2014 Volume 20 Issue 2 Pages 345-355
2014 Volume 20 Issue 2 Pages 345-355
The milk proteins were mixed with lactose in the same proportions and conditions as skim milk, which were casein-lactose (CN-L) (1: 1.6) and casein-whey protein isolate-lactose (CN-WPI-L) (1: 0.2456: 1.579) models. These two models at different pHs (6.6, 8.0) and skim milk powder were treated in a microwave field at 400 w for 15 min. Functional properties (solubility), structure, digestibility and antioxidant activities of casein-lactose Maillard reaction products were investigated. The results indicated that the digestibility of glycated milk proteins (CN-L) was higher than that of native protein. The improved solubility and enriched antioxidant peptide fraction showed that could be a promising method for dietary supplements. The fluorescence intensity was increased a lot, and environmental scanning electron microscope (ESEM) analysis showed that protein granule was changed from regular and smooth flaky to nubbly and amorphous structure. Then the results will have a great impact on the development of novel food structures with improved properties.
Milk powder is an important source of high value proteins, minerals, vitamins and bioactive components (Stanciuc et al., 2010). Along with the rhythm of life speeding up, more and more people are focusing on the quality and nutrition of the milk powder. Being alarmed at the affairs of substandard milk powder, the misuse of milk powder additives and melamine milk powder, people are paying more attention to the great many of milk powder in supermarket.
In recent years, microwave ovens have become a popular appliance due to its high efficiency and convenience. The use of microwave sterilization and microwave drying in the processing of milk powder became a universal phenomena (Beary, 1988). There are numbers of reactions in milk during microwave treatment, especially Maillard reaction (MR), which is easily occurred in milk powder processing. MR is a chemical reaction between amino groups and reducing sugars, and it is very significant for foods because it strongly affects the quality and nutrient value (Van Boekel, 1998). Caseins and whey protein are important ingredients of almost all milk from mammalian species (Rasmussen et al., 1999), thus, amino groups are mainly lysine residues in milk proteins and the reducing sugar in milk is lactose, a disaccharide of glucose and galactose. Furthermore, MR in milk protein-lactose is also a potential way to produce antioxidant products in previous study (Mcgookin and Augustin, 1991), but the reaction in milk powder is difficult to regulate and control, because it is such a complicated reaction with many parallel and consecutive steps. Then we constructed two milk-resembling models (CN-L and CN-WPI-L) to study the MR in milk powder.
The MR of milk proteins is known to occur ubiquitously during processing and storage of milk (Gaucher et al., 2008) and milk powder (Havea, 2006). The Maillad reaction products (MRPs) produced in milk protein-sugar systems in alkaline conditions have been studied by a number of investigators (Ajandouz et al., 2008; Gu et al., 2009). But to the best of our knowledge, the study of MRPs produced in acidic conditions are very scarce, and the research on the pH according to the food system is of significance (Van Boekel, 2006). Furthermore, protein type is one of the numerous factors controlling the MR rate, and its choice is critical because it will remarkably impact the biological activities or functional properties of the MRPs. Meanwhile, You et al. (2009) indicated that MRPs of casein-lactose were potential antioxidants for food applications, but the antioxidant ability in the digestive tract of the human body are unclear. Therefore, the conjugates of milk protein with lactose under different pH were selected as model systems in this study, and the aim of this research is to study the variations of these two models (CN-L and CN-WPI-L) at different pH values in a microwave field compared with that of original skim milk powder. Functional properties and structure of the final products as well as the antioxidant of these conjugates during in vitro pepsin—pancreatin simulated digestion were investigated. The results might provide some theoretical foundation for the produce antioxidants from naturally occurring substances in milk for incorporation into full-cream milk powders.
Materials Casein, 2, 2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS), O- phthaldialdehyde (OPA), 2, 2-Diphenyl-1-picrylhydrazyl (DPPH), pancreatin and L-leucine were purchased from Sigma Chemical Co. (St. Louis, MO, USA); Whey protein isolate (WPI) was purchased from Mingrui Co. Ltd (Zhengzhou, China); α-lactose was purchased from Aladdin Chemistry Co. Ltd; Pepsin (1:3000) was purchased from Solarbio; Mark (10 k-170 kDa) was purchased from Thermo Scientific (Lithuania). The other solvents/chemicals all obtained from Tianjin Chemical reagent Co., Ltd. (Tianjin, China). All other chemicals were of analytical reagent grade.
Preparation of CN-L and CN-WPI-L model system The milk-resembling model systems were assembled according to available reference (Mudgett et al., 1971; Stanciuc et al., 2010): Casein: lactose solution at a ratio of 1: 1.6 (CN-L) and casein: whey proteins isolate: lactose solution at a ratio of 1: 0.2456: 1.579 (CN-WPI-L) were prepared in 100 mL 10 mM phosphate buffer at pH 8.0 and 6.6, respectively, then the mixtures was lyophilized and stored at 4°C for further analysis.
Microwave treatment Microwave heating was carried out in a laboratory scale. The MRPs were produced by heating the milk models of CN-L and CN-WPI-L (in beaker) for 15 min at 400 W in a microwave field (Mcgookin & Augustin, 1991). The samples were treated under a vacuum in a desiccator equilibrated at aw of 0.44, achieved with a saturated K2CO3 before microwave heating. In addition, the controlled experiments were performed with casein (CN) and casein-whey protein isolate (CN-WPI) treated at the same condition without lactose. After incubation, the products were reconstituted in distilled water to a protein concentration of 1 mg/mL. To remove free carbohydrate, 2 mL portions were dialyzed (3500 Da dialyzing membrane; Solarbio, China) against fluid distilled water at 4°C for 24 h, then the solution was centrifuged at 8000 rpm for 10 min, and the supernatant was freeze-dried for later analyses (Huang et al., 2012). Incubations were performed in duplication, and all analytical determinations were done at least in duplication.
In vitro pepsin—pancreatin simulated digestion Simulated digestion using an in vitro pepsin—pancreatin hydrolysis was carried out according to (Cinq-Mars et al., 2007; Zhu et al., 2008) with a slight modification. The microwave treated milk-resembling models were re-dissolved (1% w/v, in distilled water) and adjusted to pH 2.0 with 1 M HCl. Then pepsin (4% weight as received/weight of protein in the powder (85% protein)) was added. The mixture was incubated at 37°C for 1 h. The pH was then adjusted to 5.3 with 0.9 M NaHCO3 solution and further to pH 7.5 with 1.0 M NaOH. Pancreatin was added (4% weight as received/weight of protein in the powder (85% protein)), and the mixture was further incubated at 37°C for 2 h. To terminate the digestion, the test tubes were kept in boiling water for 5 min, cooled to room temperature and centrifuged at 4000 rpm for 15 min. The supernatant was lyophilized, sealed in plastic bags and stored at 4°C before use. To investigate the changes in antioxidant activity of microwave treated milk-resembling models peptide digests during the simulated digestion, aliquots of digests were removed at 0 (Blank), 1.0 (switch from pepsin to pancreatin) and 3.0 h during the in vitro digestion (You, Zhao, Regenstein, & Ren, 2010).
Measurement of degree of hydrolysis Degree of hydrolysis (DH) was operationally defined as the percentage of free N-terminal amino groups cleaved from proteins, which was calculated from the ratio of α-amino nitrogen to total nitrogen. The DH of samples was evaluated according to the OPA method described by (Guan et al., 2010; Rao et al., 2011), and calculated as
 |
Where h = the number of peptide bonds broken and htot = the total number of bonds (8.3 mmol/g of CN and 8.8 mmol/g of WPI). L-leucine was used as a standard in the DH assays.
Measurement of UV-absorbance and browning The UV-absorbance and browning of MRPs samples were measured according to the method of (Ajandouz et al., 2001) with minor modifications. UV-absorbance at 294 nm was used to monitor the MR rate (Ajandouz et al., 2001). The final stage of the browning reaction was monitored by the increase in absorbance at 420 nm. Appropriate dilution was made using distilled water and the absorbance was measured at 294 nm (10-fold dilution) and 420 nm (5-fold dilution) using a UV-spectrophotomerer for UV-absorbance and browning intensity, respectively.
Solubility of CN-L and CN-WPI-L conjugates Protein solubility (PS) was determined according to the method of (Liu et al., 2011) with a minor modification. The pH of untreated and treated casein-lactose solutions (1 mg/mL) and casein-whey protein concentration-lactose solutions (1 mg/mL) were adjusted to pH 5.0 and pH 7.0 with 0.1 N HCl solutions. An aliquot of the solutions was centrifuged for 30 min at 8000 rpm, the protein content of the resulting supernatant fraction filtrate was determined by the BioRad protein assay method (Bradford, 1976). PS was expressed as a percentage of the total protein content of the dispersion before centrifugation. Solubility analysis was carried out in triplicate
 |
Measurement of fluorescence Fluorescence of MRPs samples were determined as described by (Lertittikul et al., 2007) with some modifications. Protein solutions (5 mg/mL) were prepared in 10 mM phosphate buffer (pH 7.0). The fluorescence intensity was measured at an excitation wavelength of 347 nm and an emission wavelength recorded from 350 nm to 550 nm at a constant slit of 5.0 nm for both excitation and emission using a Fluorescence spectrophometer (F-7000, Hitachi, Tokyo, Japan). All the determinations were conducted in triplicate.
Determination of free amino group content Free amino group content was determined by ninhydrin method of OPA described by (Guan et al., 2010; Rao et al., 2011). The OPA reagent was prepared according to (Caillard et al., 2009). Briefly, the reagent was freshly prepared before use exactly OPA (80 mg, dissolved in 2 mL 95% methanol), 50 mL 0.1 M sodium tetraborate buffer solution, 5 mL 20% SDS and 0.2 mL 2-mercaptoethanol were mixed together and then diluted with distilled water to 100 mL. The tested MRPs (200 µL, 5 mg/mL) were mixed with 4.0 mL OPA reagent. After being shaken and laid in the dark at 35°C for 2 min, the absorption at 340 nm was measured immediately. L-leucine was used to prepare standard curve for amino group measurement. The content of free amino group was calculated using the standard curve. Results were the average of three measurements and were expressed as free amino group content loss (%).
Electrophoresis (SDS-PAGE) SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 5% stacking gel and 12% running gel with a vertical gel electrophoresis unit. Protein (10 µg, 1 mg/mL 10 µL) was added into the gel. Using a power supply set 8 A/gel for the stacking gel and 15 A/gel for the resolving gel. After separation, the gels were stained using R-250 (0.125 g) in 250 mL (227 mL methanol and 23 mL acetic acid) stationary liquid for 1 h. Destaining was performed using 7% acetic acid (Guan et al., 2010). The band patterns were then photographed. Molecular weights were estimated by comparison with the migration rates of standard protein (Gambuti et al., 2006).
Antioxidant activity assays All the antioxidant methods assayed were performed as stated previously. The antioxidant activity was measured from the microwave treated samples and native samples after the in vitro digestion.
DPPH method DPPH radical scavenging activity of MRPs based on the scavenging of the stable DPPH free radical was measured according to the method of (Hwang et al., 2011), with some modifications, Aliquots of 2 mL of 0.2 mM DPPH methanolic solution were mixed with 2 mL of the samples (1 mg/mL, 10-fold dilution). The mixture was shaken vigorously and then kept at room temperature for 30 min under dark to keep away from light. The absorbance was measured at 517 nm using a TU-1900 spectrophotometer (TU-1900 PuXiTongYong, Beijing, China). The control was prepared in the same manner, except deionized water was used instead of samples. The DPPH inhibition of activity was calculated as follows:
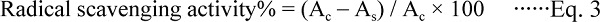 |
Where Ac was the absorbance value of the control without sample and As was the absorbance of sample. All samples were analyzed in triplicate.
Determination of Trolox equivalent antioxidant capacity (TEAC) The determination of TEAC is based on the method of (Arts et al., 2004; Liu and Kitts, 2011). The ABTS [2, 2′-Azinobis-(3-ethylbenzothiazoneline-6-sulfonic acid)] radical scavenging effect of MRPs were compared by determining the percentage of decolorization at 734 nm after 10 min of reaction at room temperature(Re, Pellegrini, Proteggente, Pannala, Yang, & Rice-Evans, 1999). The effect of MRPs on scavenging ABTS radical caution was calculated according to the following equation;
 |
The standard curve was linear between 0 and 0.3 mg/mL Trolox. Trolox equivalent antioxidant capacity (TEAC) = slope sample/slope control. Results were expressed in mmol Trolox equivalent (TE)/g freeze dried sample (Hwang et al., 2011).
Reducing power method The reducing power of the MRPs fraction was determined according to the method of (Gu et al., 2010) with modifications. One milliliter of MRPs (protein 1 mg/mL) was mixed with 2.0 mL of 0.2 M sodium phosphate buffer (pH 6.6) and 2.0 mL potassium ferricyanide (K3Fe(CN)6) (1 g/100 mL). The reaction mixtures were incubated in a temperature-controlled water bath at 50°C for 20 min, followed by addition of 2.0 mL of 10% trichloroacetic acid after cooling to room temperature. The mixtures were then centrifuged at 4000 rpm using a centrifuge for 10 min. The supernatant obtained (2.0 mL) was treated with 2.0 mL of distilled water and 0.4 mL of 0.1% FeCl3. The absorbance of the reaction mixture was measured at 700 nm with a TU-1900 spectrophotometer. Results were the average of three measurements and expressed as absorbance units.
Microstructural evaluation Samples were prepared by sticking the pectin onto double-sided adhesive tape attached to a circular specimen stub. The samples were viewed using an environmental scanning electron microscope (ESEM) (Quanta200F, FEI Deutschl and GmbH, Kassel, Germany) at 10 kV voltage and 500 × magnification. High vacuum mode was used while operating the ESEM.
Statistic analysis All the experiments were carried out in triplicate and the data obtained were subjected to statistical analysis, using One-Way analysis of variance (ANOVA); Least signicant difference (LSD) with a confidence interval of 95% was used to compare the means.
Changes in browning and A294 The color progress in MR was investigated by measuring absorbance at 294 and 420 nm, and As seen from Fig. 1, a slight increase browning and a sharp increase in UV absorbance at 294 nm of all samples were observed after microwave heating, suggesting the formation of UV-absorbing intermediate compounds prior to generation of brown pigments. All the native samples showed a zero value in absorbance at 294 nm. The sharp increase in absorbance at 294 nm suggested the formation of uncolored compound, which could be the precursor of brown pigment formation in the MR and caramelization reactions (Huang et al., 2012).

UV-vis absorbance of MRPs: CN-L pH8.0; CN-L pH6.6; CN-WPI-L pH8.0; CN-WPI-L pH6.6. Error bars show mean standard deviation of three determinations. Different letters on the top of the bars denote significant difference (p < 0.05).
In the case of pH 6.6 and pH 8.0, our results were not in agreement with previous reports, which showed alkaline environment could promote the MR and had higher absorbance than sour environment (Gu et al., 2009; Lertittikul et al., 2007). The explanation might be that casein was a open-chain, randomcoil tertiary structure (Hiller and Lorenzen, 2010), and easily to form a network structure in acidic condition and more free amino groups exposed to react with lactose (Stanciuc et al., 2010). Martins and Van Boekel, (2005) reported that the initial pH value of the reaction system was considered to affect MR significantly. The CN-L and CN-WPI-L model showed a better value in absorbance at A294 nm of pH 6.6, and followed by pH 8.0. The increased absorbance might result from the MR, and heating at pH 6.6 had more pronounced effects on the casein amino groups probably due to the disturbance of micelle structures, rendering lysine available for reticulation (Mcgookin & Augustin, 1991).
Intrinsic fluorescence emission spectroscopy Intrinsic tryptophan fluorescence emission spectra of native and treated samples (CN-L, CN-WPI-L and skim milk powder) were presented in Fig. 2, when excited at 347 nm, the samples exhibited a fluorescence emission maximum around 415 nm. Bosch et al. (2007) reported that fluorescence intensity was in consistent with the degree of MR. The maximum absorption peak was around 415 nm, which was in accordance with the absorption peak of MRPs (Obayashi et al., 1996). The fluorescence intensity of all the treated samples were increased and it was accompanied by a slight blue shift compared to the native samples, indicating that a relatively increased exposure of tryptophan toward more hydrophilic surroundings and the MR produced some fluorescent substances (Corzo-Martinez et al., 2008). In the case of milk protein-lactose and skim milk powder conjugates, the intensity was significantly increased, which can be attributed to the MRPs of milk protein-lactose with fluorescent characteristic, and high degree of MR possessed of high fluorescence intensity. Among the five conjugates, the CN-L pH 6.6 showed the highest intensity, followed by CN-WPI-L pH 6.6, suggesting that the conformational changes were more heavily affected by pH and less affected by protein pattern. Overall, the fluorescence intensity values for milk-resembling model system (CN-L) were a little higher compared with those for (CN-WPI-L), suggesting the casein heated with WPI-L was less active than heated alone with lactose. Van Boekel, (1998) remarked that lysine residues in caseins were more reactive than in serum proteins, while κ-casein seemd to be the most reactive casein. In the five conjugates, skim milk powder showed the lowest fluorescence intensity, an explanation might be the complex protein system of skim milk hided the amino terminal and disturbed the MR.

Intrinsic fluorescence intensity of milk protein conjugates with lactose and its native samples
Changes in solubility% (pH 5.0 and pH 7.0) Solubility properties is vital to casein, numerous studies have showed that this kind of protein is hardly to dissolve, limiting its applications in many aspects. Table.1. showed the solubility properties of CN-L, CN-WPI-L, skim milk powder and its conjugates at pH 5.0 and pH 7.0. The solubility of samples at pH 5.0 and pH 7.0 was increased dramatically after treated by microwave compared to the native samples. Treated samples of pH 6.6 both showed a maximum solubility at pH 7.0 and pH 5.0. The solubility at pH 5.0 was a pH which closed to isoelectric point (PI) of protein, the PI of unheated milk protein was not markedly affected by pH. The result was in agreement with Mcgookin and Augustin, (1991), Which reported that microwave caused the PI of casein changed when the pH of the casein solution was pH 6.8 but there was no substantial change in PI on heating when the pH of the casein solution was 7.8 or 8.8. All the microwave treated samples showed a significantly (p < 0.05) higher solubility than the native samples, which could attribute to the shift minimum solubility (PI) of glycated protein to a lower pH, according to the previous results derived from report (Corzo-Martinez et al., 2008).
| Solubility | CN-L pH 6.6 | CN-L pH 8.0 | CN-WPI-L pH 6.6 | CN-WPI-L pH 8.0 | Skim milk powder | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | Treated | Native | Treated | Native | Treated | Native | Treated | Native | Treated | Native |
| pH 5.0 | 52.32 ± 0.42h | 36.38 ± 0.86b | 44.80 ± 0.64e | 36.33 ± 0.23c | 45.91 ± 0.16e | 38.41 ± 0.32d | 44.93 ± 0.64e | 38.43 ± 0.32d | 46.71 ± 0.34f | 20.48 ± 0.23a |
| pH 7.0 | 85.79 ± 7.23d | 74.26 ± 1.29a | 88.29 ± 5.21e | 72.78 ± 0.65a | 83.23 ± 3.45c | 74.97 ± 3.87a | 82.60 ± 0.85bc | 75.51 ± 0.78a | 80.68 ± 0.43b | 75.64 ± 0.83a |
The values presented are mean ± S.D. Different letter indicate significant (p < 0.05) difference within the table.
Generally speaking, the solubility of proteins decreases at pH around its PI. At this point, the net charge of proteins is near zero and the proteins tend to aggregate due to the electrostatic interactions caused by the charge asymmetry of the proteins (Mu et al., 2010). With respect to the glycation effect at pH 6.6 which showed significantly (p < 0.05) increased solubility of milk protein, this might be due to the formation of soluble aggregates during the primary stages of MR. The protein type showed significant effect on the solubility of conjugates at pH 7.0, which were determined by the structure of protein. The initial pH had a higher influence on the solubility of conjugates at pH 5.0, these were determined by reaction degree of MR.
Changes in digestibility As shown in Fig. 3, the digestibility of the native and treated samples showed no significance difference (p > 0.05). They increased slightly (p > 0.05) when the peptides were digested by pepsin from 0.0 to 1.0 h. Further incubation with pancreatin (from 1.0 to 3.0 h) produced a significant in digestibility (p < 0.05). This indicated that the pepsin might break the protein into smaller fractions, but the pancreatin might have more activity in hydrolyzing some of the peptide into even smaller peptides and possibly amino acids (You et al., 2010). Overall, the digestion ability of samples was higher than that of native samples, which indicated the microwave treated samples were easily digested to some extent. Especially, the samples of CN-L gave the highest digestibility during the digestion time with a digestibility descending order: CN > CN-WPI > skim milk powder, which demonstrated that protein pattern showed significant effect on digestibility (p < 0.05). Generally, the structure of open-chain, random-coil tertiary structure (casein), of compact, globular structure (whey protein isolate), and of a more complex protein system (total milk protein in skim milk powder) (Hiller and Lorenzen, 2010). Then the different structure of milk protein rendered a different digestibility which was shown in Fig. 3. This result was in accordance with the solubility of protein at pH 7.0 which was shown in table.1, indicating a higher solubility obtained a higher digestibility. Then the microwave treated samples can be used as food additives due to its' high digestibility and the evaluated solubility.

The digestibility of milk protein conjugates with lactose and its' native samples in vitro digestion. Error bars show mean standard deviation of three determinations.
Reducing power During the reducing process where the ferric ion (Fe53+) is converted to the ferrous ion, Fe3+ acts as the electron acceptor. Thus, reducing power is indicative of the antioxidant capacity of converting radicals (electron accepters) to stable products by electron donation (Duh, 1998). An increased reducing power following proteolytic hydrolysis is attributed to the exposure of proton/electron donors of specific side-chain groups. However, pepsin digestion actually decreased the reducing potential of microwave treated samples (Fig.4). Then the reducing power was quickly recovered upon ensuing pancreatin digestion, suggesting an increased exposure of specific hydrogen donor amino acid residues. This result was consistent with report of Ma and Xiong, (2009). It was plausible that limited peptide cleavage by pepsin promoted hydrophily association of peptides and degraded some kind of reductones, therefore, lowered the availability of potential electron donors (Yen et al., 1993). Further incubation with pancreatin produced some even smaller peptides and possibly amino acids, eventually enhanced the reducing power. Overall, the milk protein-lactose and skim milk powder under microwave treatment had significant difference (p < 0.05) with each other and showed stronger reducing power, the reducing power descending order: CN-L pH 6.6 > CN-WPI-L pH 6.6 > CN-L pH 8.0 > CN-WPI-L pH 8.0 > Skim milk powder.

Reducing power of milk protein conjugates with lactose and its' native samples. Error bars show mean standard deviation of three determinations.
DPPH radical-scavenging activity A strong scavenging for the alcohol-soluble DPPH radical was shown in Fig. 5. The DPPH radical scavenging activity of the microwave treated samples was 50 – 90% (p < 0.05), while the scavenging activity were significantly (p < 0.05) decreased to around 40% during incubation with pepsin, and further incubation with pancreatin did not bring any further significant changes of activity (p > 0.05). This might be explained that MRPs hydrolysis improved the hydrophilicity of the samples while DPPH had poor dispersity in water, therefore making the digestion samples more difficult to react with the lipid-soluble DPPH radical (Zhu et al., 2008). The antioxidant activity of microwave treated samples was higher than that of native samples during digestion, which indicated that glycated protein had stronger radical-scavenging activity (Chevalier et al., 2001). Overall, samples of CN-L and CN-WPI-L prepared at pH 6.6 showed higher DPPH scavenging activity, suggesting a higher degree of MR leading to stronger scavenging activity. The possible reason might be that casein is easily to form a network structure in acidic condition and more free amino groups exposed to react with lactose (Stanciuc et al., 2010). The complex composition of skim milk powder and the electrostatic interactions occurring in the micelle structures of casein might disturb the MR in skim milk powder (Ajandouz et al., 2008), then showed a lower DPPH radical-scavenging activity.

DPPH radical-scavenging activity of milk protein conjugates with lactose and its' native samples. Error bars show mean standard deviation of three determinations.
ABTS+ radical-scavenging activity A strong scavenging for the water-soluble ABTS+ radical expressed as TEAC is shown in the Fig. 6. The TEAC value of milk protein-lactose conjugates digests increased sharply (p < 0.05) and reached to a maximum value of 1.15 mmol/g at 3 h digestion by pancreatin. The result indicated that the DH was improved during the digestion process. More and more polar or charged amino groups were emerged, resulting in the unbalanced ratio of polarity to nonpolarity. ABTS had a fairly good dispersity in water, the ABTS+ scavenging capability was increased gradually during digestion. These results were consistent with Ma and Xiong, (2009). The structure of proteins were hydrolyzed into polypeptide chain and further destroyed during the pancreatin incubation, forming lots of short peptides and free amino acids, which would make the digestion products more accessible to the ABTS+ radicals and allow them to trap the radical more easily. Therefore, the improved hydrophilicity of digestion product enhanced the ABTS+ radical scavenging activity. Overall, the microwave treated sample and native sample both showed the same trend of ABTS+ radical-scavenging activity. Samples of CN-L at pH 6.6 and 8.0 showed higher antioxidant activity during the digestion, suggesting that casein was more easily digested by pancreatin.

The ABTS+ scavenging capacity TEAC (mmol/g) of in vitro sequential digests of milk protein conjugates with lactose. Error bars show mean standard deviation of three determinations.
The digestion products of microwave treated samples showed different radical scavenging capacity of DPPH and ABTS. The radical scavenging principle had the most connection with the structure of digestion products. Chen et al. (1998) reported that the lack of protein hydrophobic led to relatively poor interaction with hydrophobic radical. Mendis et al. (2005) reported that the antioxidant activity of peptides extracted from squid skin had a connection with polarity-nonpolarity ratio of peptide chain.
Changes in free amino group content loss Fig. 7 showed the content of group amino loss of heated protein and protein-lactose compared with native samples. It reflected that when protein was heated without lactose under different pH, the free amino group content was reduced compared with the unheated samples, this may be due to the disulfide covalent bond between OVA and steric obstruction (Gu et al., 2009). Previous research had also found that amino losses in proteins when heated alone (Smith and Friedman, 1984).

Lines 1–4 (CN pH 6.6, CN pH 8.0, CN-WPI pH 6.6, CN-WPI pH 8.0) were the free amino group content loss of milk protein, the free amino group content loss of milk protein conjugates with lactose 5–9 (CN-L pH 6.6, CN-L pH 8.0, CN-WPI-L pH 6.6, CN-WPI-L pH 8.0, Skim milk powder). Error bars show mean standard deviation of three determinations. Different letters on the top of the bars denote significant difference (p < 0.05).
When milk protein was conjugated with lactose, the free amino group content was decreased significantly, especially the casein-lactose pH 6.6. In five conjugates, the loss of free amino group content descending order: CN-L pH 6.6 > CN-WPI-L pH 6.6 > CN-L pH 8.0 > CN-WPI-L pH 8.0 > skim milk powder, which suggested that MR rate was significant influenced by protein type and initial pH. Generally, pH 6.6 was help with the MR of milk protein-lactose, this result was in accordance with the previous report (Stanciuc et al., 2010). The skim milk powder showed the lowest loss of free amino group content. An explanation may be the complex composition of skim milk powder prevented the MR, which was proved in SDS-PAGE (Fig. 8). The decrease in amino group content was coincidental with increase in browning intensity and fluorescence intensity, which was in accordance with Lertittikul et al. (2007), who reported that free amino groups in protein-reducing sugar model system were decreased during the MR.

SDS-PAGE pattern of native and microwave treated samples. Lanes 1–5 were the microwave treated samples (CN-L pH8.0, CN-L pH6.6, CN-WPI-L pH8.0, CN-WPI-L pH6.6 and Skim milk powder), Lanes 6–10 were the native samples compared to channel 1–5.
Changes in protein pattern All the native (milk protein-lactose) and treated samples were analyzed by reducing SDS-PAGE shown in Fig. 8. For milk protein, the major milk proteins were CN and WPI. Previous study showed that protein bands were diminished by enzymatic digestion consistent with the DH analysis, especially when subjected to pancreatin where most protein bands vanished (Ma and Xiong, 2009), which indicated the good digestion of samples. In the condition of reducing condition, the aggregates were failed to dissociate, it meaned that some aggregates were linked by covalent, inter-molecular bonds, possibly conjugates of proteins and lactose. The new smear band with much higher molecular weight in lanes 1–5 from (Fig. 8) indicated the formation of milk protein-lactose conjugates compared with individual milk protein (Diftis and Kiosseoglou, 2006). The initial pH showed little significant difference on protein pattern of native samples, no native milk proteins were detected after 15 min microwave heating, skim milk powder seemed less active compared to the other four conjugates during microwave heating, the reason would be the condition in milk powder prevented the reaction of MR. These structural changes in the proteins were in good agreement with the amino group loss.
ESEM analysis Scanning microscopy analysis was used to characterize the topography modification of milk protein-lactose and skim milk powder under a microwave field. The microstructure of microwave treated samples and native samples were observed with ESEM and the images were presented in Fig. 9. The granules of native samples were seen as a regular and smooth flaky structure (Fig. 9. A2, B2, C2). As shown by ESEM, the images (Fig. 9. A1, B1, C1) revealed that microwave radiation resulted in the modification of milk protein structure, which was irregular and shape with nubbly and amorphous structure. Microwave radiation damaged the structure and caused it to be rugged. The pH and protein pattern showed little influence on the microscopy structure. The microscopy structure of treated skim milk powder was different from the other conjugates, which just overlaid milk protein with granules, but the A1 and B1 showed that the vesicular pore bulky grain by microwave heating. The change of microstructure may be connected with the solubility of milk protein-lactose. The higher degree of MR, the more obvious microstructure changed.

A1, B1, C1 were represented of treated CN-L pH 6.6, treated CN-WPI-L pH 6.6 and skim milk powder, the following Fig. 9. A2, B2, C2 were the native samples
The results indicated that the microscopy structure modification in treated samples was mainly caused by the function of assembled behavior and heating effect of microwave, which produced powerful microwave energy and caused temperature increase through acute rub of polar molecules.
In this study, milk protein-lactose and milk powder mixture in solution were subjected to microwave treatment at 400 W for 15 min. After microwave heating, PI of milk proteins shifted to more acidic environment, which may broaden the application of milk protein, allowing its use in acidic foods.
The milk protein-lactose conjugates at pH 6.6 exhibited stronger UV-vis absorbance and browning intensity as well as improved antioxidant activity (reducing power, DPPH scavenging activity and ABTS+ scavenging activity) in vitro digestion. The result of free amino group content loss was consistent with the improved fluorescence intensity and A294 nm, and the descending order: CN-L pH 6.6 > CN-WPI-L pH 6.6 > CN-L pH 8.0 > CN-WPI-L pH 8.0 > skim milk powder. The SDS-PAGE proved that the samples formed polymeric compound after microwave heating, and skim milk powder seemed less active compared to the other four conjugates. The ESEM analysis showed that microstructure become irregular, nubbly and amorphous after microwave heating, and the irregular microstructure render better solubility of conjugates. Future work will be focused on the mechanism of MR in milk-resembling model, the safety and nutrition of milk protein-lactose conjugates after microwave heating. Then the use of microwave dry process to prepare MRPs of milk protein-lactose as a new milk powder additive is very feasible.
Acknowledgements The authors gratefully acknowledge the financial support of the National Key Basic Research Program of China (973 Program) (No, 2012CB126314) and the National High Technology Research and Development Program of China (863 Program) (No, 2013AA102205). The freedom explore Program of State Key Laboratory of Food Science and Technology of Nanchang University (No. SKLF-ZZB-201310).