2014 Volume 20 Issue 2 Pages 207-216
2014 Volume 20 Issue 2 Pages 207-216
Lipase-catalyzed geraniol synthesis of geranyl acetate via transesterification with vinyl acetate in organic solvents was investigated. The lipase from Pseudomonas fluorescens was identified as the optimal enzyme and vinyl acetate was employed as both acyl donor and solvent through transesterification. The effects of various parameters on transesterification, including enzyme loading, temperature, and agitation speed, were optimized. The optimal reaction temperature was 30°C. The external diffusion limitation could be greatly reduced by increasing the agitation speed to 240 rpm, and the internal diffusion could be ignored. Lipase operational stability research revealed that lipase activity has no obvious loss after nine batch cycles. A kinetic model based on the ping-pong bi-bi mechanism without inhibition by geraniol and geranyl acetate using the King-Altman method was proposed. Matlab was applied to simulate the model parameters. The experimental values could be satisfactorily fitted to the simulated values with a relative error of 1.88%.
Geraniol is a commercially important terpene alcohol found in the essential oils of several aromatic plants. One of the most important components in the flavor and fragrance industries, this alcohol is a common ingredient in consumer products produced by these industries. In addition to its pleasant odor, geraniol is known to exhibit insecticidal and repellent properties, and it is used as a natural pest-control agent with low toxicity. Geraniol has been suggested to represent a new class of chemoprevention agents for cancer (Chen and Viljoen, 2010). However, geraniol possesses a certain toxicity, which limits its use in the food industry. Geranyl esters are often used as alternative to edible spices, and research on geranyl esters has an important practical significance (Tang et al., 2012). Geranyl acetate, the most common and most valuable geranyl ester, has a sweet lemon fruity flavor and the fragrance of rose and lavender, which is an important flavor component. The Council of Europe classifies it as an artificial edible flavoring that can be used in food without endangering human health (Liu, 2009). Geranyl acetate naturally exists in essential oils, including Marca eucalyptus oil, citronella oil, geranium oil, sassafras oil, palmarosa oil, petitgrain oil, lemongrass oil, lemon oil, and lavender oil. Geranyl acetate is also found in plants such as lemon, tomato, almond, coffee, nutmeg, and ginger. Geranyl acetate is mainly used to prepare fruit-flavored essences with aromas of lemon, lavender, rose, convallaria majalis, osmanthus fragrans, and flores aurantii. It can also be prepared as edible essences that have the fruit flavor of apricot, peach, banana, apple, grape, and lemon (Chen, 2008). Thus, geranyl acetate has been widely applied in the food, perfume, detergent, cosmetics, soap, brewing, and pharmaceutical industries as an important flavor and fragrance component (Chen et al., 2002).
Geranyl acetate can be isolated by vacuum fractionation from natural essential oils. However, this method is not suitable for large-scale industrial production of geranyl esters because of the limited availability of natural raw materials, the separation and purification of the product, the low conversion, and the high cost of vacuum fractionation (Yee et al., 1995). Geranyl acetate production by traditional chemical synthesis is often performed using acetic anhydride and geraniol in the presence of acetic acid, or it can also be synthesized via direct acetic acid esterification and geraniol catalysis by concentrated sulfuric acid (Zhang, 1991). However, traditional chemical synthesis has several disadvantages. First, concentrated sulfuric acid, which acts as the chemical catalyst, can simultaneously u-ndergo esterification, dehydration, and oxidation. This possibility will cause a series of side reactions, resulting in a number of difficulties in terms of refining the final product and recovering the raw material, especially for food spices, which may conform to a certain hygiene index. Second, spice esters produced by chemical synthesis are not natural and do not meet the requirements for natural flavors. The benefit is significantly low. Considering the aforementioned production issues, we aim to seek a novel approach for producing natural perfumery compounds. One such method is lipase-catalyzed esterification or transesterification of terpene alcohol to prepare terpene esters, the aromatic components of which can be released slowly. Based on the legal rules of the United States and the European Union, aromatic esters produced from biological transformation are regarded as a natural product, and the added value of the products is significantly greater than that produced from chemical synthesis. Thus, the synthesis of spices using the biological method has potential application value (Abbas and Comeau, 2003). Geranyl acetate prepared through lipase-catalyzed transesterification or esterification is considered as a “natural” product, thereby attracting increasing interest.
The lipase-catalyzed synthesis of geranyl acetate in non-aqueous systems has been reported. Several researchers prepared geranyl acetate via direct esterification of geraniol and acetic acid catalyzed by lipase in non-aqueous system, including ionic liquid, supercritical carbon dioxide, supercritical ethane, organic solvent, and solvent-free system (Barahona et al., 2006; Couto et al.; 2011, Peres et al.; 2003, Verma and Kanwar, 2008). However, acetic acid has an inhibitory effect on most lipases; it lowers the microenvironment and interference pH with their aqueous layer. Therefore, esterification by geraniol and acetic acid is not the optimum reaction for the synthesis of geranyl acetate. Yee and Akoh (1996) studied lipase from Pseudomonas sp. (PS) as a catalyst for the synthesis of geranyl acetate via esterification of geraniol and acetic anhydride in hexane. However, the strong acidity and stimulation of acetic anhydride make it unsuitable for lipase-catalyzed reaction. Moreover, acetic acid is a byproduct of the reaction. Chulalaksananukul et al. (1992, 1993) investigated the lipase produced from the Mucor miehei lipase that catalyzed the geraniol transesterification synthesis of geranyl acetate with n-propyl acetate in n-hexane and supercritical carbon dioxide, respectively. The transesterification was a reversible reaction that cannot reach full completion when n-propyl acetate was used as the acyl donor. The reaction conversion was only 85% after 3 days. Wang et al. (2006) investigated Novozym 435-catalyzed geraniol transesterification with vinyl acetate synthesis of geranyl acetate in ionic liquid, and the conversion was 95% in 4 h. The ionic liquid is significantly expensive, and the high cost is not suitable for the industrial production of the product. Gupta et al. (2013) reported the transesterification of geraniol and vinyl acetate catalyzed by lipase from Thermomyces lanuginosus immobilized by physical adsorption. The yield can reach 90%, but the paper did not study the kinetics of the reaction. Mahapatra et al. (2009) conducted a research on the synthesis of geranyl acetate via geraniol transesterification with vinyl acetate as the acyl donor catalyzed by Rhizopus oligosporus NRRL 5905 lipase in a solvent-free system. However, the maximum molar conversion obtained only 67%. The transesterification reaction kinetic research revealed a pseudo first-order reaction, which was described by the Michaelis-Menten kinetics of single substrate. Thus, it was not deduced from the reaction mechanism.
The lipase with the highest activity was screened in the current study to catalyze the transesterification of geranyl acetate in organic solvents (Scheme 1). The effects of various factors were studied to establish a more efficient biosynthesis system for geranyl acetate. Moreover, the transesterification mechanism was studied, and the kinetic equation was deduced to provide the necessary basis for its application in industrial production.

Lipase catalyzed transesterification of geraniol with vinyl acetate
Chemicals Lipases that were derived from Pseudomonas fluorescens, Aspergillus niger, Candida rugosa, Rhizopus oryzae, Mucor javanicus, and Burkholderia cepacia were used in the experiments. All lipases were purchased from Amano Enzyme Ltd. The geraniol raw materials and geranyl acetate standards were purchased from Sigma-Aldrich Trading Co, Ltd. Other commercially available chemicals and solvents were of analytical grade. All chemicals and enzymes were used without further modification.
Experimental procedure The synthesis was performed in a plugged bottle, which was then placed in a constant-temperature shaker to maintain the temperature and to achieve effective mass transfer. In the absence of special instructions, the reaction was conducted as follows: a solution of 300 mmol L™1 geraniol was mixed with 3 mL of vinyl acetate, which functioned as both reactant and solvent. The reaction liquid was placed in a constant-temperature shaker and preheated for 10 min. A solution of 12 mg mL−1 lipase was then added to the mixture to initiate the reaction, which was subsequently incubated for 3 h. The following parameters of the shaker incubator were set: the reaction temperature was 30°C, and the agitation speed was 240 rpm. Clear liquid samples that were free from catalyst particles were periodically withdrawn from the reaction mixture and analyzed through GC. The initial reaction rate was defined as the amount of geranyl acetate generated per unit time and per unit volume.
Analytical method The analyses of the liquid samples were performed using a GC apparatus equipped with a flame ionization detector and an SGE AC10 stainless steel column. The area of internal standard method was adopted, and hexadecane was used as internal standard to determine geranyl acetate concentration. The following specific test conditions were used: the N2 flow rate was 30 mL min−1, the H2 flow rate was 40 mL min−1, the air flow rate was 400 mL min−1, the split ratio was 10:1, the column temperature was kept at 150°C, the injector temperature was 220°C, and the detector temperature was 250°C. Typical retention times of geraniol and geranyl acetate are 4.734min and 6.427min, respectively.
Lipase selection from different microorganisms Lipases from various microbial origins exhibit different catalytic activities and selectivities. Six kinds of free lipases from different sources, including P. fluorescens, A. niger, C. rugosa, R. oryzae, M. javanicus, and B. cepacia, were tested to catalyze the geraniol transesterification synthesis of geranyl acetate with vinyl acetate. Table 1 shows the catalytic activity of lipase and geranyl acetate yield in 3 h. The lipase from P. fluorescens exhibited the most suitable catalytic activity under the same reaction conditions, with conversion as high as 96% in 3 h. C. rugosa and B. cepacia lipases achieved only 23.3% and 40.1% conversion, respectively. Lipases from A. niger, R. oryzae, and M. javanicus almost had no catalytic effect on the reaction. As Berger, B. and Franken, B. reported, the byproduct released during the reaction, namely, acetaldehyde, may inactivate several lipases (Berger and Faber, 1991, Franken et al., 2011). For example, C. cylindracea lipase forms a Schiff base with lysine residue, thus limiting its use (Franken et al., 2011). The poor activity of these enzymes could be accounted for. Consequently, the lipase from P. fluorescens was used throughout the remaining experiments.
| Number | Lipase source | Yield (%) | Activity (U g−1) |
|---|---|---|---|
| 1 | Pseudomonas fluorescens | 96.30 ± 0.40 | 121.79 ± 2.08 |
| 2 | Aspergillus niger | 2.34 ± 0.04 | 1.46 ± 0.02 |
| 3 | Candida rugosa | 40.35 ± 0.74 | 23.25 ± 0.19 |
| 4 | Rhizopus oryzae | 0.78 ± 0.02 | 0.57 ± 0.01 |
| 5 | Mucor javanicus | 6.50 ± 0.09 | 4.21 ± 0.11 |
| 6 | Burkholderia cepacia | 42.36 ± 0.82 | 40.05 ± 1.49 |
Reaction condition: geraniol, 100 mmol L−1; vinyl acetate, 3 mL; speed of agitation, 200 rpm; temperature, 35°C; catalyst loading, 10 mg mL−1; reaction time, 3h
Reaction media effects The properties of solvents are important for the catalytic activity of an enzyme in a non-aqueous medium. The solvent affects the catalytic power of the enzyme by changing the three-dimensional conformation of protein, and, therefore, significantly alters the conversion and initial rate (Yadav and Devendran, 2012). As demonstrated in other studies, logP (the partition coefficient of the solvent for the standard octanol/water two-phase system) is a widely used parameter to describe solvent hydrophobicity and its possible effects on enzyme activity. Therefore, organic solvents with different polarities, including toluene, benzene, acetone, dichloromethane, n-hexane, and vinyl acetate, were c-hosen to investigate the effect of organic solvents on the enzymatic reaction. Table 2 shows the yield of geranyl acetate catalyzed by lipase from P. fluorescens in different organic solvents. The yield of geranyl acetate generated in n-hexane (logP=3.5), an organic solvent of medium polarity, was significantly high and can reach 85.48% after 3 h. The enzyme activity is usually very high when the organic solvent logP is between 2.0 and 3.0 (Akoh and Yee, 1998, Gandhi and Mukherjee, 2000). The conversion in benzene and toluene were 50.18% and 49.16%, respectively, but the toxicity and odor of benzene and toluene limit its application in perfume synthesis. For organic solvents with logP < 2, such as dichloromethane (logP = 1.25), the reaction yield was only 22.54%. This result is attributed to the polar organic solvent, leading to the natural conformation change in lipase, thus reducing the reaction conversion (Yadav and Devendran, 2012). Polar organic solvents, due to their higher affinity to water, tend to strip essential water from enzyme molecules. The results lead to the inactivation of enzyme because of enzyme molecular dehydration or the reaction departing from the optimum water activity. For other polar solvents, considering that acetone is an aprotic polar solvent, its “attack” to protein may not be very strong. In other words, acetone does not inactivate or denaturate enzymes as in the case of other polar solvents. Therefore, geranyl acetate yield can reach 65.68% with acetone as the reaction solvent. In this experiment, the reaction effect is enhanced when vinyl acetate was used as both the reactant and solvent compared with that using n-hexane as solvent. Another advantage of using vinyl acetate is the simplification of the reaction system, which prevents the recovery problem with the mixture of various solvents. Such an advantage is beneficial to the separation and purification of the product, thereby enabling the separation and recovery of vinyl acetate by simple fractionation. Thus, the solvent-free system with vinyl acetate was used for the subsequent experiments.
| Number | organic solvent | logP | Yield (%) |
|---|---|---|---|
| 1 | toluene | 2.52 | 49.16 ± 0.84 |
| 2 | benzene | 2.03 | 50.18 ± 0.19 |
| 3 | dichloromethane | 1.25 | 22.54 ± 0.38 |
| 4 | acetone | −0.24 | 65.68 ± 0.17 |
| 5 | n-hexane | 3.50 | 85.48 ± 1.66 |
| 6 | vinyl acetate | − | 96.30 ± 0.40 |
Reaction condition: geraniol, 100 mmol L−1; organic solvent, 3 mL; speed of agitation, 200 rpm; temperature, 35°C; catalyst loading, 10 mg mL−1; reaction time, 3h
Enzyme concentration effects The decrease in the reaction yield after 240 min caused by enzyme concentration includes two aspects: the enzyme concentration is significantly low and the excess enzyme in the reaction medium may increase the mass transfer resistance. Therefore, selecting an appropriate amount of enzyme is necessary from the aspect of yield or economy. The effects of different enzyme concentrations on the transesterification reaction were studied in the range of 3 mg mL−1 to 15 mg mL−1 under the same reaction conditions. The reaction process curve of different enzyme concentrations is shown in Fig. 1. Initially, the conversion and initial rate increased with increasing enzyme concentrations from 3 mg mL−1 to 12 mg mL−1. The reaction yield exceeded 97% after 3 h when the enzyme concentration was 12 mg mL−1. However, no significant increase but a slight reduction in the final yield was observed when the catalyst loading was increased to 15 mg mL−1 after 3 h. The reason is attributed to the increase in probability of enzyme contact with the substrate when the enzyme concentration was increased to a certain range of enzyme concentration. However, at high enzyme loading, excess enzyme particles have a tendency to attract one another and form enzyme aggregates, which may reduce the accessibility of enzyme particles to reactants. The enzyme particles found on the external surface of such aggregates are easily exposed to the substrate. However, the mass transfer rate could drastically diminish the substrate availability to enzyme particles present inside the aggregates. Another interpretation is that enzyme concentration approaches saturation compared with substrate concentration. The conversion cannot be effectively improved because the effective collision between molecules are reduced, which was caused by the increased viscosity in the reaction system (Yadav and Devendran, 2012). As shown in the relationship of the initial rate and enzyme concentration (Fig. 2), the initial rate of the reaction linearly increased with increasing enzyme concentration in the reaction mixture, thereby suggesting that the reaction system was determined by kinetics. Furthermore, the internal diffusion limitation did not exist in the system. Further experiments were performed at an enzyme loading of 12 mg mL-1 after simultaneously considering the final yield, the reaction time, and the high cost of the enzyme.

Effect of enzyme loading (Reaction condition: geraniol, 300 mmol L−1; vinyl acetate, 3 mL; speed of agitation, 200 rpm; temperature, 35°C; catalyst loading, 3 – 15 mg mL−1; (■) 3 mg mL−1, (▲) 6 mg mL−1, (★) 9 mg mL−1, (◆) 12 mg mL−1, (×) 15 mg mL−1.)

Initial rate versus enzyme loading (Reaction condition: geraniol, 300 mmol L−1; vinyl acetate, 3 mL; speed of agitation, 200 rpm; temperature, 35°C; catalyst loading, 3 – 15 mg mL−1; (■) 3 mg mL−1, (▲) 6 mg mL−1, (★) 9 mg mL−1, (◆) 12 mg mL−1, (×) 15 mg mL−1.)
Temperature effects Temperature is an important parameter that affects the reaction catalyzed by lipase. Selecting an appropriate reaction temperature can not only accelerate the reaction but also prolong enzyme stability. The influence of temperature on the transesterification reaction was investigated within the range of 20°C to 40°C (Fig. 3). The results showed that the initial rate and yield did not significantly increase as the temperature rose from 20°C to 25°C. When the temperature increased to 30°C, the geranyl acetate yield obviously increased, and the reaction was completed after 3 h. This result is due to the increase in temperature that reduced activation energy and mixture viscosity, enhanced mutual solubility, and improved the diffusion process of the substrate, thus reducing mass transfer limitations and favoring interactions between enzyme particles and substrates (Xin et al., 2011). Above this temperature, the initial rate and conversion did not change significantly. Enzyme activity loss was not found in the temperature range of the experiment. The heat inactivation caused by temperature is greatly weakened in the almost dry reaction system, which is a significant advantage of the solvent-free system catalyzed by lipase. Nevertheless, extremely high temperatures not only increase the economic cost but also lead to the deactivation of the lipase, which would limit the reusability of the enzyme. Therefore, 30°C was selected as the subsequent reaction temperature for further experiments.

Effect of temperature (Reaction condition: geraniol, 300 mmol L−1; vinyl acetate, 3 mL; speed of agitation, 200 rpm; catalyst loading, 12 mg mL−1; temperature, 20 – 40°C; (■) 20°C, (▲) 25°C, (★) 30°C, (◆) 35°C, (×) 40°C.)
The Arrhenius plot was made on the basis of log of initial rates vs. reciprocal of temperature and shown in (Fig. 4). Based on the Arrhenius law, the activation energy of the system was calculated to be 7.712 kJ mol−1. The activation energy of the enzyme-catalyzed reaction was previously reported to be between 3.762 and 37.62 kJ mol−1 (Uppenberg et al., 1994). Therefore, the derived activation energy for the current system is reasonable for the given enzymatic reaction.

Arrhenius plot
Agitation speed effects Enzymes are often insoluble in most organic solvents. Thus, the interaction between the enzyme and substrate molecules is limited by external diffusion, consequently affecting the catalytic activity of enzymes in organic solvents. The effect of external mass transfer can be minimized by increasing the agitation speed. The effect of the agitation speed was studied within the range of 80 rpm to 240 rpm to select the optimum agitation speed (Fig. 5). During low agitation speed, the enzyme in the reaction flask deposited at the bottom of the bottle and was not well dispersed in the reaction system, resulting in mass transfer resistance increase and initial rate decrease. At this time, the e-xternal diffusion speed became the main factor limiting reaction. Thus, the reaction effect remained almost unchanged when the speed was increased from 80 rpm to 160 rpm. The enzyme distribution state in the reaction system significantly changed, and the enzymes were dispersed in the whole system uniformly when the agitation speed increased. Fig. 5 and Fig. 6 show that the initial rate and yield clearly increased when the agitation speed increased to 200 rpm. The reaction effect was optimum and the external diffusion effect was greatly reduced when the agitation speed reached 240 rpm. In view of the limitation of the experimental instruments, 240 rpm was determined as the follow-up experiment rotation speed.

Effect of speed of agitation (Reaction condition: geraniol, 300 mmol L−1; vinyl acetate, 3 mL; catalyst loading, 12 mg mL−1; speed of agitation, 80 – 240 rpm; temperature, 30°C; (■) 80 rpm, (▲) 120 rpm, (★) 160 rpm, (◆) 200 rpm, (×) 240 rpm.)
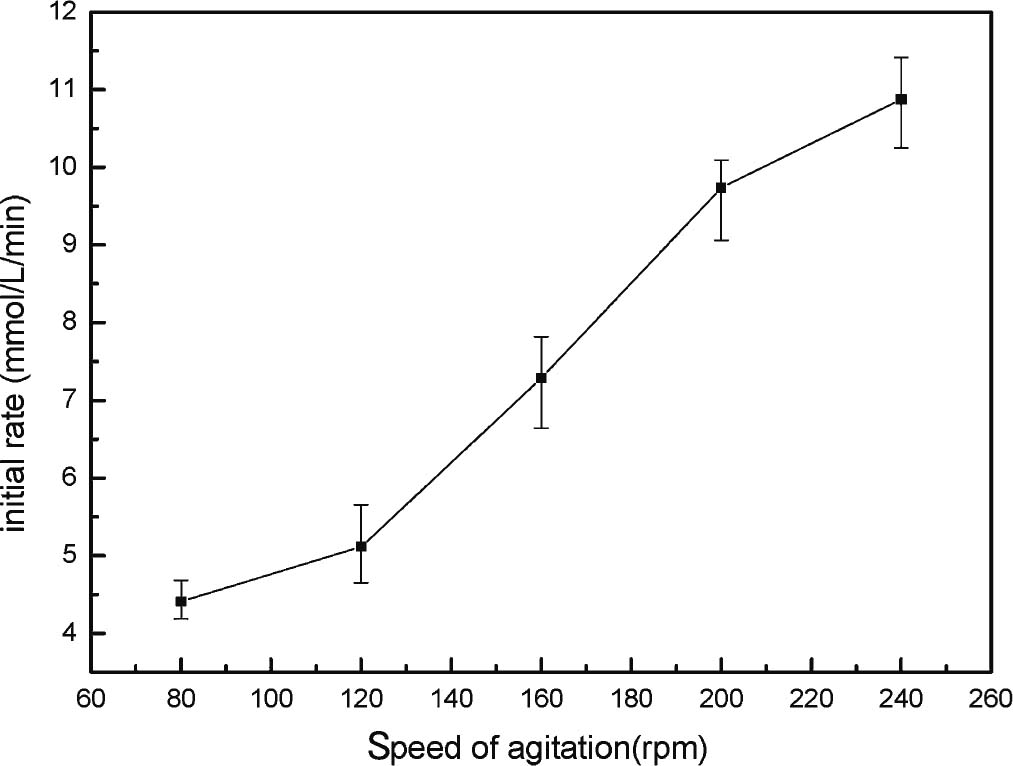
Initial rate versus speed of agitation (Reaction condition: geraniol, 300 mmol L−1; vinyl acetate, 3 mL; catalyst loading, 12 mg mL−1; temperature, 30°C.)
Effect of additional amount of water Water content in the reaction system is a key factor of the catalytic activity of the lipase in organic solvent. The organic solvent used in the reaction was not completely anhydrous but with lower moisture content. The water molecule layer combined with the enzyme molecule is essential for the catalytic activity of the enzyme. Klibanov et al. explained that a minimum amount of water was required to preserve the conformation of the enzyme in an organic reaction system (Zaks and Klibanov, 1984). The conformation of an enzyme is significantly “rigid” to preserve its activity when the water content is significantly low. An appropriate amount of water added to the reaction system can improve the polarity of the active center of the enzyme as well as its flexibility, thereby increasing the activity of the enzyme. This case illustrates that a certain amount of water is necessary for the enzyme to maintain its proper conformation required for its catalytic activity (Herbst et al., 2012). Therefore, we can adjust the enzyme catalytic activity by controlling the water content in the reaction system. The effect of adding various amounts of water to the enzyme-catalyzed reaction was studied in this experiment and the result is shown in Fig. 7. The initial rate and yield of geranyl acetate slightly decreased when the water content in the reaction system was 0.2%. The initial reaction rate significantly decreased as the water content increased to 0.5%, but the reaction yield can also reach more than 99% in 3 h. As the water content increased to 1% and 2%, the reaction effect significantly decreased. No reaction occurred when the water content was 5%, thereby confirming that the enzyme may have been inactivated completely. The results indicated that the initial rate and conversion were maximum in the reaction system without additional water. This condition may be attributed to the water content of the system being sufficient in maintaining the natural conformation of the enzyme, which is necessary for its activity. One possible reason for the observed trend is that the excess water led to the accumulation of free enzymes, which prevented the hydrophobic substrates from easily entering the active center of the enzyme. Therefore, the diffusion mass transfer resistance of the substrate and the product increased. Another reason is that the generation of water clusters within the active center of the enzyme changed its structure, which eventually led to a decrease in enzyme activity.

Effect of water concentration (Reaction condition: geraniol, 300 mmol L−1; vinyl acetate, 3 mL; catalyst loading, 12 mg mL−1; speed of agitation, 240 rpm; temperature, 30°C; (■) 0%, (▲) 0.2%, (★) 0.5%, (◆) 1%, (×) 2%, (+) 5%.)
Substrate concentration effects In organic solvents, t-he synthesis of esters catalyzed by enzymes occurs at the interface of the water phase adsorbed on the surface of the enzyme and the organic phase. Thus, the substrate concentration has a direct effect on the transesterification reaction. Substrate molecules dissolved in an organic solvent should first diffuse in the aqueous phase and then be catalyzed by enzyme molecules. When the substrate concentration is too low, the diffusion rate of the substrate molecules into the water phase is significantly low, which leads to a decline of dynamics of the transesterification reaction and yield. As the substrate concentration increases, the transesterification reaction efficiency increases, although it can also have an inhibitory effect on the enzyme (Oliveira et al., 2009). The substrate concentration process curve was investigated in the range of 100 mmol L−1 to 500 mmol L−1 (Fig. 8). Fig. 8 shows that the reaction rate was enhanced when the initial substrate concentration increased. This common phenomenon in enzymatic reactions revealed that no serious substrate inhibition occurred within the studied range of substrate concentrations. Reciprocal initial reaction rates were plotted versus the inverse geraniol concentration (Fig. 9). Based on the double reciprocal plot, the curve is determined to be almost linear, confirming the deduction that substrate inhibition is negligible.

Effect of concentration of geraniol (Reaction condition: geraniol, 100 – 500 mmol L−1; vinyl acetate, 3 mL; catalyst loading, 12 mg mL−1; speed of agitation, 240 rpm; temperature, 30°C; (■) 100 mmol L−1, (▲) 200 mmol L−1, (★) 300 mmol L−1, (◆) 400 mmol L−1, (×) 500 mmol L−1.)

Lineweaver-Burk (Reaction condition: geraniol, 100 – 500 mmol L−1; vinyl acetate, 3 mL; catalyst loading, 12 mg mL−1; speed of agitation, 240 rpm; temperature, 30°C.)
Enzyme reusability The cost of lipase is one of the limitations in producing terpene esters using the biological enzyme method. If lipase exhibits higher stability during the catalytic reaction and it can be repeatedly used numerous times, then the economic cost can be reduced to a certain extent. Therefore, reusing enzymes during the biological process has an economic significance. The stability and reusability of lipase from P. fluorescens was tested by the reaction of geraniol and vinyl acetate via transesterification, and the concentration of geranyl acetate was used to express the activity. After each cycle, the reaction mixture was removed and the lipase was rinsed, filtered, and washed three times with vinyl acetate. The solvent was evaporated before being used with fresh substrates under similar conditions. The enzyme activity retention after repeated use was assessed in terms of conversion at the end of each cycle. Fig. 10 shows the results. A marginal decrease in conversion was found, and the lipase retained almost complete activity after nine cycles, which was due to enzyme loss during filtration and drying. No loss of activity was noted, but material was lost during fiultration. Thus, the lipase is quite stable.

The reuse of lipase (Reaction condition: geraniol, 300 mmol L-1; vinyl acetate, 3 mL; catalyst loading, 12 mg mL-1; speed of agitation, 240 rpm; temperature, 30°C.)
Kinetic model based on initial rate measurements Many reactions catalyzed by lipase in solvent-free systems such as hydrolysis, esterification, and transesterification occur with two substrates and two products. Such reactions can be modeled by using the ping-pong bi-bi mechanism (Ganapati et al., 2004; Wang et al.; 2012, Xin et al., 2011). During the course of the experiment, the effect of product concentration on the transesterification reaction was investigated in the range of 0 mmol L−1 to 250 mmol L−1 under the same conditions. Fig. 11 exhibits that the initial rate had no obvious change with the increase in product concentration, which indicated that product inhibition is negligible. Therefore, the kinetics of lipase-catalyzed transesterification synthesis of geranyl acetate binds to the free enzyme (E) and forms a non-covalent enzyme-ester complex (EA), which produces the acyl-enzyme intermediate (FP) upon isomerization. The modified enzyme (F) then binds to the released first product and enol (P). The second substrate, geraniol (B), reacts with the activated enzyme (F) to produce another complex (FB), which produces the ester-enzyme complex (EQ) upon isomerization. EQ finally disassociates into the second product geranyl acetate (Q) and the free enzyme (E).

Initial rate versus concentration of geranyl acetate (Reaction condition: geraniol, 300 mmol L−1; vinyl acetate, 3 mL; geranyl acetate, 0 – 250 mmol L−1; catalyst loading, 12 mg mL−1; speed of agitation, 240 rpm; temperature, 30°C.)

King-Altman scheme of enzyme-catalyzed bi-bi transesterification (A, vinyl acetate; E, enzyme; (EA = FP), non-covalent complex; F, acyl-enzyme intermediate; P, enol; B, geraniol; (EQ = BF), another complex; Q, geranyl acetate)
The reaction rate equation is postulated using the King-Altman method. The total enzyme in the system is as follows:
 |
because,
 |
 |
The reaction rate can be expressed as follows:
 |
Therefore,
 |
The reaction was carried out in a solvent-free system. Thus, the vinyl acetate concentration was constant. The final rate equation is expressed as follows:
 |
Eq. (6) shows the rate equation for the lipase-catalyzed transesterification synthesis of geranyl acetate in the solvent-free system.
Based on the experimental data, Matlab was used to simulate the optimal model parameters. Table 3 summarizes the results. Fig. 12 shows that the experimental values could be satisfactorily fitted to the simulated values. The relative error of this model was calculated to be 1.88%, which is acceptable for the kinetic study in this system. Therefore, the model presented in this study is useful for simulating the lipase-catalyzed transesterification in solvent-free systems within a given range of experimental substrate concentrations.
| Parameter | Value |
|---|---|
| Vm (mmol L−1 min−1) | 0.1154 |
| KA (mmol L−1) | 1.886 × 102 |
| KB | 0.7842 |
| KQA | 0.1335 |
| KBQA(L mmol−1) | 1.400 × 10−3 |

Comparison of simulated values with the experimental data (The initial concentration of geraniol: experimental: (■) 100 mmol L−1, (▲) 200 mmol L−1, (★) 300 mmol L−1, (◆) 400 mmol L−1, (×) 500 mmol L−1; simulated: lines.)
The lipase-catalyzed transesterification synthesis of geranyl acetate in a solvent-free system was systematically studied in this research, including the effects of various param-eters. Among the different lipases, the lipase from P. fluorescens was found to be the most active catalyst, with vinyl acetate used as the acyl donor in a solvent-free system. Under the optimal reaction conditions, the geranyl acetate yield reached up to 99% in 3 h. The research on lipase operational stability indicated that geranyl acetate yield could reach 98.78% after being repeatedly used nine times, which suggests the high stability of lipase from P. fluorescens. Moreover, a mechanism for the transesterification synthesis of geranyl acetate was proposed based on the ping-pong bi-bi model without substrate and product inhibition. A kinetic model was established, and the corresponding optimal model parameters were simulated. The relative error of the simulated values and the experimental values was 1.88%, demonstrating the feasibility of the kinetic model.
Acknowledgments We gratefully acknowledge the Chinese National Natural Science Foundation (No. 21006051) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions for their financial support. All the authors do not have any possible conflicts of interest.