the kinetic data were modeled using first-order (Lagergren 1898), second-order (Ho and McKay, 1999) and intraparticle diffusion kinetic equations (Guibal et al., 1998; El-Ghaffar et al., 2009).
Pseudo-first order model:
 |
Pseudo-second order model :
 |
Particle diffusion kinetics model:
 |
where qe (mg/g) and qt (mg/g) are the amounts of perillaldehyde adsorbed onto adsorbents at equilibrium and at contact time t (min), respectively, k1 (min−1) is the pseudo-first order rate constant, k2 (g/mg min) is the pseudo-second order rate constant, kp (mg/g min1/2) is the intraparticle diffusion rate constant, C (mg/g), the constant, represents boundary layer thickness.
Fig. 4 presents the plot of perillaldehyde adsorbed (qt) versus contact time (t) for DM-21, LS-305, LS-305A, XAD-16 and HPD-100 at 25°C. After 30 min for the five resins, no significant changes occur, indicating that the rates of adsorption of perillaldehyde achieved equilibrium.

Kinetic curves of the adsorption of perillaldehyde on five resins ((▪) DM-21, (•) LS-305, (▴) LS-305A, (▾) XAD-16 and (♦) HPD-100) from 1503.0 mg/L aqueous solution shaking at 100 rpm at 25°C.
The kinetic parameters for the pseudo-first and pseudo-second models are determined from the linear plots of log (qe − qt) vs t or (t/qt) vs t, respectively. The validity of each model could be checked by the fitness of the straight lines (r2 values). As listed in Table 5, all the correlation coefficients for the pseudo first order, pseudo-second order and intraparticle diffusion kinetic models are high. In addition, the calculated qe values of the three kinetics also agree with the experimental data. Best fit was observed by pseudo-second order kinetics model for the adsorption of perillaldehyde on these five resins. Similar phenomena have been observed in the adsorption of phenol, phenolics and rosmarinic acid on polymeric adsorbents (Zeng et al., 2009; Lin et al., 2012). According to the pseudo-second order rate constant, k2, the adsorption rate of the five reins is ordered as follows: HPD-100 > LS-305A > DM-21 = LS-305 > XAD-16.
| Resin | qe (exptl) (mg/g) | kp (mg/g min1/2) | r2 | Pseudo-first order model | Pseudo-second order model | ||||
|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe (calcd) (mg/g) | r2 | k2 (g/mg min) | qe (calcd) (mg/g) | r2 | ||||
| DM-21 | 757.81 ± 33.52 | 33.37 | 0.9196 | 1.890 | 714.71 | 0.9657 | 2.37 × 10−9 | 777.12 | 0.9997 |
| LS-305 | 748.65 ± 31.56 | 33.02 | 0.9078 | 1.964 | 706.56 | 0.9613 | 2.37 × 10−9 | 770.42 | 0.9994 |
| LS-305A | 739.05 ± 28.36 | 31.83 | 0.9256 | 1.851 | 688.54 | 0.9723 | 2.71 × 10−9 | 755.74 | 0.9985 |
| XAD-16 | 725.58 ± 30.25 | 25.98 | 0.9037 | 2.426 | 690.15 | 0.9697 | 2.03 × 10−9 | 743.44 | 0.9994 |
| HPD-100 | 714.52 ± 28.34 | 29.22 | 0.9087 | 2.590 | 651.14 | 0.9603 | 4.12 × 10−9 | 734.38 | 0.9969 |
The pseudo-first order and pseudo-second order models basically consider external film diffusion, intraparticle diffusion and interaction step for adsorption process. Although the particle diffusion kinetics models cannot represent the whole adsorption processes, they can be applied to give a definite mechanism of adsorption. The straight line obtained in Fig. 5 indicates that the rate of adsorption is controlled by intraparticle diffusion process (Guibal et al., 1998; McKay and Poots, 1980). The positive value C is far from the origin of coordinate for the five resins, which is proportional to the boundary layer thickness (Guibal et al., 1998), indicates that the intraparticle diffusion is not the only rate-controlled step and the external film diffusion controls the adsorption to a large degree (Lin et al., 2012; El-Ghaffar et al., 2009).

Intraparitcle diffusion model for perillaldehyde adsorption on five resins ((▪) DM-21, (•) LS-305, (▴) LS-305A, (▾) XAD-16 and (♦) HPD-100) shaking at 100 rpm at T = 25°C.
Desorption kinetics of perillaldehyde on DM-21, LS-305, LS-305A, XAD-16 and HPD-100 resins were investigated at 25°C and shown in Fig. 6. Compared with Fig. 4, the adsorbate was completely desorbed after 10 min, which suggested that the process of desorption was faster than adsorption rate.

Kinetic curves of the desorption of perillaldehyde from five resins ((▪) DM-21, (•) LS-305, (▴) LS-305A, (▾) XAD-16 and (♦) HPD-100) shaking at 100 rpm at 25°C.
In our results, the adsorption and desorption kinetics for the five resins showed similar tendency. Among the five resins HPD-100 has the lowest adsorption capacity and XAD-16 entails the highest material cost. In the comprehensive consideration of the adsorption capacity and cost, DM-21, LS-305 and LS-305A resins were fit to enrich perillaldehyde and were used in the following tests.
Dynamic adsorption and desorption Fig. 7 shows the experimental breakthrough curves. As expected the results indicated that for higher flow rate the breakthrough time and the saturation time are achieved faster. The adsorption parameters are listed in Table 6. As can been seen from Table 6, DM-21 has the highest breakthrough capacity among the three resins. The breakthrough adsorption capacities and efficiency of resin utilization differed significantly (p < 0.05) at the flow rates of 3 and 5 BV/h, therefore, 3 BV/h was used as the proper adsorption flow rate. LS-305A shows the lowest breakthrough adsorption capacity and efficiency of resin utilization, it was not considered in dynamic desorption investigation.

Dynamic adsorption breakthrough curves of perillaldehyde on three resins ((▪) DM-21, (•) LS-305, (▴) LS-305A) at different flow rates. (T = 25°C, C0 = 873.6 mg/L). Closed, open and crossed symbols for flow rate of 1, 3 and 5 BV/h respectively.
| Resin | Flow rate (BV/h) | qba (mg/mL wet resin) | qsb (mg/mL wet resin) | Rc (%) |
|---|---|---|---|---|
| DM-21 | 1 | 132.25 ± 4.76 | 140.60 ± 3.28 | 94.6 |
| LS-305 | 1 | 126.28 ± 4.14 | 135.78 ± 4.19 | 93.0 |
| LS-305A | 1 | 122.65 ± 3.27 | 132.48 ± 3.23 | 92.6 |
| DM-21 | 3 | 117.4 ± 3.17 | 130.07 ± 2.87 | 90.3 |
| LS-305 | 3 | 106.40 ± 2.19 | 121.08 ± 3.01 | 87.9 |
| LS-305A | 3 | 95.40 ± 2.13 | 114.27 ± 2.58 | 83.5 |
| DM-21 | 5 | 66.57 ± 2.33 | 99.85 ± 3.56 | 66.7 |
| LS-305 | 5 | 60.54 ± 1.49 | 94.22 ± 2.87 | 64.3 |
| LS-305A | 5 | 49.27 ± 2.47 | 85.96 ± 2.69 | 57.3 |
a qb: breakthrough adsorption capacity, mg/mL wet resin.
b qs: saturation adsorption capacity, mg/mL wet resin.
c R: the efficiency of resin utilization = qb/qs × 100%.
Fig. 8 shows the elution curves of perillaldehyde adsorbed on DM-21 and LS-305 resins. Narrow and relatively symmetric peaks were observed for the two resins. The slightly broad peak for DM-21 may be due to the more difficult desorption of perillaldehyde from DM-21. At the flow rate of 1 BV/h, perillaldehyde was totally desorbed from the two resins with a solvent volume of about 3.0 BV. By comparison, at the flow rates of 2 and 3 BV/h, perillaldehyde was totally desorbed from the two resins with a solvent volume of about 4.5 and 6.0 BV, respectively. These results indicate that the lower desorption flow rate produced the most concentrated product among the flow rates tested. Therefore, this flow rate of 1 BV/h is better for desorption in terms of lower solvent use and high efficiency.

Elution of perillaldehyde adsorbed on two resins ((▪) DM-21, (•) LS-305) with absolute ethanol at different flow rates. (T = 25°C). Closed, open and crossed symbols for flow rate of 1, 2 and 3 BV/h respectively.
The dynamic adsorption and desorption tests were repeated for three times to test its reusability (Table 6 and Table 7). In this case, adsorption test was terminated at breakpoint. As shown in Table 6, LS-305 can be completely regenerated for repeated use without any significant capacity loss, which is significant for practical application.
| Items | Type | Run 1 | Run 2 | Run 3 |
|---|---|---|---|---|
| Breakthrough capacity (mg/mL wet resin) | DM-21 | 115.61 ± 6.96 | 108.43 ± 5.85 | 104.63 ± 4.33 |
| LS-305 | 111.78 ± 5.74 | 109.27 ± 6.54 | 108.96 ± 5.46 | |
| Desorption ratio (%) | DM-21 | 92.34 ± 2.61 | 88.73 ± 3.43 | 85.16 ± 3.16 |
| LS-305 | 95.51 ± 2.48 | 97.89 ± 3.27 | 98.15 ± 4.27 |
In this study, a combination of hydrodistillation and adsorption by macroporous resins system for recovery of perillaldehyde from hydrodistillation condense of Perilla frutescens (L.) Britton var. crispa f. viridis leaves was developed. Hydrodistillation is an efficient method to increase the purity of perillaldehyde by one step. By comparing the material state to be processed, including fresh leaves, air-dried leaves in shade, sun-dried leaves, frozen leaves and salted leaves, air-dried in shade was the optimal choice for prolonging the pre-processing storage with the advantage of low cost and simplicity.
The separation characteristics of nine kinds of macroporous resins were systematically investigated by means of the static adsorption/desorption experiments. DM-21, LS-305, LS-305A, XAD-16 and HPD-100 exhibited higher adsorption capacity of perillaldehyde than other four adsorbents, which resulted from larger specific surface area and apolar charicteristics. Based on the static experimental results, it was found that the experimental data fitted best to the pseudo second-order kinetics model and Freundlich isotherm model coupled better correlation coefficients. Absolute ethanol was determined as the appropriate desorption solution with the advantage of avoiding forming an emulsion of water and perillaldehyde thus decreasing the loss of perillaldehyde at the concentration stage.
By comparison of the further dynamic adsorption/desorption experiments using a column packed with DM-21, LS-305 and LS-305A resins, LS-305 was selected as a suitable resin for perillaldehyde recovery, owing to its good efficiency for repeated use. This method could be utilized in large-scale production of perillaldehyde recovery from hydrodistillation condense of Perilla frutescens (L.) Britton var. crispa f. viridis leaves in industry, due to the prominent advantages of macroporous resin method such as the procedural simplicity, lower material cost, less labor intensiveness, higher purification efficiency and easier scale-up.
Acknowledgements We gratefully acknowledge the generous support provided by Shandong Province Nature Science Fund (ZR2010BL027, Y2005B06), and Yantai Science and Technology research project fund (2011072).