2014 年 20 巻 2 号 p. 505-507
2014 年 20 巻 2 号 p. 505-507
Cyanobacteria contain substances of high biological value and are used to meet the high demands of the global food and pharmaceutical industries. In this study, we prepared a hot water extract of the edible cyanobacterium, Nostochopsis, and evaluated its possible use as an ingredient in cosmetics. Specifically, a hot water extract of Nostochopsis was assayed for tyrosinase inhibitory activity. Tyrosinase, a key enzyme in skin melanin synthesis, was strongly inhibited by the Nostochopsis hot water extract, which reduced α-melanocyte-stimulating hormone-induced melanin synthesis in B16 mouse melanoma cells. Thus, Nostochopsis hot water extract may be suitable as an ingredient in cosmetics.
Cyanobacteria produce numerous bioactive compounds, most of which have therapeutic properties (Pulz and Gross, 2004). Cyanobacteria are thus attracting attention in the feld of microalgal biotechnology to meet the high demands of the food and pharmaceutical industries (Pulz and Gross, 2004). In northern Thailand, particularly in the Nan and Mekong rivers, the edible cyanobacterium, Nostochopsis sp., which is known as “Lon”, is highly abundant. Nostochopsis have a thick gelatinous covering. Peerapornpisal et al. (2006) reported that an ethanol extract of Nostochopsis exhibits various therapeutic properties, including anti-gastric ulcer, anti-infammatory, and anti-oxidant effects. Our recent study (Hashimoto et al., 2012) showed that Nostochopsis contains substantial amounts of pycobiliproteins, which act as light-harvesting accessory pigments. In particular, C-phycocyanin, a pigment protein (Eriksen, 2008), is a potent peroxy radical scavenger (Bhat and Madyastha, 2000), a platelet aggregation inhibitor (Chiu et al., 2006), and a proliferation inhibitor of cancer cells (Liu et al., 2000) both in vitro and in vivo. However, the pigment protein is heat labile and thus loses its therapeutic activities upon sterilization. Heat-stable and hydrophilic compounds are more suitable for use in manufactured health food products or cosmetics that are heat sterilized.
Melanin plays an important role in protecting skin from UV-induced injury; however, the excessive formation and accumulation of melanin in the skin cause hyperpigmentation disorders such as melasma, freckles, and geriatric pigment spots (Kim et al., 2005). Tyrosinase is a key enzyme that catalyzes the first two steps of melanin synthesis, i.e., the hydroxylation of tyrosine to 3-(3,4-dihydroxyphenyl)-L-alanin (DOPA) and the oxidation of DOPA to dopaquinone. Several tyrosinase inhibitors have been used as skin-lightening agents in the cosmetics industry (Lim et al., 2009); therefore, we examined the inhibitory effects of a Nostochopsis hot water extract on tyrosinase.
Materials Dulbecco's Modified Eagle's Medium (DMEM), mushroom tyrosinase, and synthetic melanin were obtained from Sigma-Aldrich Japan, Inc., (Tokyo, Japan). α-Melanocyte stimulating hormone (α-MSH) and 3-(3,4-dihydroxyphenyl)-L-alanin (DOPA) were purchased from Wako Pure Chemical, (Osaka, Japan). Phosphate-buffered saline (PBS) and trypsin solution used for cell culture were obtained from Life Technologies Corp., (Carlsband, CA). All other reagents were of the highest commercially-available purity. Nostochopsis sp. was typically grown for 10 days in BG11-N medium under illumination, and the collected cells were stored at −30°C until use.
Cell culture B16 melanoma (mouse melanoma-producing melanin, RCB1283) cells were purchased from Riken Bio Resource Center (Tsukuba, Japan). B16 cells were cultured in DMEM supplemented with 10% (v/v) fetal bovine serum (Biowest, Paris, France) and penicillin/streptomycin (Wako) in air containing 5% CO2 at 37°C. During incubation, the medium was changed every 3 days.
Preparation of the hot water extract of Nostochopsis sp. Nostochopsis (1 kg wet weight) was added to 500 mL of distilled water and autoclaved for 20 min at 121°C. The solution was fltered through a bleached cotton cloth, and the filtrate was then lyophilized in a freeze-dryer (Yamato Co., DC800) and used in the experiments.
Assay of tyrosinase inhibitory activity The lyophilized hot water extract was dissolved in distilled water to prepare a 10 g/L extract solution, which was used for the experiments. The tyrosinase reaction mixture contained 500 µL of 0.5 mg/L DOPA, which was dissolved in 0.1 M 4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid (HEPES) buffer, pH 7.0, and the indicated amount of the hot water extract solution. The control mixture contained distilled water instead of the hot water extract. The mixtures were preincubated for 1 min at 25°C, and then the enzymatic reaction was initiated by the addition of 100 µL of mushroom tyrosinase (50 unit/mL), which was dissolved in 10 mM HEPES buffer (pH 7.0) and allowed to stand for 6 min. Changes in absorbance at 475 nm during the experimental time period were measured using a Shimadzu spectrophotometer (UV-2550). Tyrosinase inhibitory activity was calculated on the basis of the enzyme activities in the presence and in the absence of the hot water extract.
Assay for inhibitory activity of melanin synthesis using a cell culture system The Nostochopsis hot water extract solution (10 g/L) was sterilized in an autoclave and used for this experiment. Melanin content was determined as follows. In brief, B16 cells were cultured in the presence of 10 nM α-MSH with 5.0 × 104 cells/well in 12-well plates. After 24 h, the cells were treated with the indicated amount of the hot water extract or arbutin as a positive control for 72 h. After washing with PBS, the cells were harvested by trypsinization. Each cell pellet was treated with 1 mL of 1M NaOH at 80°C for 1 h. The absorbance of each cell extract was measured at 475 nm using a Shimadzu spectrophotometer. Synthetic melanin was used as a standard. Cell viability was analyzed by trypan blue dye exclusion staining.
Statistical analyses B16 cell viability and melanin concentration were analyzed using a one-way ANOVA with Tukey's multiple comparisons test. The analyses were performed using GraphPad Prism® for Windows, version 5.03 (GraphPad Software Inc., La Jolla, CA). All data were expressed as means ± standard deviation, and significant differences were defined as p < 0.05.
Effects of the Nostochopsis hot water extract on mushroom tyrosinase activity As shown in Fig. 1, mushroom tyrosinase activity was significantly inhibited by the addition of the Nostochopsis hot water extract up to 1.0 mg/mL, after which the enzyme inhibition rate gradually decreased up to 4.0 mg/mL (approximately 80% inhibition). The results indicated that tyrosinase inhibitors were present in the Nostochopsis hot water extract.
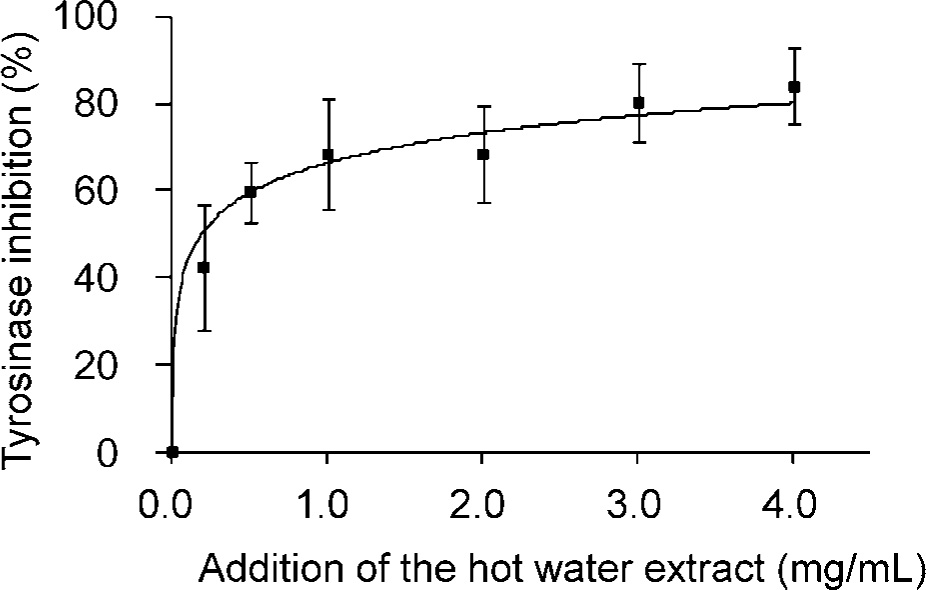
Inhibitory effect of the Nostochopsis hot water extract on mushroom tyrosinase.
Nostochopsis hot water extract (10 g/L) was used for the experiments. The tyrosinase reaction mixture containing 500 µL of 0.5 mg/L DOPA was dissolved in 0.1 M HEPES buffer (pH 7.0) and the indicated amount of the hot water extract was added (n = 3). Tyrosinase inhibitory activity was calculated on the basis of enzyme activity in the presence or absence of the Nostochopsis hot water extract. The values represent the mean ± SD from three independent experiments.
Effects of the Nostochopsis hot water extract on α-MSH-induced melanin synthesis in B16 cells We evaluated the effects of the Nostochopsis hot water extract on α-MSH-induced melanin synthesis in B16 cells (Fig. 2). The Nostochopsis hot water extract had no significant cytotoxic effects on B16 cells under the experimental conditions (Fig. 2 A). After stimulating B16 cells with α-MSH (10 nM) for 24 h, the cellular melanin content increased approximately three-fold in the presence of α-MSH compared to in the absence of this hormone. When B16 cells were cultured in the presence of 33 – 100 µg of the hot water extract per 1 mL of medium, α-MSH -induced melanin production was reduced in a dose-dependent manner by the hot water extract (Fig. 2 B). Nostochopsis extract (100 µg/mL) had an inhibitory effect of approximately 59.1%, which is identical to that of arbutin (100 µg/mL), a popular skin-lightening agent (Lim et al., 2009).
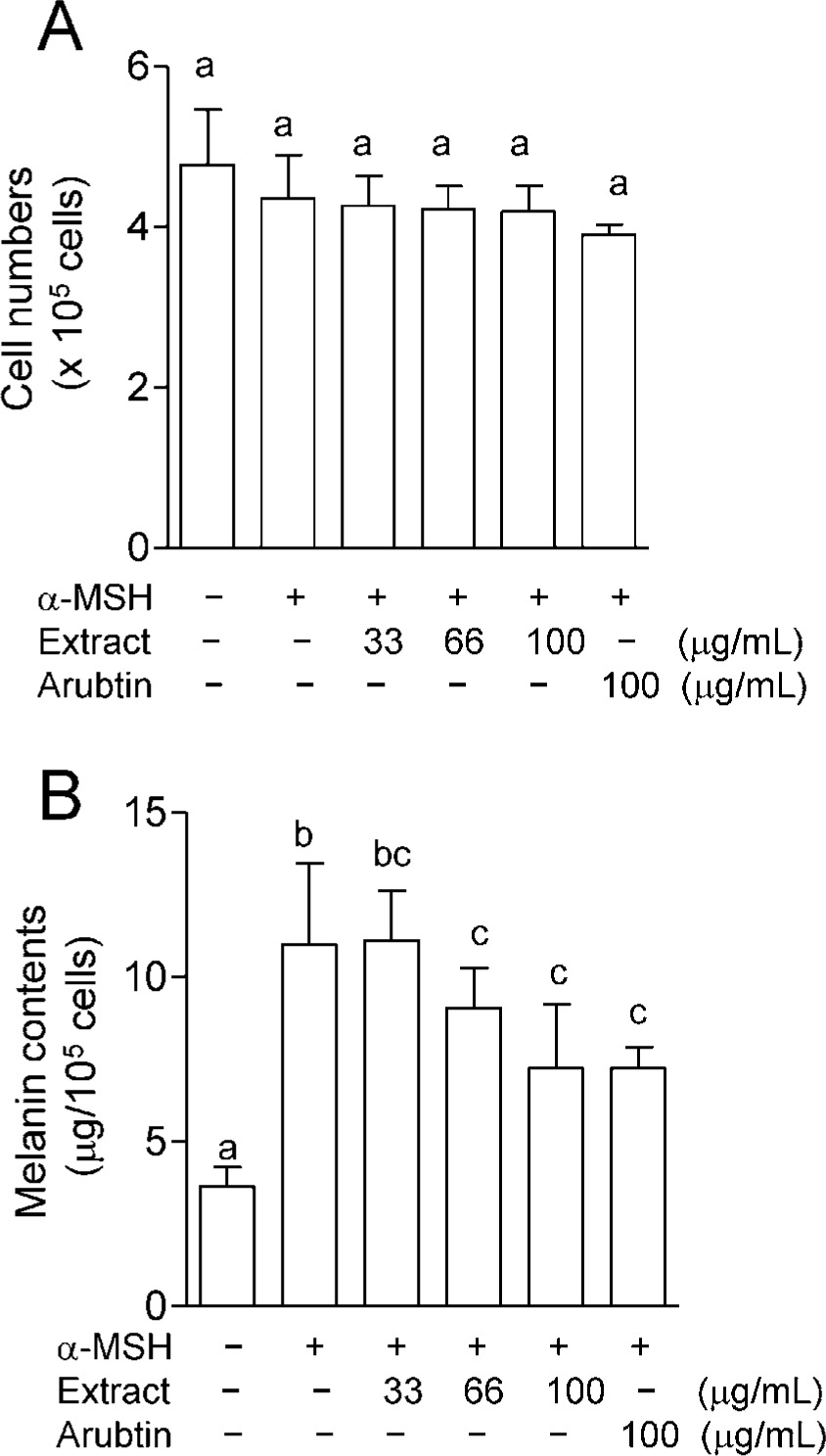
Effects of the Nostochopsis hot water extract on α-melanocyte stimulating hormone (α-MSH) -induced melanin synthesis by B16 cells.
The Nostochopsis hot water extract (10 g/L) was sterilized in an autoclave and used for the experiments. B16 cells were cultured at 5.0 × 104 cells/well in the presence or absence of 10 nM α-MSH. After 24 h, the cells were treated with the indicated amount of hot water extract, or with arubtin as the positive control, for 72 h (n = 3). After washing with phosphate-buffered saline, the cells were harvested by trypsinization. The cell pellets were treated with 1 mL of 1M NaOH at 80°C for 1 h. A) Cell viability. B) Melanin content. The melanin content of each cell pellet was measured at 475 nm using a spectrophotometer. Cell viability was determined by trypan blue dye exclusion staining. Values represent the mean ± SD from three independent experiments. Different letters indicate values that are significantly different (p < 0.05).
When the hot water extract was passed through a Sep-Pak C18 cartridge, most of the tyrosinase inhibitory activity was recovered in the non-absorbed fraction. This fraction was concentrated and loaded onto a Sephadex G-25 fine column. The active compounds were eluted in the low molecular weight fractions (data not shown). These preliminary experiments suggest that the inhibitory effects on mushroom tyrosinase activity and α-MSH-induced melanin synthesis in B16 mouse melanoma cells are attributable to low molecular compounds. One compound is probably a biline moiety (a potent peroxy radical scavenger) released from Nostochopsis pycobiliproteins by heat treatment (Yabuta et al, 2010).
These results presented here indicate that Nostochopsis hot water extract may be suitable as an ingredient in cosmetics, but further biochemical studies are needed to identify the chemical structures of the bioactive compounds.