2014 Volume 20 Issue 2 Pages 247-253
2014 Volume 20 Issue 2 Pages 247-253
‘Oushuu’ is a late ripening cultivar of Pyrus pyrifolia. In the present study some of the physical and gas transport properties of this cultivar have been measured or estimated from a mathematical model written with COMSOL Multiphysics 4.2a, using the exact 3D geometry of the pear obtained with NextEngine 3D laser scanner. The experiments were done at 5, 15 and 25°C. The model was validated by conducting experiments to measure the internal concentration at different points of the fruit and comparing them with the model results. The model was shown to be successful in predicting the internal gas concentrations. Gas diffusivity of ‘Oushuu’ at 5°C was found to be 5.97 × 10−7 m2/s and its skin resistance to be 4.04 × 105 s/m. However the results at higher temperatures seem to be less accurate. As a result, a co-diffusion model was used to estimate the gas diffusivity and skin resistance of ‘Oushuu’ at higher temperatures.
The knowledge of gas transport properties is essential for calculating the internal concentration of O2 and CO2 in the fruit when the storage gas concentrations are known. These properties are important in design and optimization of cold storage, and transportation systems for agricultural products. In past years numerous studies have been conducted to obtain such properties of many fruits and vegetables. The reported skin resistance to diffusion of ethane are 6.3 × 105 s/m for ‘Gala’ apples and 11 × 105 s/m for ‘Golden delicious’ apples (Banks, 1985), 16 × 105 s/m for ‘McIntosh’ apples (Solomos, 1987), 8 × 105 s/m and 4.1 × 105 s/m for ‘Golden delicious’ (Knee, 1991), 21 × 105 s/m for ‘Braeburn’ apples and 6.8 × 105 s/m for ‘Barlett’ pears (Elgar et al., 1999). The reported values of gas diffusivity are between 0.2 × 10−8 m2/s and 21.2 × 10−8 m2/s (Mannapperuma et al., 1991; Rajapakse et al., 1989; Solomos, 1987; Zhang and Bunn, 2000). The diffusivity of ethane in ‘Jonica’ apples was reported to be in the range of 1.76 × 10−8 m2/s to 121 × 10−8 m2/s (Pham et al., 2009). These differences in gas diffusivity can be attributed to a difference in intercellular volume and cell size which can be a property of the cultivar (Schotsmans et al., 2002; Schotsmans et al., 2004). There is limited information on physical properties of Japanese pear such as its gas transport characteristics and it is therefore necessary to conduct the present study on this fruit.
Mathematical models are quantitative tools allowing us to design and optimize the food processing systems, permitting the generation of high quality products, and diminishing the time and cost of laboratory tests (Simpson et al., 2004). However, geometrical characterization of an object is required to make a model for analytical or numerical solutions while most agricultural products have irregular 3D shapes. The traditional approach in modeling studies is assuming they are homogenous, isotropic products with regular shapes (Borsa et al., 2002; Uyar and Erdoğdu, 2009). Using the exact shape of an object is a tedious and difficult task, especially for irregular shaped food products and there are not many studies published based on exact geometry of these products (Celik et al., 2011; Fabbri et al., 2011; Goñi et al., 2007; Kelkar et al., 2011; Uyar and Erdoğdu, 2009). Using the 3D laser scanners is one of the new and advanced methods of obtaining the geometry of a product with any shape.
In the present study efflux method is used to estimate the gas transport properties of Japanese pear (Pyrus pyrifolia cultivar ‘Oushuu') at 5, 15 and 25°C. The modeling is done with COMSOL Multiphysics 4.2a and the results are compared with a co-diffusion model to confirm the effect of respiration in the estimation of gas diffusivity and skin resistance.
A cultivar of Japanese pear called ‘Oushuu’ was used for the present study. ‘Oushuu’ is a late-ripening cultivar that arose from crossbreeding of cultivars with reduced occurrence of water core. The harvest time of ‘Oushuu’ is around late October up until November. The fruits of this cultivar are large and generally spindle-shaped. ‘Oushuu’ is a hybrid of Japanese and Chinese pear, registered as a Japanese pear, with a long shelf life and very low loss of nutritional compounds during storage due to low respiration (Kotobuki et al., 2004).
All fruit samples were obtained from the same batch of fruits at the same time from Fukuoka Agricultural Research Center and kept in a 2°C refrigerator to keep their respiration at the least possible level until the experiments were completed.
To study gas diffusion, the fruits were soaked in ethane as a tracer gas in a sealed jar and later the rate at which ethane diffuses out of the fruit was measured in an efflux jar. Ethane was chosen as the tracer gas because it is inert with low solubility in water which is not consumed or produced by the fruit (Pham et al., 2009). In the present study, the pear samples with initial internal ethane concentration of zero were placed in a permeation jar and ethane was injected to the jar with a concentration of 1.3 mg/L. The fruits were soaked in ethane at 5, 15 and 25°C for three days. This long period was chosen to ensure that the equilibrium conditions will be reached and that ethane will be distributed uniformly throughout the fruit.
After three days the fruits were quickly transferred to the efflux jar filled with air without any ethane. The permeation jar was opened outside the experiment room and the pear samples were quickly wiped with a tissue, transferred to the experiment room and placed in efflux jars. This is to ensure that the second jar was not contaminated by ethane from the first jar. The efflux jars were sealed and taken to a controlled temperature room with temperature equal to permeation step (5, 15 or 25°C). Changes of ethane, CO2 and O2 concentrations were monitored using gas chromatography (GL Science GC390, Japan) with a WG-100 column, FID-TCD detector and helium as the carrier gas with a flow rate of 30 mL/min. Column temperature was set at 60°C, injection temperature at 100°C, FID temperature at 150°C and TCD temperature at 80°C. Gas concentration measurements were done for 12 hours, followed by three more samplings the next morning to obtain the equilibrium concentrations. All experiments were done with three replications.
After finishing the efflux experiments, the 3D image of each individual pear was obtained using NextEngine 3D Laser Scanner (NextEngine Inc.) with standard speed settings (about 6.45 × 108 points/m2) and macro range settings with 0.0001 m accuracy. Other configurations have been explained in author's previous publication (Ebrahim Rezagah et al., 2013). Volume of the pear can be measured with ScanStudio CAD TOOLS in the scanner's accompanying software, NextEngine ScanStudio HD 1.3.2, and can be used to calculate the density of the fruit (ρb = m/v). To calculate the porosity of the pear from Eq. 1, first we need to obtain solid-liquid density of the fruit which can be calculated from measuring the weight of 100 cm3 of a pulped, homogenized and de-aerated sample. A vacuum pump was used to eliminate pores and air in the pulp (Nieto et al., 2004).
 |
ε is the porosity (dimensionless), ρb is the bulk density of the whole fruit (g/cm3) and ρs is solid-liquid density of the fruit flesh (g/cm3).
The geometry file can be saved in “.stl” format and imported in COMSOL Multiphysics 4.2a to do the gas transfer modeling (Fig. 1). The obtained geometry only consists of surface mesh. The volume mesh construction was created in COMSOL Multiphysics 4.2a code with tetrahedral solid element type. About 14000 tetrahedral elements were obtained for mesh construction of the model. The model was written in the “Transport of Diluted Species” node of COMSOL. Fick's law can be written for ethane in the fruit in the form of Eq. 2 and the boundary condition as in Eq. 3.

Geometry of ‘Oushuu’ as appeared in COMSOL
 |
 |
where c is concentration of ethane in the pear (mol/m3) and D is effective diffusivity (m2/s), n is the unit normal vector, K is the distribution coefficient ((mol/m3 in air)/(mol/m3 in fruit)), hc is the mass transfer coefficient (m/s), cext is the concentration of ethane in the jar (mol/m3) and csurf is the ethane concentration at the fruit's surface (mol/m3).
The external concentration (cext) in the efflux jar can be obtained from the material balance and by rearranging it to the following form:
 |
where V is the volume of the pear (m3), Vext is the volume of the jar minus that of the fruit (m3), cavg is the average concentration of ethane in the whole fruit (mol/m3) and cavg,o is the average concentration at the beginning of efflux period (mol/m3).
For a pear that was initially equilibrated with a mixture of ethane-air at the ethane concentration of cext,0 in permeation jar, the internal concentration of ethane will be uniformly distributed (Eq. 5). At an infinite time the whole fruit reaches equilibrium with the air in the jar so cext,eq = Kcavg:
 |
 |
Substituting cavg,0 from Eq. 5 gives Eq. 7 from which we can calculate the distribution coefficient.
 |
 |
Eq. 2, Eq. 4, Eq. 7 and Eq. 8 were used in writing the gas transfer model in Transport of Diluted Species node of COMSOL Multiphysics 4.2a based on finite element method to obtain the estimated values for D, hc and skin resistance for ‘Oushuu’ at 5, 15 and 25°C.
The resistance of fruit's surface to mass transfer, rskin (s/m) was defined as the inverse of mass transfer coefficient (hc) (Pham et al., 2009):
 |
Henry's law constant ((mol/m3 in dense phase)/(mol/m3 in gas phase)) can be determined from Eq. 10, knowing the porosity and distribution coefficient:
 |
Basic properties of ‘Oushuu’ such as weight, volume, bulk density, solid density and porosity were measured from experiments at all temperatures for all pears and the average of these measurements together with their standard deviation values are shown in Table 1. In general, the difference in internal space volume can be a property of the cultivar but it can also be a function of the growing season and the number and size of the cells (Schotsmans et al., 2002). In the present study the fruits were harvested at the same time, so the difference is limited to the natural differences between individual fruits. However, in case of porosity, the temperature has a strong effect. The porosity of the fruit increased along with the increase of temperature which can be related to the higher rate of respiration at higher temperatures, as well as dehydration of the fruit which both can affect the porosity of the fruit.
| Temperature (°C) | Weight (g) | Volume (cm3) | Bulk density (g/cm3) | Solid density (g/cm3) | Porosity (-) |
|---|---|---|---|---|---|
| 5 | 716.0 ± 72.3 | 695.3 ± 74.6 | 1.030 ± 0.010 | 1.047 ± 0.007 | 0.016 ± 0.010 |
| 15 | 710.0 ± 48.3 | 689.6 ± 48.7 | 1.030 ± 0.011 | 1.060 ± 0.005 | 0.029 ± 0.007 |
| 25 | 700.3 ± 72.3 | 686.6 ± 43.6 | 1.019 ± 0.010 | 1.059 ± 0.001 | 0.038 ± 0.010 |
The accuracy of the volume measurements has been investigated before by measuring the volume of a spherical object with a known diameter obtained from the scanner and by comparing it with the calculated volume of the sphere. The error in volume measurement was found to be 3.1% (Ebrahim Rezagah et al., 2013). The accuracy of the volume measurements with NextEngline 3D laser scanner was also validated in previous studies (Raz-Bahat et al., 2009; Uyar and Erdoğdu, 2009).
Fig. 2 shows one of the graphs of the changes in external concentration of ethane measured from the efflux jar in comparison with the model estimations. Root mean square error values of all experiments were calculated using Eq. 11 for cext values obtained from the efflux experiments  and estimated from the model
and estimated from the model  .
.

Changes of external concentration of ethane in efflux jar (experimental data and the model estimations)
 |
The values of RMSE were found to be in the range of 1.25 × 10−8 to 2.06 × 10−8 mol/L, much smaller compared to the concentrations of ethane which are in orders of 10−7 mol/L.
The mesh independency of the model was examined by solving the same model for the same geometry with different mesh numbers. Fig. 3 shows the model estimations from the same parameter values. The numbers indicate the mesh numbers. Low mesh numbers (6750) do not give accurate results but with 15700 and 38000, the concentration values are almost the same, which means we can choose the lower number of mesh among them in order to save time while the accuracy remains the same.

Effect of the number of mesh on model estimations
The model had been validated in authors' previous study using the method introduced by Kawano and Shimokawa (1994). Ethane was extracted from tissue samples that were cut from different locations in pears and injected into the gas chromatograph. From the model estimations, points of the geometry equivalent to the locations of these tissue samples were picked to determine their internal ethane concentration values. The comparison was made between these values (Ebrahim Rezagah et al., 2014).
The estimated values of diffusivity (m2/s), mass transfer coefficient (m/s) and skin resistance (s/m) obtained from the models are shown in Table 2. There are limited studies published on Japanese pear, therefore in lack of such results for cultivars of Japanese pear, the comparison was made with apple and pear cultivars reported by Schotsmans et al. (2002). The average values obtained from the present study are 4.04 × 105 s/m at 5°C, 2.94 × 105 s/m at 15°C and 1.87 × 105 s/m at 25°C, which are comparable to previous findings. Another comparison was made between present findings and gas diffusivity reported by Schotsmans et al. (2004) in different cultivars of apple. The values found in the present research are well within these limits.
| Temperature (°C) | D (×10−8 m2/s) | hc (×10−6 m/s) | rskin (×105 s/m) |
|---|---|---|---|
| 5 | 5.97 ± 0.42 | 2.48 ± 0.13 | 4.04 ± 0.22 |
| 15 | 5.77 ± 0.81 | 3.45 ± 0.46 | 2.94 ± 0.42 |
| 25 | 5.60 ± 0.14 | 5.37 ± 0.33 | 1.87 ± 0.11 |
Fig. 4 shows the distribution of ethane inside the pear during efflux experiment. The images were exported from COMSOL Multiphysics software with intervals of one hour starting from the first hour and continuing until eight hours.

Changes in distribution of internal ethane concentration during efflux experiment
At initial hours, ethane emits from the outer layers and by passing time, the gas diffuses from the inner parts to the surface to leave the fruit flesh. Fig. 3 shows that the equilibrium condition for external ethane concentration in the efflux jar happens at around seven hours which is the time the internal concentration of ethane inside the pear remains constant.
The results of experiments done at 25°C show the lowest effective diffusivity and skin resistance. Comparing the data shown in Table 1 and Table 2 shows that these values belong to the samples with the highest porosity. Unlike what might be expected, higher porosity did not lead to easier transport of gases inside the fruit because it seems the porosity at higher temperature is made of the ineffective or closed porosity that cannot actively contribute in gas transport.
Another reason can be the effect of respiration rate on transport of other gases such as ethane. Fig. 6 shows the concentration of CO2 in the efflux jar at different temperatures.

Changes of ethane diffusivity of ‘Oushuu’ in different temperatures
The rate of the production of CO2 was much higher at 25°C while at 5°C the concentration of CO2 was so low that it was undetectable during the first hours of the experiments.
To confirm this idea the diffusivity and skin resistance of ethane was calculated based on the co-diffusion model presented in Eq. 12 (Ho et al., 2006; Pham et al., 2008; Pham et al., 2009):
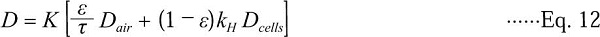 |
where Dair is diffusivity of gas in free air (m2/s), Dcells is diffusivity of gas in cells (m2/s) and τ is a reduction factor that accounts for the tortuosity of the diffusing paths, variations in their cross-section areas and connectivity (dead pores). The first term in the brackets represents pore-phase diffusion and the second term represents cell-phase diffusion. Tortuosity factor can be calculated by re-arranging Eq. 13 into the following form (Pham et al., 2009):
 |

Concentration of CO2 measured from efflux jar at 5, 15 and 25°C
Porosity and tortuosity factor are the same for all gases since they are determined by geometry, while Dair, Dcell and kH for different gases can be found from literature (Pham et al., 2009). In order to calculate Henry's law constant we need to know the concentration of ethane in gas-phase and in dense-phase (water). Ideal gas law can be used to obtain the concentration of ethane in gas phase, while the concentration of ethane in water was calculated from the graph for solubility of ethane in water at different temperatures(i).
Applying exponential regressions to these known data allows us to obtain the relationship between Henry's law constant and temperature as in Eq. 14 for ethane (R2 = 0.99).
 |
Dair is related to temperature according to Eq. 15.
 |
where Dair (m2/s) is diffusivity of gas in air at T (K) and P (Pa), D0 is gas diffusivity (m2/s) at 273.15 K and atmospheric pressure (Pa) and m is the equation parameter (-). The values of D0 is 1.22 × 10−5 m2/s for ethane and the value of m is 2.00 which is a dimensionless value. The obtained linear regression for temperature dependency of Dair is the following (R2 = 0.999):
 |
On the other hand, since the biggest part of a cell is made of water, the values of diffusivity in water can be used for Dcells in Eq. 17.
 |
where Dcell is diffusivity of gas in cells (m2/s), Dwater is diffusivity of gas in water (m2/s), F is the equation parameter (K/m2Pa) and µ is the viscosity of water (Pa.s). Viscosity of water at 0, 5, 10, 15, 20, 25 and 30°C are 1.792, 1.520, 1.307, 1.138, 1.002, 0.890 and 0.797 mPa·s, respectively (Tokyo Astronomical Observatory, 1987), while F equals 2.79 K/m2Pa for ethane (Kagaku Kougaku Kyoukai, 1958). With these values the temperature dependency of Dcell for ethane can be predicted from the following linear regression (R2 = 0.996).
 |
Fig. 6 shows the estimated gas diffusivity of ethane using the co-diffusion model together with experimental data. The gas diffusivity at 5°C is close to co-diffusion model estimation but for 15 and 25°C the gas diffusivity decreases while the model estimations increase. This can confirm the effect of respiration that comes on the way of gas diffusion measurements.
Calculation of skin resistance is more problematic than diffusivity. The co-diffusion model, Eq. 12, can be used together with the assumption that skin resistance is inversely proportional to the diffusivity in the denser layer of tissue at the surface. The problem is both εskin and τskin are not known and since εskin is much smaller in the skin region than the rest of the fruit, the second term, [(1 − εskin) kH Dcells], may not be negligible. If this term is neglected anyway, then
 |
Since εskin and τskin are common to all gases, the value of εskin/τskin can be calculated from the measurements of ethane. This means the skin resistance is inversely proportional to the diffusivity of the gas in air (Pham et al., 2009).
Fig. 7 gives a comparison between the temperature dependency of skin resistance estimated from co-diffusion model and the experimental data. The values of skin resistance measured from experiments decrease much faster with increasing of temperature compared to the model. Skin resistance is dependent on free space volume which depends on the cultivar, the growing season and the number and the size of the cells (Elgar et al., 1999; Schotsmans et al., 2002). Free space volume can be also affected by the respiration and moisture loss.

Changes of skin resistance of ‘Oushuu’ in different temperatures
Some of the basic physical properties of Japanese pear cultivar ‘Oushuu’ as well as its gas transfer properties were measured or estimated at three temperatures (5, 15 and 25°C) in the present study. The 3D geometry of the pear was imported in COMSOL Multiphysics 4.2a to perform the mathematical modeling. Gas diffusivity of ‘Oushuu’ at 5°C was found to be 5.97 × 10−7 m2/s and its skin resistance to be 4.04 × 105 s/m. In lack of such information for cultivars of Japanese pear, the comparison was made with cultivars of apple and pear. These values are well within the range of previous findings. However for higher temperatures the diffusivity estimated from the measurements decreases while it is expected to have an increasing trend. The co-diffusion model was used to estimate the diffusivity of ethane in different temperatures based on 5°C findings. It showed the measurement of gas transport properties in high temperatures cannot be accurate and such measurements must be done in low temperatures so that respiration and moisture loss are at the lowest possible level.