2014 年 20 巻 2 号 p. 337-343
2014 年 20 巻 2 号 p. 337-343
Industrial wastewater containing heavy metals can become a serious environmental pollutant if not treated appropriately. Conventional treatment to remove toxic heavy metals can be expensive and may generate large amounts of toxic sludge. Regenerated natural wastes such as eggshells and eggshell membrane which are easily available, inexpensive, biodegradable, and have high adsorbability, can act as promising ‘green’ alternatives to remove heavy metal pollutants from wastewater. Here, we studied the adsorption capacity of eggshells with membrane (ESWM), eggshell membrane (ESM), and eggshells (ES) for the removal of nickel and silver ions in synthetic wastewater. Reaction time (1 to 72 h), metal ion concentration (25 to 200 mg/L), adsorbent dosage (0.1 to 0.8 g/20 mL), temperature (15°C to 45°C), and pH (1 to 9) were evaluated. Post-treatment nickel and silver concentrations were later analyzed using a spectrophotometer. Our results indicated increased removal of nickel and silver ions with increased adsorbent (all three ESWM, ESM, and ES) dosage, whereas the removal of nickel and silver ion decreased with increasing initial metal concentration. Among ESWM, ESM, and ES, ESM has the highest removal capacity and was the best adsorbent. The 0.8 g of ESM could remove 90.91% of nickel ions (100 mg/L) at 25°C, pH 5.76 and 24 h. On the other hand, approximately 100% of silver ions (25 mg/L) could be removed by 0.2 g of ESM at 25°C, pH 5.2, and 24 h. There was no difference in the adsorbability of ES and ESWM on nickel and silver ions. In summary, all three adsorbents, ESWM, ES, and ESM, can remove heavy metal ions from aqueous solution, with ESM having the highest efficiency. Hence, eggshell and its derivatives can be promising ‘green’ adsorbent materials for treating wastewater containing nickel and silver ions.
The removal of toxic and heavy metals from wastewater is a matter of great interest in the field of water management. Recently, removal of heavy metal ions from industrial waste effluents has become more challenging due to the implementation of stricter laws and regulations to restrict the concentration of pollutants discharged into waters and soil to less than 1 mg/kg. Traditional methods of metal ion removal become inefficient at eliminating metal ions below this concentration. Therefore, there is an urgent need to develop alternative ways to feasibly remove ion pollutants to reach the required low concentration (Yang, et al., 2009). One possible approach is to use sorbents of biological origin to remove toxic metals from dilute aqueous solutions. Biological sorbents generated from food and agricultural industry waste, such as waste eggshells and its derivatives, are considered to be an economical source. If low-cost biological sorbents can be used in the wastewater pre-treatment process, the overall management cost will be reduced significantly (Chojnacka, 2005). Moreover, it is a ‘green’ approach as these sorbents are generally biodegradable and hence, more environmentally friendly.
Nickel is a common, non-biodegradable toxic heavy metal ion present in wastewater. The main source of nickel pollution in the water derives from industrial production processes such as galvanization, smelting, mining, batteries manufacturing, and metal finishing. The presence and accumulation of nickel in industrial effluents is known to have toxic or carcinogenic effect on living species. Therefore, it is crucial to eliminate nickel ions from wastewaters. As an economical and efficient method, the adsorption technique has been widely applied to remove heavy metal ions such as nickel from wastewater (Yang et al., 2009). Different adsorbents such as activated carbon (Hasar, 2003), husk of Lathyrus sativus (Panda et al., 2007), olive stone waste (Fiol et al., 2006), bagasse and fly ash (Rao et al., 2002), mordenite (Wang et al., 2007) and crab shells (Pradhan et al., 2005) have been reported as workable adsorbents for Ni2+ ion removal from wastewaterwever, further applications for these adsorbents are limited because of their low adsorption capacities. Therefore, evaluation and development of new biological adsorbents with high adsorption capacities and efficiencies for nickel is still an on- going effort.
With the development of electroplating, coinage, medication, and chemical engineering, pollution caused by silver-bearing wastewater has emerged rapidly. Silver in aquatic discharges may occur in a variety of chemical forms, though the concentration of free ionic Ag+ is probably very low in most circumstances. Silver ions can be accumulated in animal organisms through their food chain to cause harm to themselves (Hou et al., 2009). It is known that Ag+ exposure can cause inhibition of active Na+ and Cl− uptake by compromising branchial Na+-ATPase and K+-ATPase to cause ion regulatory failure in crayfish and daphnids (Bianchini et al., 2003; Grosell et al., rid="bib2002" ref-type="bibr">2002). More recently, Ag+ has been shown to impair neurodevelopment in neuronotypic cells (Power et al., 2010). Since silver is widely used in various industries due to its excellent malleability, conductivity, thermal conductivity, etc., sources for silver have become rarer as its future usage increases (Purcell et al., 1998). Its wide applications have produced a fair amount of silver waste. Therefore, effective removal and recovery of Ag+ has also become a critical task.
Hen eggshell waste is widely produced in households and food industries such as restaurants and bakeries. In this study, we proposed to use hen eggshells as a low-cost biological adsorbent of nickel and silver ions. Whole eggshells or eggshells with membrane (ESWM) consist of ceramic materials constituted by a three-layered structure, namely the cuticle on the outer surface, the spongy (calcareous) layer in the center and the inner lamellar (or mammillary) layer (Tullett, 1987; Stadelman, 2000). Eggshells (ES) have little porosity and pure CaCO3 is their main constituent. By-product eggshells generated from food processing and manufacturing plants are inevitably composed of calcium carbonate (ES) and eggshell membrane (ESM). ESM resides between the egg white and the inner surface of the eggshell. There are two shell membranes around the egg: a thick outer membrane attached to the shell and a thin inner membrane (Parsons, 1982; Nakano et al., 2003). Each of these membranes is composed of protein fibers that are arranged to form a double-layered, semi-permeable membrane. Therefore, ESM possesses an intricate lattice network of stable, water-insoluble fibers and has a high surface area for various applications such as an adsorbent (Tsai et al., 2006; Tsai et al., 2008).
In general, ‘green’ industrial processing has three main missions: minimizing harmful wastes, recovering precious materials and heavy metals, and maximizing regeneration of potentially useful value-added wastes and energy. Here, we reused eggshells (recycled wastes) to minimize heavy metal ion contaminants. The aim of this study was to examine the removal efficiency of less-researched heavy metals, nickel and silver, from synthetic wastewater using whole eggshells (ESWM) or their derivatives (ES and ESM) as bio-adsorbents. A comprehensive panel of experimental parameters with various adsorption times, initial concentration of nickel and silver ions, pH, and temperatures were evaluated.
Preparation of adsorbent The natural raw eggshells were obtained from a campus breakfast store in Tunghai University. After cleaning the eggshells, they were divided into three groups: eggshell with membrane (ESWM), eggshell membrane (ESM), and eggshell (ES), as adsorbents in this study. These samples were dried at 55°C in an oven and subsequently ground into powder by high speed grinder RT-02B (Rong Tsong, Taiwan). Then, these samples were further screened by No. 200 meshes to obtain their final adsorbent samples for experimental use. The resulting adsorbent particle size of diameter should be lower than 0.074 mm. Besides, in order to simplify the separation procedure of ESM from eggshell, and observe whether the ESWM has the better capacity for metal ion adsorption, we kept the ESWM to be used as one of adsorbents in this study.
Adsorption experiment design
1. Effect of adsorption time
To test the effect of adsorption time of each 0.2 g of adsorbents (ESWM, ESM, and ES), each sample was added into 20 mL of either 100 mg/L nickel (II) chloride (Katayama, Japan) or silver nitrate (Katayama, Japan) single-ion aqueous solution. Each mixture was shaken at 100 r/min in a 25°C water bath for 1, 2, 3, 24, 48, and 72 h. The adsorbed amount of each Ni2+ and Ag+ was analyzed by a spectrophotometer (Jasco V-530, Japan). For all experiments (except the one evaluating various pH), pH of nickel and silver solutions were 5.76 and 5.2, respectively.
2. Effect of adsorbate dosage
Adsorbate dosage was evaluated by having each 0.2 g of adsorbents (ESWM, ESM, and ES) added into 20 mL of 25, 50, 100, and 200 mg/L Ni2+ or Ag+ single-ion solution. Each treatment was shaken at 100 r/min in a 25°C water bath for 24 h, and metal adsorption was analyzed by spectrophotometry.
3. Effect of adsorbent dosage
Each sample of 0.1, 0.2, 0.4, and 0.8 g of adsorbents (ESWM, ESM, and ES) was added into 20 mL of 100 mg/L Ni2+ or Ag+ single-ion solution, shaken at 100 r/min in a 25°C water bath for 24 h. The post-treatment Ni2+- and Ag+ concentrations were measured by a spectrophotometer.
4. Effect of adsorption temperature
Each 0.2 g of adsorbents (ESWM, ESM, and ES) was added into 20 mL of either 100 mg/L Ni2+ or Ag+ single-ion solution, incubated at various temperatures (15°C, 25°C, 35°C, and 45°C), and shaken at 100 r/min for 24 h in a water bath. Again, post-treatment Ni2+- or Ag+ were analyzed using a spectrophotometer.
5. Effect of pH
Each 0.2 g of adsorbents (ESWM, ESM, and ES) was added into 20 mL of either 100 mg/L Ni2+ or Ag+ single-ion solution under various pH parameters (1, 2, 3, 4, 5, 6, 7, 8, and 9), adjusted with HNO3 or NH3, then shaken at 100 r/min in a 25°C water bath for 24 h. The amount of Ni2+ and Ag+ adsorbed with different adsorbents was then analyzed by a spectrophotometer.
Spectrophotometer analysis To measure the post-treatment concentration of each ion, each post-treatment solution was filtered with filter paper (Whatman No. 1) and centrifuged at 3500 r/min for 15 min. For Ni2+ ions detection, the supernatant of 2.5 mL was aspirated and mixed with 4 mL NaOH, 5 mL ammonium persulfate, 2 mL dimethyglyoxine, and 12.5 mL DDW (double distilled water). After 30 min of reaction time, 1 mL of the mixed solution was taken to detect ion concentration at 465 nm wavelength by a spectrophotometer. For Ag+ ions detection, the supernatant of 5 mL was aspirated and mixed with 2 mL citric acid-monopotassium phosphate buffer, 1 mL arsenazo-III, 1.2 mL L-ascorbic acid, and 5 mL DDW. After 4.5 min of reaction time at 90°C followed by cooling to room temperature, the ion concentration in 1 mL of mixed solution was detected at 540 nm wavelength. Standard curve of Ni2+ and Ag+ were established from 25–200 mg/L and 6.25–100 mg/L, respectively (Dong, 2006).
The percentage of adsorption for Ni2+ and Ag+ with each adsorbent was calculated as follows:
 |
Cin = initial concentration of metal ions in the aqueous solution and Crem = remaining concentration of metal ions in the aqueous solution.
Statistical analysis Each experimental set was performed in ten replicates. Each testing parameter for ESWM, ESM, and ES was analyzed by analysis of variance (ANOVA) and Duncan's new multiple range test in the Statistical Analysis Software (SAS®) to compare the adsorption capacity of Ni2+ or Ag+. P < 0.05 is taken as statistically significant.
Effect of reaction time The effect of reaction time (1 – 72 h) on adsorption for 100 mg/L Ni2+ and 100 mg/L Ag+ with ESWM, ESM, and ES is shown in Figs. 1a and 1b. Overall adsorption of Ag+ was higher than Ni2+, with ESM exhibiting the best performance of adsorbing both Ni2+ and Ag+ (p < 0.05). The maximum adsorption of Ni2+ and Ag+ with ESM was 34.9% and 88.7% at 48 h and 72 h reaction time, respectively. The reaction time curve showed that each Ni2+ and Ag+ adsorptions were rapid within the first hour and then the adsorption values changed only slightly after that (within 24 h). We found no correlation between percentage of adsorption and reaction time. Unlike the report by Misra et al. (2011) that indicated removal of Ni2+ under low ion concentration of iminodiacetic acid functionalized cation exchange resin could achieve above 55% within 4 h, adsorption of Ni2+ and Ag+ did not change over time with adsorbents derived from eggshell materials in our study. Therefore, application of egg-shell adsorbents on industrial wastewater treatment we recommended 3 h for the optimum reaction time based on our study. However, the adsorption capacity may depend on many other factors as measured in our next few experiments.

Percentage of adsorption for (a) nickel and (b) silver ions with different adsorbents for various reaction times. Nickel concentration: 100 mg/L, adsorbent dosage: 0.2 g/20 mL, temperature: 25°C, particle size < 0.74 mm, pH = 5.76; silver concentration: 100 mg/L, adsorbent dosage: 0.2 g/20 mL, temperature: 25°C, particle size < 0.74 mm, pH = 5.2; ESWM, ESM, and ES = eggshells with membrane, eggshell membrane, and eggshells (without membrane), respectively.
Effect of adsorbate dosage Experiments conducted with different initial dosage of Ni2+ or Ag+ concentration (range from 25 to 200 mg/L) showed that ESM exhibited the highest adsorption capacity, with the percentage of adsorption decreasing as the metal ion concentration increased (Figs. 2a and 2b). With 0.2 g/20 mL of ESM, the highest percentage of adsorption on Ni2+ or Ag+ was 86.1 and 100%, respectively, at 25 mg/L of adsorbate dosage. This reversed correlation between adsorbate dosage and adsorption capacity was similar to the phosphate adsorption research reported by Mezenner and Bensmaili (2009), who indicated that at a fixed dosage of adsorbent, the number of active sites that an adsorbate can bind to on the adsorbent surface is constant. Therefore, although adsorption capacity is highest with the initial adsorbate concentration of 25 mg/L, as adsorbate concentration increased, the active sites of adsorbents gradually reached saturation, progressively decreasing the adsorption rate of adsorbates.

Percentage of adsorption for (a) nickel and (b) silver ions with different adsorbents at various initial ion concentrations. Reaction time: 24 hr; adsorbent dosage: 0.2 g/20 mL; temperature: 25°C; particle size <0.74 mm. Nickel single-ion solution pH = 5.76; Silver single-ion solution pH = 5.2.
Effect of adsorbent dosage From experiment 2.2, we can hypothesize that the total active sites of adsorbents increased with the dosage of adsorbents. The effect of adsorbent dosage (0.1 – 0.8 g/20 mL) on Ni2+ or Ag+ adsorption is shown in Figs. 3a and 3b. Again, among the three adsorbents, ESM has the highest adsorption capacity toward nickel and silver, regardless of its dosage. The maximum adsorption of Ni2+ and Ag+ with 0.8 g ESM was 90.9% and 88.1%, respectively. The direct correlation between adsorbent dosage and adsorption capacity was clearer in nickel than silver single-ion solution. For Ag+, the percentage of adsorption increased rapidly from 0.1 g and plateaued at 0.4 g of adsorbent dosage. This observation is likely due to the overlapping and overcrowding of aggregated particles at a higher dosage, resulting in limited availability of the surface area and exposed active binding sites (Yeddou and Bensmaili, 2007).

Percentage of adsorption for (a) nickel and (b) silver ions with different adsorbents at various dosages. The rest of the parameters for Ni2+ and Ag+ are the same as in Fig. 1.
Effect of reaction temperature on adsorption of nickel and silver with eggshell samples Generally, the temperature of industry wastewater ranges from 25°C to 40°C. Therefore, the effect of temperature on Ni2+ or Ag+ adsorption by eggshell samples was studied in the range from 15°C to 45°C (Figs. 4a and 4b). As seen in Fig. 4a, adsorption of Ni2+ achieved its highest capacity at 45°C at 45.5%, 79.1%, and 73.2%, with ESM, ESWM, and ES, respectively. For ESWM and ES, the removal of Ni2+ increased more rapidly from 35°C to 45°C, when compared with ESM. A similar phenomenon was observed in Ni2+ adsorption by wheat straw (Dona et al., 2008) and phosphate adsorption by iron hydroxide-eggshell (Mezenner and Bensmaili, 2009), suggesting that adsorption of Ni2+ with ESWM and ES was attributed to an endothermic process, as a high temperature favored Ni2+ removal from wastewater. We showed that ESM has the highest adsorption capacity of 93.2 % at 45°C for Ag+ ion. Since the adsorption of Ag+ changed slightly over the temperature range of 15°C to 45°C in all three eggshell samples (Fig. 4b), the effect of temperature on Ag+ adsorption could be ignored, as presented by Huo et al. (2009), or affected by an endothermic reaction as in amino methylene phosphonic acid resin (Shu et al., 2006), depending on the types of adsorbents.
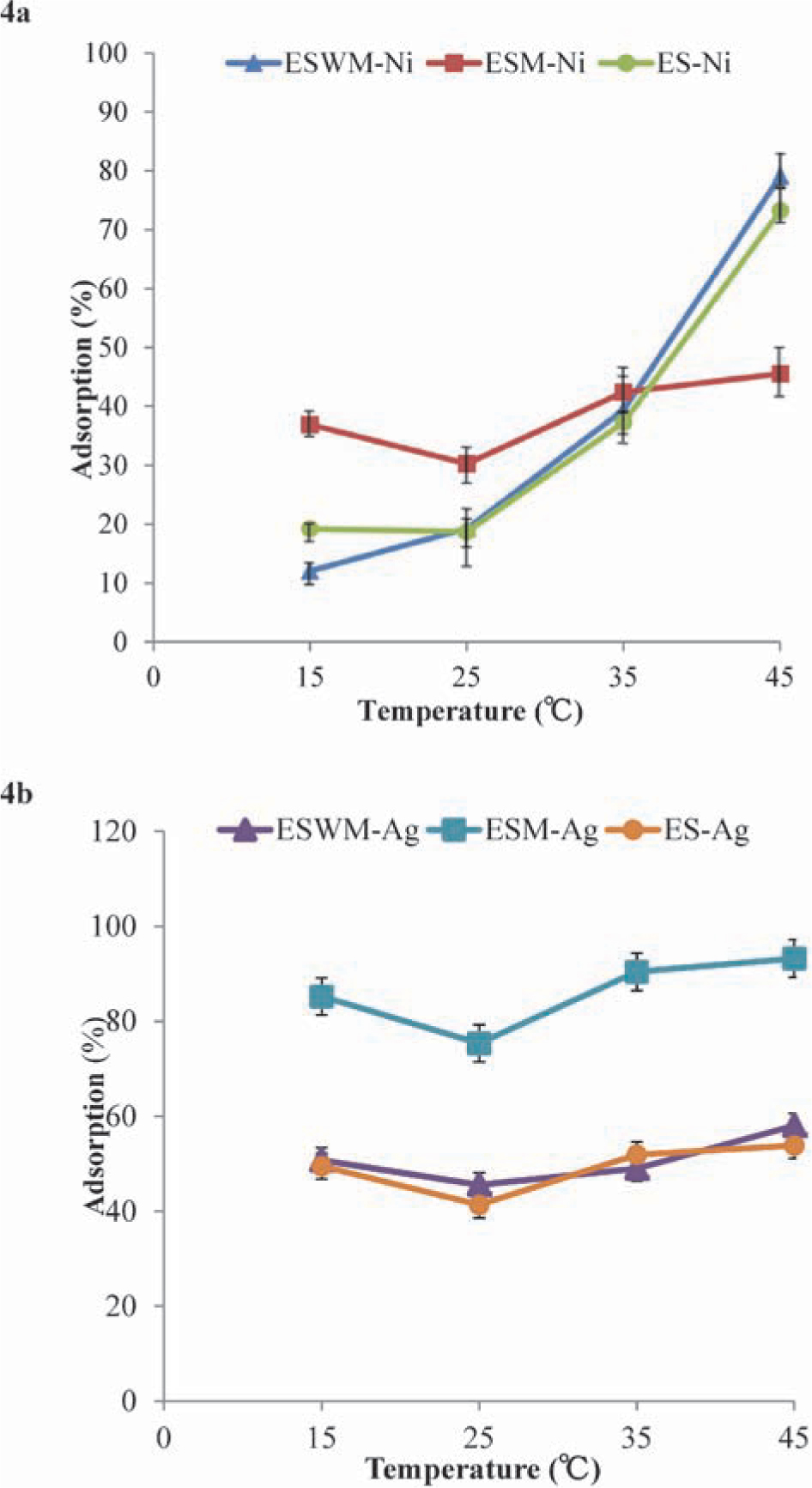
Percentage of adsorption for (a) nickel and (b) silver ions with different adsorbents at various temperatures. The rest of the parameters for Ni2+ and Ag+ are as in Fig. 1.
Effect of pH on adsorption of nickel and silver with eggshell samples The pH of a solution is known to play an important part in metal adsorption by adsorbents. The pH influences not only the chemical forms of metals, but also the adsorption behavior of adsorbents. The effect of pH (1–9) on Ni2+ or Ag+ adsorption by eggshell samples is shown in Figs. 5a and 5b. At pH 9, ESM, ESWM, and ES reached the highest Ni2+ adsorption of 85.5%, 73.0%, and 64.3%, respectively, while Ag+ adsorption was the lowest at this pH. Each ESM, ESWM, and ES performed its best (92.3%, 53.2% and 59.8%, respectively) toward Ag+ adsorption at pH 4. From Fig. 5a, we showed that increased pH also increased Ni2+ adsorption, and our results coincided with findings from Yang et al. (2009) and Dona et al. (2008). They indicated that at a low pH environment, the predominant specie of nickel, Ni2+, will compete with the high concentration of H+ to bind to the active sites of adsorbents, resulting in a less favored environment for Ni2+ adsorption.

Percentage of adsorption for (a) nickel and (b) silver ions with different adsorbents at various pH. The rest of the parameters for Ni2+ and Ag+ are as in Fig. 1.
From the results of Ni2+ and Ag+ adsorption by ESWM, ESM, or ES under different experimental conditions, we can conclude as follows:
Acknowledgements This research was supported by Tunghai Green Energy Development and Management Institute (TGEI) and instrumental analysis by Department of Nutritional Science, Toko University, Taiwan.