2014 Volume 20 Issue 2 Pages 309-316
2014 Volume 20 Issue 2 Pages 309-316
A rod-shaped and Gram-positive anaerobic S-equol-producing intestinal bacterium, TM-30, was isolated from the feces of a healthy human. The 16S rRNA partial sequence of strain TM-30 exhibited 99% identity to that of Slackia sp. NATTS. TM-30 produced S-equol from daidzein. The in vitro incubation of β-estradiol with TM-30 yielded estrone, and incubation of estrone with TM-30 produced β-estradiol. The estrone producing activity was higher than the β-estradiol producing activity. The results presented herein demonstrate that the S-equol-producing human intestinal bacterium, TM-30, likely plays a role in estrone and β-estradiol metabolism in vitro.
The health benefits of soy-based foods are largely attributed to isoflavones. Daidzin, genistin, daizein (the aglycone of daidzin), and genistein (the aglycone of genistin) are the most common isoflavones found in soy products. Human gastrointestinal bacteria play important roles in isoflavone metabolism (Bowey et al., 2003; Chang et al., 1995). S-equol is a metabolite of daidzein that is produced by the intestinal microbiota (Bowey et al., 2003). S-equol has higher affinity than daidzein when competing with 3H-estradiol for binding to the estrogen receptor (ER) (Sathyamoorthy et al., 1997). A case-control study involving residents in Japan and Korea demonstrated that the ability to produce S-equol is closely related to a lower prevalence of prostate cancer (Akaza et al., 2004). A long-term, randomized controlled trial that characterized postmenopausal women by their equol-producing status showed lumbar spine BMD (bone mineral density) was better after 2 years in equol producers compared with non-equol producers (Lydeking-Olsen et al., 2004). Natural S-equol might play a role in glycemic control and in the prevention of cardiovascular disease by its abilities to lower LDL-C levels and CAVI scores in overweight or obese individuals (Usui et al., 2013). Thus, S-equol is an important bacterial metabolite in the gut. S-equol production by the microbiota seems to contribute to host health. However, inter-individual variations in S-equol production have been identified. Only 30% to 50% of humans are equol producers (Cassidy et al., 2006; Song et al., 2006). The isoflavones in modest amounts of ingested soy protein are biotransformed by intestinal microflora, are absorbed, and undergo enterohepatic recycling (Setchell, 1998). It has been reported that estrogens are conjugated with glucuronic and/or sulfuric acid in the liver prior to excretion in urine or bile. Deconjugation of estrogen conjugates in the gut increases the enterohepatic circulation of the steroids and has important implications for their bioavailability and hormonal activity (Rowland et al., 1999). More than 99% of biliary estrogens are conjugated. Having entered the gut, about 80% are reabsorbed mainly as free steroids after extensive hydrolysis by enzymes of mainly bacterial origin (Hämäläinen et al., 1987). It has been demonstrated that S-equol excretion, but not total isoflavone excretion, correlates positively with the 2-OH E(1):16alpha-OH E(1) ratio, suggesting that the colonic bacterial profile associated with S-equol production may be related with estrogen metabolism, and may therefore influence breast cancer risk. Estimation of S-equol production and/or enumeration of the S-equol-producing bacteria may be a biomarker of host health. S-equol-producing bacteria have been previously isolated from human feces (Wang et al., 2005; Jin et al., 2010; Matthies et al., 2009; Yokoyama et al., 2011; Tsuji et al., 2010) or from the cecal content of a rat (Minamida et al., 2008). However, few reports have focused on the role of S-equol-producing intestinal bacteria in estrogen metabolism.
In this study, we isolated an S-equol-producing intestinal bacterium from the feces of a healthy human and tested the hypothesis that the S-equol-producing intestinal bacterium modulates the metabolism of estrogen through in vitro assays.
Chemicals Daidzein was purchased from LC Laboratories (Woburn, MA, USA). S-equol was kindly provided from Daicel Corporation (Osaka, Japan). β-Estradiol and estrone were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Isolation of fecal bacteria Fecal samples from a male S-equol producer were collected in sterilized stainless-steel cups. The samples were processed within 30 min of defecation. Approximately 1 g of feces was transferred to a sterilized glass homogenizer containing 9 mL of a prereduced anaerobic medium and a serial 10-fold dilution was performed. The diluted samples were spread onto the surface of Eggerth-Gagnon (EG) agar (Mitsuoka et al., 1965) non-selective medium plates (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). The plates were anaerobically incubated under an atmosphere of CO2 generated using an AnaeroPack® system (Mitsubishi Gas Chemical Company Inc., Tokyo, Japan) for 3 d at 37°C. The colonies were then selected from the plates of a 106 dilution using an inoculating loop and spread across the surface of the EG agar medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). The plates were further incubated for 3 d at 37°C under the anaerobic conditions described above. This study was performed under the guidelines of the Helsinki Declaration. The Human Investigations Review Board of the National Food Research Institute approved the study protocol and informed consent was obtained from the subject.
In vitro qualitative incubation of intestinal bacteria with daidzein Daidzein (20 mg) was dissolved in 1 mL dimethyl sulfoxide (DMSO). The daidzein solution (1 µL of each) was then transferred into 0.2 mL anaerobic medium, which was subsequently used as the reaction mixture. An inoculating loop bearing bacteria isolated from the EG agar medium was suspended in the reaction mixture and incubated anaerobically at 37°C for 24 h. After incubation, methanol-acetic acid (100:5, v/v) was added to the reaction mixture to a total volume of 1.0 mL. The mixture was vortexed for 60 s and centrifuged at 5,000 g at 4°C for 10 min. The supernatant was filtered through a 0.2-µm filter. The filtrate was subjected to high-performance liquid chromatography (HPLC) analysis: 20 µL of each preparation was injected into a 250 × 4.6 mm Capcell Pak C18 5-µm column (Shiseido, Tokyo, Japan). To detect the isoflavonoids, a JASCO MD-1515 photodiode array detector (JASCO, Co., Ltd., Tokyo, Japan) was used to monitor the spectral data from 200 to 400 nm for each peak. Daidzein and S-equol were used as standard samples to quantitate the isoflavonoids. The spectral data at 254 nm was used to quantify daidzein and the data at 280 nm was used to quantify S-equol. The mobile phase consisted of methanol/acetic acid/water (35:5:60, v/v/v). The HPLC system was operated at a column temperature of 40°C and a flow rate of 1 mL/min. The anaerobic medium that was used in this experiment was prepared as follows. Brain heart infusion (37 g), agar (1 g), l-cysteine HCl (0.5 g) and Na2CO3 (4 g) were dissolved in 1,000 mL distilled water. Aliquots of the medium (9 mL) were then distributed into test tubes, gassed with O2-free CO2, sealed with a butyl rubber stopper, and sterilized by autoclaving.
In vitro incubation of strain TM-30 with daidzein, β-estradiol and estrone Among the 200 tested bacteria, only strain TM-30 had equol-producing activity. TM-30 bacteria previously anaerobically incubated for 72 h on a BL agar plate were suspended in an anaerobic medium as described above, and adjusted to a final concentration of 1.0 × 109 CFU/mL. For the in vitro incubation of strain TM-30 with daidzein, daidzein (5 mg) was dissolved in 1 mL DMSO. The daidzein solution (0.5 mL) was then transferred into 0.2 mL of anaerobic medium containing the suspended bacteria. Then, 2 µL of 10% arginine, 2 µL of 5% formic acid and 4 µL of 10% sodium thioglycolate were aseptically added to the medium. The reaction mixture was incubated anaerobically at 37°C for 72 h. After incubation, methanol-acetic acid (100:5, v/v) was added to make a total volume of 1.0 mL. The mixture was vortexed for 60 s, and centrifuged at 5,000 g at 4°C for 10 min. The supernatant was filtered through a 0.2-µm filter and the filtrate was used for HPLC analysis as described above. For the in vitro incubation of strain TM-30 with β-estradiol, estrone, or β-estradiol plus estrone, 1 µL of β-estradiol (0.1 mg/mL in DMSO) or 1 µL of estrone (0.1 mg/mL in DMSO) or 1 µL of β-estradiol (0.1 mg/mL in DMSO) plus 1 µL of estrone (0.1 mg/mL in DMSO) was transferred into 0.2 mL of anaerobic medium containing the suspended bacteria or 0.2 mL of anaerobic medium (as a control). Then, 2 µL of 10% arginine, 2 µL of 5% formic acid and 4 µL of 10% sodium thioglycolate were aseptically added to the medium. The reaction mixture was incubated anaerobically at 37°C for 72 h. After incubation, 0.3 mL distilled water was added to the reaction mixture, followed by treatment with 500 µL of ethyl acetate, vortexing for 30 sec, and centrifugation at 5000 g for 5 min at 4°C. The supernatants were transferred to an eggplant-type flask. The same volume of ethyl acetate as used in the first extraction was added to the sediment, and the procedure was repeated. The supernatants from both extractions were pooled in the eggplant-type flask and the solvent was evaporated completely using a rotary evaporator. The residue was dissolved in 800 µL of 80% methanol and filtrated through a 0.2-µm filter. Filtrates were used for LC-MS/MS analysis. For LC-MS/MS analysis, detection and quantification were performed with a 4000 QTRAP LC/MS/MS system (AB Sciex, Foster City CA, USA) equipped with an atmospheric pressure chemical ionization (APCI) source (heated nebulizer) and 1100 Series HPLC system (Agilent, Waldbronn, Germany). Chromatographic separation was performed at 40°C on a ZORBAX Eclipse Plus C18 column, 150 × 2.1 mm i.d., 3.5 µm particle size (Agilent). Eluents were composed of water/acetic acid (99.9:0.1, v/v) containing 0.5 mM ammonium acetate (eluent A), and acetonitrile/acetic acid (99.9:0.1, v/v) (eluent B). Both solvents were prepared with chemicals of LC/MS grade (water, acetonitrile) and HPLC grade (acetic acid). Elution was conducted at a flow rate of 0.2 mL/min with a linear gradient of acetonitrile. After maintaining B at 5% for 2 min, it was increased linearly to 95% over 15 min, followed by a hold time of 5 min at 95% B. Thereafter, the portion of B was decreased to 5% over 1 min, and kept at 5% for 10 min prior to the next sample injection. APCI-MS/MS was performed in multiple reaction monitoring (MRM) mode at positive polarity with the following settings: source temperature 400°C, curtain gas 10 psi, ion source gas 1 (sheath gas) 60 psi, ion source gas 2 (drying gas) 10 psi, needle current 3 µA, dwell time 20 ms. The analyte-dependent MS/MS parameters were optimized by direct infusion of the standards. Each analyte dissolved in methanol (1 – 10 mg/L) was supplied at a rate of 10 µL/min with a syringe pump, mixed via a “mixing T” with the carrier solvent adjusted to 50% B, and introduced into the mass spectrometer at a flow rate of 0.2 mL/min. MS and MS/MS parameters (such as the selection of the most abundant MRM transitions, declustering potentials, collision energies, and cell exit potentials) were optimized for all analytes in the positive APCI mode. Table 1 summarizes the parameters of the optimized MRM transitions. Calibration curves were shown to be linear between 10 ng/mL and 400 ng/mL with correlation coefficients >0.999 for β-estradiol. Calibration curves were shown to be linear between 10 ng/mL and 400 ng/mL with correlation coefficients >0.999 for estrone.
| Compound | Precursor ion (m/z) | Product ion (m/z) | Declustering potential (V)a | Collision energy (V)a | Cell exit potential (V)a |
|---|---|---|---|---|---|
| Estrone | 271.1 | 253.1/159.1 | 51 | 19/33 | 10/16 |
| β-Estradiol | 255.1 | 159.1/133.1 | 76 | 31/27 | 8/22 |
aNumerical values are given in the order quantifier/qualifier.
DNA extraction from bacteria TM-30 bacteria were selected from EG agar plates that had previously been incubated with this strain. The isolated bacteria were suspended in 1 mL sterilized distilled water. Bacterial template DNA for the polymerase chain reaction (PCR) was extracted using InstaGene matrix (Bio-Rad Laboratories, CA, USA) in accordance with the manufacturer's instructions.
PCR amplification of 16S rRNA PCR was used to amplify the 16S rRNA. The PCR mixture (50 µL) was composed of each deoxynucleoside triphosphate at a concentration of 200 µM, the primers 27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-GGCTACCTTGTTACGACTT-3′) at a concentration of 0.30 µM, template DNA and 1.25 U of Takara EX Taq™ DNA polymerase and EX Taq™ buffer (Takara Bio Inc., Otsu, Japan). This process was carried out using the Dice PCR System (Takara Bio Inc.). The amplification program consisted of one cycle at 94°C for 1 min, followed by 30 cycles at 94°C for 1 min, 65°C for 1 min, 72°C for 1.5 min, and finally one cycle at 72°C for 2 min. The amplification products were subjected to gel electrophoresis in 0.7% agarose followed by ethidium bromide staining.
16S rRNA sequence analysis The PCR products were purified using QIAquick spin columns (Qiagen KK, Tokyo, Japan) according to the manufacturer's instructions. The purified DNA was used for 16S rRNA sequence analysis, which was performed using an ABI Prism dye terminator cycle sequencing ready reaction kit (Applied Biosystems, Foster City, CA, USA) and the following sequencing primers (Shinoda et al., 2000): r1L (5′-GTATTACCGCGGCTGCTGG-3′), r2L (5′-CATCGTTTACGGCGTGGAC-3′), r3L (5′-TTGCGCTCGTTGCGGGACT-3′), r4L (5′-ACGGGCGGTGTGTACAAG-3′), 926f (5′-AAACTCAAAGGAATTGACGG-3′) and f3L (5′-GTCCCGCAACGAGCGCAAC-3′). The sequences were automatically analyzed using an ABI Prism 310 DNA sequencer (Applied Biosystems). The assembled partial 16S rRNA sequences were compared with sequences from the GenBank database (Altschul et al., 1990). The CLUSTAL W program (Thompson et al., 1994) was used to construct a phylogenetic dendrogram, which was visualized using the Tree View program.
Identification of the isolated bacterium The TM-30 colonies on EG agar medium and on glucose blood liver (BL) agar medium were grayish white. The cells were rod-shaped and Gram reaction positive. The sequence data were aligned and the assembled partial 16S rRNA sequences were compared with those available in the GenBank database. A phylogenetic tree was constructed by using the neighbor-joining method based on the partial 16S rRNA sequences of strain TM-30 and related bacteria. The 16S rRNA partial sequence (1402bp) of strain TM-30 (Accession no: AB727353) that was isolated from human feces exhibited a 99% identity to the analogous sequence of Slackia sp. NATTS (Accession no: AB505075) (Fig. 1). Therefore, this strain was identified as belonging to the genus Slackia.

Phylogenetic tree constructed by using the neighbor-joining method based on the partial 16S rRNA sequences of strain TM-30 and related bacteria.
The phylogenetic tree was constructed using the Tree View program.
Bar, 0.1 substitutions per nucleotide position.
In vitro incubation of strain TM-30 with daidzein The in vitro incubation of daidzein with the intestinal bacterium TM-30 yielded S-equol and dihydrodaidzein (Fig. 2). Strain TM-30 produced S-equol from daidzein (S-equol concentration: 6.23 µg/mL) and dihydrodaidzein from daidzein (dihydrodaidzein concentration: 5.19 µg/mL). In the daidzein incubation solutions, the S-equol and dihydrodaidzein concentrations were higher than the daidzein concentration (daidzein concentration: 0.40 µg/mL). The amount of S-equol produced from this amount of daidzein substrate was about 52% and the amount of dihydrodaidzein produced from this amount of daidzein substrate was about 43.3%.

Metabolism of daidzein by strain TM-30. Strain TM-30 was anaerobically incubated with daidzein in the medium. Concentration of the isoflavonoids (µg/mL) Data from one experiment of 2 with similar results are presented.
In vitro incubation of strain TM-30 with β-estradiol and estrone In vitro incubation of β-estradiol with the intestinal bacterium TM-30 resulted in the production and detection of β-estradiol and estrone. The peak areas of β-estradiol (a) and estrone (b) are shown in Fig. 3. β-Estradiol was detected in both incubation solutions of strain TM-30 with β-estradiol or estrone. Estrone was detected in both incubation solutions of strain TM-30 with β-estradiol or estrone. The in vitro incubation of β-estradiol with the intestinal bacterium TM-30 yielded estrone (287.5 ng/mL) (Fig. 4), as well as β-estradiol from estrone (148.5 ng/mL) (Fig. 4). The estrone producing activity from β-estradiol was higher than the β-estradiol producing activity from estrone. In in vitro incubation of β-estradiol and estrone with TM-30, the estrone concentration (506.7 ng/mL) was higher than the β-estradiol concentration (249.1 ng/mL) in the reaction mixture (Fig. 5). These results suggest that TM-30 is involved in not only isoflavone metabolism, but also in estrogen metabolism.

LC-MS/MS analysis of the incubation solution of strain TM-30 incubated with β-estradiol (a) Peak area of β-estradiol, monitor ion; 255.1/159.1), (b) Peak area of estrone, monitor ion; 271.1/159.1).

Metabolism of β-estradiol and estrone by strain TM-30. Strain TM-30 was anaerobically incubated with β-estradiol or estrone in the medium.
Data from one experiment of 2 with similar results are presented.

Metabolism of β-estradiol and estrone by strain TM-30. Strain TM-30 was anaerobically incubated with β-estradiol and estrone in the medium.
Data from one experiment of 2 with similar results are presented.
Much attention has been focused on the biological activity of isoflavones and their metabolites by intestinal microbiota. The major intestinal bacterial metabolites of daidzein are S-equol and O-desmethylangolensin (Rowland et al., 2003). Metabolites of daidzein may be important because of the different biological effects of these compounds. Thus, intestinal microbiota likely play important roles in the biological activities of isoflavones in humans. However, few reports have focused on the role of S-equol-producing intestinal bacteria in estrogen metabolism. We isolated the S-equol-producing bacterium TM-30 from the feces of a healthy human and tested the hypothesis that the S-equol-producing intestinal bacterium modulates the metabolism of estrogen
S-equol-producing bacteria have been previously isolated from human feces (Wang et al., 2005; Jin et al., 2010; Matthies et al., 2009; Yokoyama et al., 2011; Tsuji et al., 2010) or from the cecal content of a rat (Minamida et al., 2008). In particular, bacteria belonging to the genus Slackia may play important roles in isoflavone metabolism in the gut. For example, a Gram-positive, non-spore-forming rod bacterium (Slackia sp. strain NATTS) with the ability to convert daidzein to S-equol has been isolated from human feces (Tsuji et al., 2010). When Slackia sp. strain NATTS was incubated anaerobically in the presence of 100 µM of daidzein, it converted 90% or more of the daidzein into equol during an 8-h culture (Tsuji et al., 2010). Thus, the equol-producing activity of intestinal bacterium TM-30 seems to be lower than that of Slackia sp. strain NATTS. However, there are no reports on the effects of an equol-producing bacterium on the metabolism of estrogen. This is the first report on the effects of an equol-producing bacterium on estrogen metabolism.
The 16S rRNA partial sequence of strain TM-30 isolated from human feces exhibited a 98% identity to that of Slackia isoflavoniconvertens JCM16137T. Slackia isoflavoniconvertens is capable of transforming daidzein to S-equol, and genistein to 5-hydroxy-S-equol (Matthies et al., 2009). The prevalence of the Slackia sp. in Japanese adults was examined by reverse transcription-quantitative PCR (RT-qPCR). It was found to be present at 40% of the mean population level, at 106 cells per gram of feces (Tsuji et al., 2010).
S-equol is considerably more estrogenic than daidzein. It has also been demonstrated to be a more effective antioxidant than daidzein or genistein (Mitchell et al., 1998; Song et al., 2006). S-equol or the ability to produce S-equol is related to anticarcinogenic characteristics in humans. A case-control study involving residents in Japan and Korea demonstrated that the ability to produce S-equol is closely related to a lower prevalence of prostate cancer (Akaza et al., 2004). Thus, S-equol is an important bacterial metabolite in the gut. However, inter-individual variations in S-equol production have not yet been identified. Only 30% to 50% of humans are S-equol producers (Cassidy et al., 2006; Song et al., 2006).
The concentrations of serum hormones, sex hormone-binding globulin (SHBG), urinary 2-hydroxyestrone (2-OH E1) and 16α-hydroxyestrone (16α-OH E1) in relation to S-equol-producer and O-DMA-producer phenotypes in 89 post-menopausal women have been reported (Frankenfeld et al., 2004). The results suggested that inter-individual variability in intestinal bacteria may be related to differences in the products of hormone metabolism in post-menopausal women (Frankenfeld et al., 2004). It has been demonstrated that S-equol excretion, but not total isoflavone excretion, correlates positively with the 2-OH E(1):16alpha-OH E(1) ratio, suggesting that the colonic bacterial profile associated with S-equol production may be related with estrogen metabolism, and may therefore influence breast cancer risk. Results of prior studies suggest beneficial effects of producing S-equol in relation to breast cancer risk, potentially through effects on endogenous hormones (Atkinson et al., 2003). The enterohepatic circulation of estrogen in women has been reported (Schindler et al., 1982). Thus, intestinal microbiota affect the amount of circulating estrogen by modulating the amount of estrogen in the gut. S-equol productivity by microbiota in humans might be one way that the host endogenous hormone status can be beneficially modulated by varying the metabolism of circulating estrogens in the gut. Our results indicated that the S-equol-producing bacterium, TM-30, can interconvert estrone and β-estradiol and thus, the production of S-equol might be an important function of the gut microbiota. A proposed pathway for isoflavone daidzein reduction by intestinal microbiota is shown in Fig. 6, which also shows the interconversion of β-estradiol and estrone.
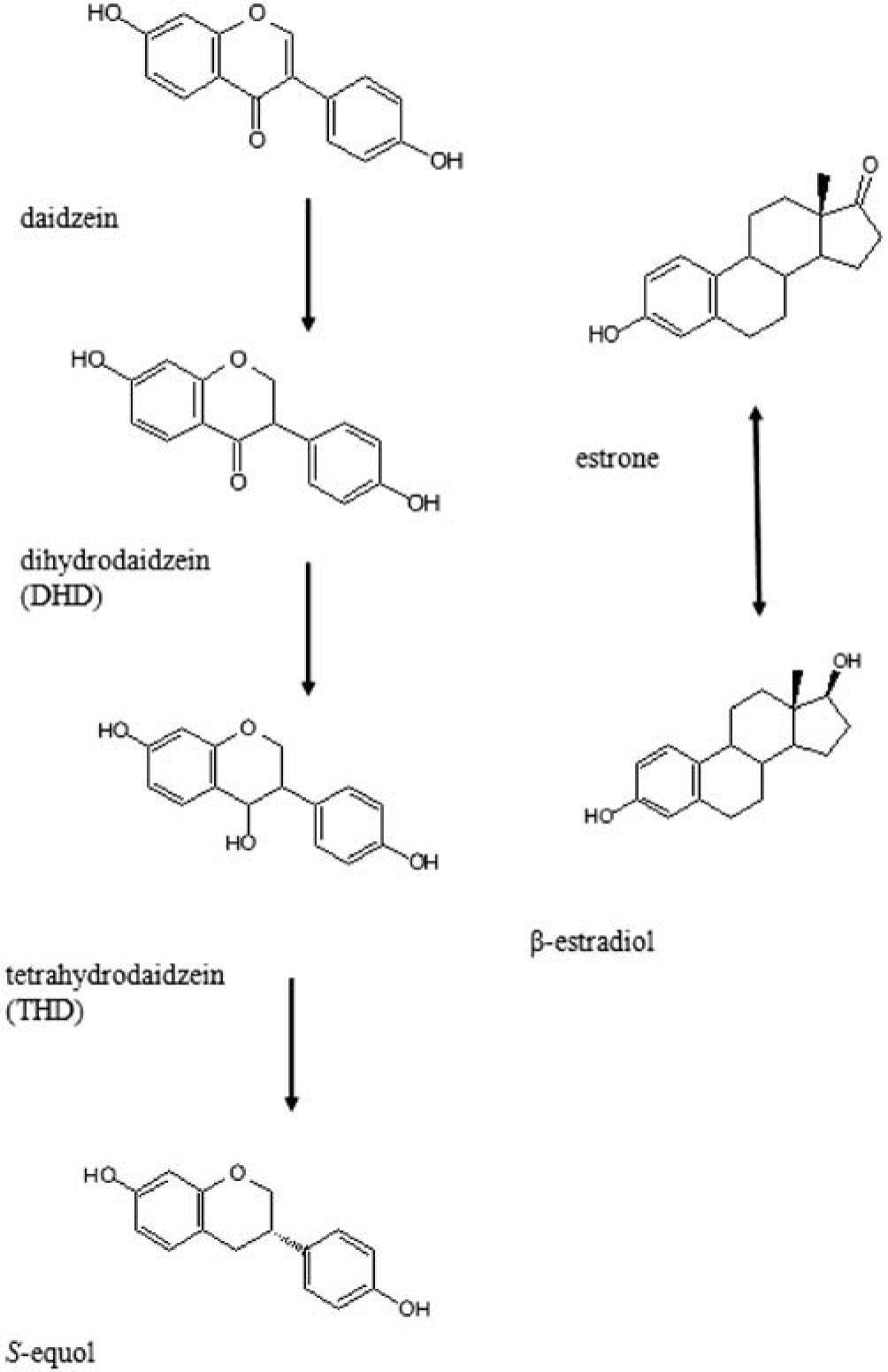
Proposed pathway for isoflavone daidzein reduction by intestinal microbiota (Kim et al., 2009) and the interconversion of β-estradiol and estrone.
Some intestinal bacteria that can reduce daidzein all the way to S-equol were recently isolated from mice (Matthies et al., 2008), rats (Minamida et al., 2006), pigs (Yu et al., 2008), and humans (Maruo et al., 2008). Dihydrodaidzein, a bacterial metabolite of the widespread isoflavone daidzein (Rowland et al., 2003), has been proposed as a precursor of S-equol (Rowland et al., 2003). Stereochemical reduction of isoflavanone dihydrodaidzein (DHD) to the isoflavan (3S)-S-equol via tetrahydrodaidzein (THD) by the human intestinal anaerobic bacterium Eggerthella strain Julong 732 was reported (Kim et al., 2009). During the S-equol production from daidzein, the S-equol-producing bacterium seems to reduce the ketone of daidzein to the hydroxy of tetrahydrodaidzein (THD) by hydrogenation. On the other hand, β-estradiol has a structure similar to daidzein and is a ketone (Fig 6). The S-equol-producing bacterium, TM-30, might reduce the ketone of estrone to the hydroxy of β-estradiol by hydrogenation. However, β-estradiol production from estrone is a reversible reaction. In our experiments, TM-30 also produced β-estradiol from estrone, with the estrone producing activity being higher than the β-estradiol producing activity. It has been reported that estrogens are conjugated with glucuronic and/or sulfuric acid in the liver prior to excretion in urine or bile. Deconjugation of estrogen conjugates in the gut increases the enterohepatic circulation of the steroids and has important implications for their bioavailability and hormonal activity (Rowland et al., 1999). S-equol was negatively associated with plasma estradiol in 1,998 healthy women (Low et al., 2007). It has been reported that dietary seaweed increases equol production in healthy postmenopausal women (Teas et al., 2009). In that report, for five equol excretors, the combination of seaweed and soy protein isolate consumption increased urinary equol excretion (P = 0.0001). There was an inverse correlation between seaweed dose (mg/kg body weight) and serum estradiol (E2). S-equol-producing bacteria might affect both estrogen and phytoestrogen metabolism in the gut, thereby changing the estrogen status of the host. However, there is little information regarding the effects of S-equol-producing bacteria on the metabolism of estrogen.
Further studies are needed to clarify the mechanism of the effects of S-equol-producing bacteria on the reversible reaction of estrone and β-estradiol.
S-equol might also have a role in glycemic control and in the prevention of cardiovascular disease by its effects on lowering LDL-C levels and CAVI scores in overweight or obese individuals (Usui et al., 2012). Recently, much attention has also been focused on the relationship between obesity and intestinal microbiota (Turnbaugh et al., 2006; Conterno et al., 2011). Further studies are needed to clarify the effects of S-equol-production by microbiota on obesity.
A limitation of our study was that we could not identify whether this S-equol-producing bacterium affects estrogen metabolism in the intestinal microbiota from S-equol-non-producers. However, this is the first study indicating that an S-equol-producing intestinal bacterium likely modulates the metabolism of estrogen.
In conclusion, a rod-shaped and Gram-positive anaerobic S-equol-producing intestinal bacterium, TM-30, was isolated from the feces of a healthy human. The 16S rRNA partial sequence of strain TM-30 (Accession no: AB727353) exhibited 99% identity to the analogous sequence of Slackia sp. NATTS (Accession no: AB505075). TM-30 produced S-equol from daidzein. Surprisingly, the in vitro incubation of β-estradiol with TM-30 yielded estrone, and TM-30 also produced β-estradiol from estrone. The estrone producing activity was higher than the β-estradiol producing activity. The results presented demonstrate that the S-equol-producing human intestinal bacterium TM-30 likely plays a role in estrone and β-estradiol metabolism in vitro. This is the first study indicating that an S-equol-producing intestinal bacterium likely modulates the metabolism of estrogen.
Acknowledgements This study was financially supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science.