2014 年 20 巻 3 号 p. 655-661
2014 年 20 巻 3 号 p. 655-661
Effect of cooking temperature (40–100°C) on changes in protein composition of big head carp muscle and exudate was investigated. The results showed that the proportion of salt-soluble and water-soluble proteins in cooked muscle decreased with temperature up to 50°C and 60°C, respectively. Alkali-soluble proteins content in muscle increased with increasing temperature in the range of 40–98°C, while both non-protein nitrogen (NPN) and alkali- insoluble proteins content showed no significant change. Content of soluble collagen in muscle and collagen in exudates increased with increasing temperature. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS- PAGE) patterns revealed disappearance of most muscle protein bands when the temperature reached 60°C for water-soluble proteins, and 50°C for salt-soluble proteins, but proteins with molecular weight (MW) about 37 kDa was observed when heated to 98°C. Visible β and a band of collagen in exudates were clear.
Fish is a source of high quality protein, vitamins and essential minerals. China is the largest producer of freshwater fish in the world. According to the statistical data from the Fishery Bureau of Ministry of Agriculture, the yield of freshwater fish was 21,854,100 tons in 2010 (China Fishery Statistic Yearbook, 2011). Big head carp is one of the major species produced in China and has a unique taste and texture, and is very popular among consumers especially in China, as well as in other Asian countries. It is rich in protein, carbohydrates, vitamin A, B, minerals, etc. Protein is the main nutritional ingredient of fish meat and the main supporting composition of muscle structure, and the changes of protein will directly affect the structure, textural and sensory properties of fish meat.
Thermal processing is one of the major steps in cooking or the process of food production, which can provide specific texture, flavor, and color of the food, as well the main measure to ensure food hygiene and safety. Different heating temperatures can cause varying degrees of degeneration or degradation of proteins in the muscle, leading to the differences in organizational characteristics, texture and sensory qualities (Palka and Daun, 1999). It is reported that the solubility of protein during heating is close related to its structure, while sarcomere length and collagen solubility are key factors that affect the cooking loss and texture of chicken (Wattanachant et al., 2005). Cooking loss and area shrinkage of salmon muscle are significantly correlated with shear force change during heating (Kong et al., 2007).
Changes in texture, flavor, color and other sensory qualities, also the protein solubility and collagen content of various muscles, including beef, pork, chicken, rabbit, shrimp, sea fish, etc. during heat treatment have been reported in other literatures (Cambero et al., 1998; Combes et al., 2004; Hashimoto et al. 1979; Kong et al. 2007; Light et al., 1985; Wattanachant et al., 2005). But few studies involved in the changes of protein composition in fresh water fish heated at different temperatures. The results obtained from different specie muscles could have something to do with the muscle type and structure, and thermal properties of protein, such as the protein compositions and solubility, as well as the content and nature of the intramuscular collagen.
In our previous study, the effects of heating temperature on the texture, color of big head muscle were studied, and the objective of this paper was to discuss the effects of heat treatments on the protein components of fish muscle, and further understand the relationship between the texture and protein composition. In addition, the biochemical characteristics changes of collagen in big head carp during heating were also studied by solubility analysis and SDS- PAGE.
Chemicals Tetramethylethylenediamine (TEMED) was purchased from Sigma- Aldrich (Shanghai, China). Protein molecular weight marker was obtained from TaKaRa Biotechnology Co., Ltd. (Dalian, China). Other reagents used were at least analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Sample treatments Big head carp (Aristichthys nobilis) weighing between 2.0–2.5 kg, was captured from Tai Lake (Wuxi, China). Then was transferred to the laboratory, the fish were beheaded, gutted and washed. The skin was removed and the muscles were trimmed of visible fat and connective tissue. The muscles were cut to the size 2.5 × 2.5 × 1.0 cm with the fibers parallel to the longest axis. The fish blocks were individually weighed, and packed into polyethylene bags with laboratory vacuum sealing machine (Golden Bridge Technology Co., Ltd., Guangdong, China). Samples were heated in water baths at 42 ± 0.5, 52 ± 0.5, 62 ± 0.5, 72 ± 0.5, 82 ± 0.5, 92 ± 0.5, 100°C until their core temperature reached 40, 50, 60, 70, 80, 90, 98°C, respectively.
Determination of crude protein content Crude protein content was determined by the Kjeldahl method (McGill, 1981) and was expressed as the percentage of the weight of fish blocks.
Determination of cooking loss Cooking loss was calculated from differences in the weight of raw and cooked fish blocks as Equation (1). The measurements were conducted in six replications.
 | Eq. 1 |
Where M1 is the weight of raw fish blocks before heated, M2 is the weight of both raw fish block and bag before heated. After heated, the liquid in the bag is removed and collected, and the weight of both the left fish block and bag is M3.
Determination of protein composition The protein components in fish were fractionated according to the method of Hashimoto et al. (1979) with a slight modification. Samples (4 g) were extracted with 10 volumes of solution A (15.6 mM Na2HPO4, 3.5 mM KH2PO4, pH7.5) using an Ultra-Turrax homogenizer (IKA, Guangzhou, China) at 8000 rpm for 1 min. The homogenate was centrifuged at 5000 x g for 15min. The extraction was repeated twice. The supernatants were combined and mixed with cold 50% TCA to a final concentration of 10%. The resulting precipitate was collected by centrifugation at 5000 × g for 15 min (the water- soluble fraction). The filtrate was the NPN fraction. The pellet was homogenized with 10 volumes of solution B (0.45 M KCl, 15.6 mM Na2HPO4, 3.5 mM KH2PO4, pH7.5) at 8000 rpm for 1 min and centrifuged at 5000 × g for 15 min. The extraction was repeated twice. The supernatants were combined (the salt-soluble fraction). The pellet obtained was extracted with 10 volumes of 0.1 M NaOH with continuous stirring for 2 h. The mixture was centrifuged and the supernatant was the alkali-soluble fraction. The final residue was the alkali-insoluble fraction. The nitrogen content of all protein and non-protein fractions was determined by the Kjeldahl method (AOAC, 2000). The water-soluble fraction and the salt-soluble fraction were analyzed by SDS-PAGE according to the method of Laemmli (1970).
SDS-PAGE analysis Raw and cooked muscle samples (3 g) were homogenized in 5% (w/v) SDS (27 mL) at 11,000 rpm for 60 s with a homogenizer. The water-soluble fraction (including water-soluble proteins and NPN), the salt-soluble fraction, and exudates from cooked muscle samples were solubilized in 5% (w/v) SDS. All mixtures were incubated at 85°C for 1 h, the extract was then centrifuged at 6100 × g for 10 min. The protein content of the supernatant was analyzed according to the Biuret method (Robinson & Hogden, 1940). SDS-PAGE was carried out by the method reported in the literature(Fritz et al., 1989; Laemmli, 1970). The supernatants were mixed at a ratio of 1:1 (v/v) with the SDS-PAGE sample buffer (0.125 M Tris-HCl(pH6.8), containing 4%(w/v)SDS, 10%(v/v)β-mercaptoethanol, 20%(v/v)glycerol and 0.005%(w/v) bromophenol blue) and boiled for 5 min. The samples (10 μL) were loaded onto the gel made of 4% stacking and 10% separating gels and then subjected to electrophoresis using a mini vertical apparatus (Bio-Rad, USA). After electrophoresis, the gels were stained with 0.125%(w/v) Coomassie Brilliant Blue R-250 in 50%(v/v) methanol and 10%(v/v) acetic acid, and decolorized with 50% methanol and 10% acetic acid for 2~3 h until the background is clear.
Extraction of soluble collagen Soluble collagen in heated muscles was extracted according to the method of Eilert and Mandigo (1993) with a slight modification. Muscle samples (2 g) were homogenized with 8 mL of 25% Ringer's solution (32.8 mM NaCl, 1.5 mM KCl, and 0.5 mM CaCl2). The homogenates were heated for 15 min at 50°C and centrifuged for 30 min at 2300 × g. The supernatant was decanted, and the pellet was suspended with the same solution and re-centrifuged. The supernatant solutions, which were the soluble collagen solution, were combined. The final residue was the insoluble collagen.
Analysis of collagen The extracted soluble collagen solution and insoluble collagen, as well as the exudates, were separately hydrolyzed with 3 M H2SO4 at 115°C for 16 h. The hydroxyproline content in the hydrolysates was analyzed by the standard of "Meat and meat products – Determination of Hydroxyproline Content (ISO 3496:1994, IDT)" and converted to collagen content using the factor of 9.75 (Zeng and Huang, 1982). While, the total collagen content in the cooked muscle was the sum of both extracted soluble collagen content and insoluble collagen content.
Statistical analyses A completely randomized design was used in this study. For all parameters, the determinations were done in duplicate, except for cooking loss, where the analyses were carried out with 6 determinations. Statistical analysis was done using SAS version 8.0 (1999, SAS Institute, Inc., Cary, NC, USA). Analysis of variance (ANOVA) using the General Linear Model procedure.
Changes of total protein and cooking loss in bighead muscle heated at different temperatures Crude protein content is the main nutritional index of fish muscle, and the changes of crude protein in cooked fish muscle were showed in Fig.1. A light increase in crude protein content was found, and reached to about 21.28 g/100 g when the core temperature above 90°C, which can be attributed to the cooking loss as shown in Fig.2. A significant increase in cooking loss was observed. In the range of 40~60°C, the cooking loss reached to the largest at the temperature of 50°C, which was mainly caused by the partial denaturation of myosin and the shrinkage of muscle fiber, leading to a loose gel network or failing to form a gel network, allowing the juice dripping out easily. This result was also corroborated by the phenomenon of pale red turbid exudates observed during the experiment, and the denaturation of water- soluble proteins above 50°C (Fig.5). With heat treatment above 60°C, myofibrillar proteins and water-soluble proteins almost entirely denatured, and formed a complete gel structure, which impeded the loss of juice. In the range of 60–80°C, the cooking loss increased with the increasing temperature due to the exacerbated shrinkage of the gel network. Above 80°C, the increase of cooking
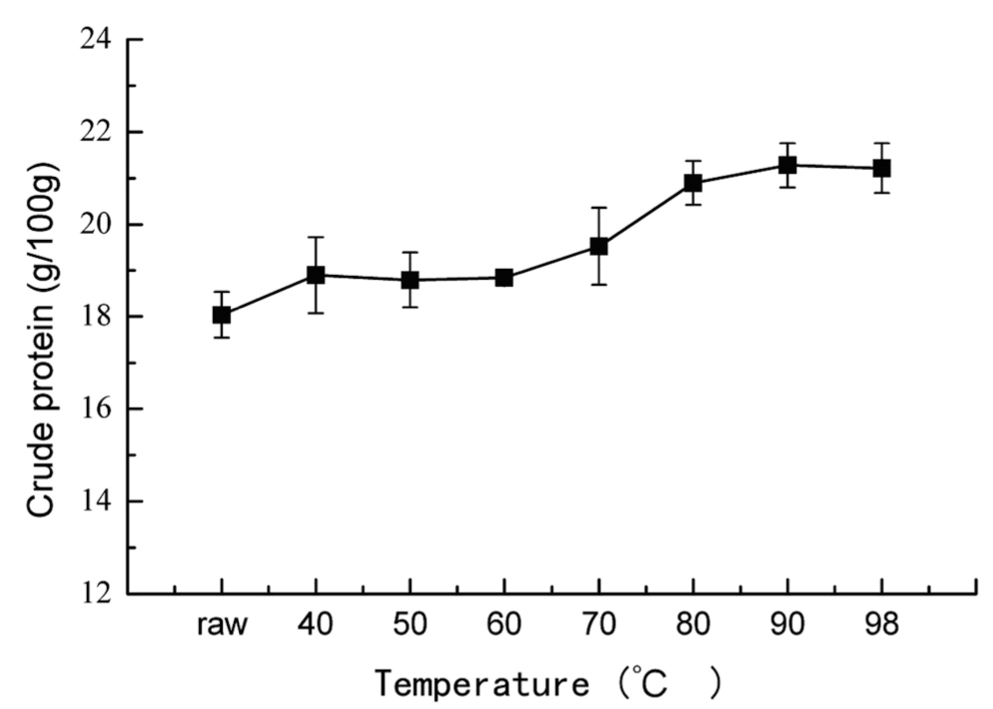
Effect of heating temperature on crude protein content in big head muscle.

Effect of heating temperature on cooking loss

Effect of heating temperature on the protein composition of big head muscle; Water-soluble proteins (Square), Non-protein nitrogen (Circle), Alkali-soluble proteins (Down Triangle), Alkali-insoluble proteins (Left Triangle), Salt-soluble proteins (Up Triangle). (Protein compositional changes in muscle)
loss decreased, possibly due to the denaturation of actin.
Electrophoretic studies of each protein fraction revealed the different protein component in big head muscle after heated at different temperatures. SDS-PAGE patterns of total protein from big head carp muscle during heating are shown in Fig.3. As can be seen, there are a variety of proteins in big head carp, mainly actin (43 kDa) and myosin heavy chain (200 kDa). No remarkable disappear in bands of protein was observed in the cooked muscle with increasing temperature, which indicated that the major protein in the bighead muscle did not show significant loss or degradation that can be detected by gel electrophoresis. Similar results in broiler muscle have also been reported in other studies (Wattanachant et al. 2005), in which the changes in SDS-PAGE patterns for broiler muscle during heating were not noticeable.
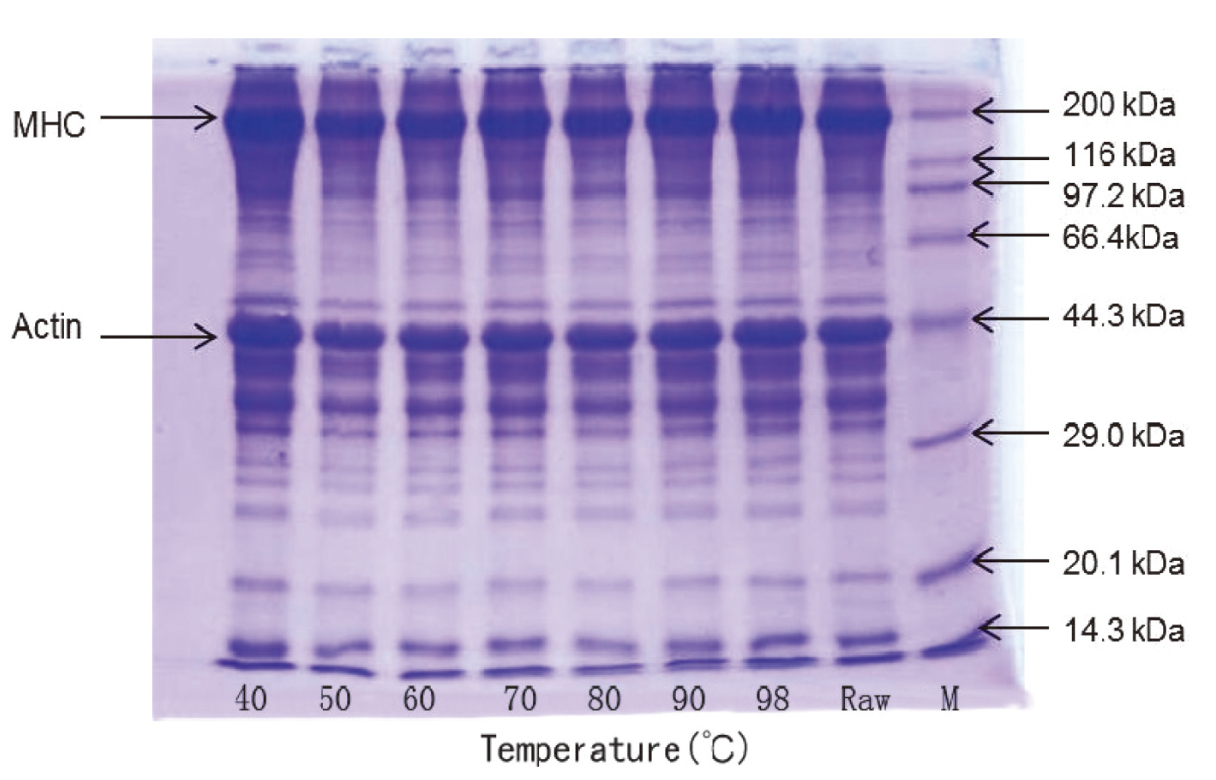
SDS-PAGE pattern of total protein from big head muscle heated at different temperature; M-protein molecular weight marker. (Total protein in muscle)
Changes of protein composition in bighead muscle heated at different temperatures Protein composition was classified into five fractions based on solubility, including water-soluble proteins, NPN, salt-soluble proteins, alkali-soluble proteins and alkali- insoluble proteins. Effects of heating temperature on the content of each fraction were shown in Fig.4. With the increase of temperature, significant changes in content of water-soluble proteins, salt-soluble proteins and alkali-soluble proteins were observed, and little changes for NPN and alkali-insoluble proteins.
In Fig.4, the content of NPN showed no significant change with the increasing temperature. This might be explained that NPN consisted of some AA and peptides with small MW, which were relatively stable to heat treatment.
As shown in Fig.4, the contents of water-soluble proteins in the cooked big head carp muscle decreased slightly with increasing temperature at the beginning, and showed a sharp decline at 60°C, followed by slow decrease in the range of 60 – 80°C and almost no changes when heated to above 80°C. The results could be attributed to the denaturation of sarcoplasmic proteins, which can be confirmed by the electrophoretic analysis. As can be seen from Fig.5, there were many clear bands of proteins with MW from 20 kDa to 97.2 kDa at the range of 40 – 60°C. All these water- soluble proteins in the muscle, also known as sarcoplasmic proteins, mainly include enzymes of the glycolytic pathway, creatine kinase and myoglobin. Tornberg (2005) reported that about 100 different proteins are known to be present in the sarcoplasmic fraction and they are globular proteins of relatively low MW ranging from 17 kDa (Myoglobin) to 92.5 kDa (Phophorylase b), this supports our observations. When the temperature reached above 60°C, the most distinct bands for protein with MW about 40 kDa, and the band of protein with MW about 97 kDa disappeared. With the increasing temperature, more and more protein bands were found to be thinner and lighter or even disappeared because of denaturation. However, protein bands with MW of about 37 kDa and 20 kDa were still noticeable even heated to 98°C, which were probably heat stable components. The results were similarly found in the exudates.

SDS-PAGE pattern of water-soluble proteins from big head muscle heated at different temperature; M-protein molecular weight marker. (Water-soluble proteins in muscle)
The salt-soluble proteins in the muscle, also known as myofibrillar proteins, mainly consist of myosin heavy chain (480 kDa) and actin (43 kDa), and also contain tropomyosin (65 kDa), troponin (70 kDa), connective protein (100 kDa), myosin light chain and a variety of micro-regulatory proteins, etc. Myofibrillar proteins play the most critical role during meat processing as they are responsible for cohesive structure and the firm texture of meat products (Visessanguan et al. 2004). As can be seen from Fig.4, changes in content of salt-soluble proteins were similar to water-soluble proteins, which exhibited a significantly decrease at 50°C, followed by slow decrease in the range of 50 – 80°C, and then insignificant change when heated to above 80°C. The extracted salt-soluble proteins were analyzed by SDS- PAGE electrophoresis and the results were shown in Fig.6. Salt- soluble proteins in the raw material were distributed in a broad range of molecular weights, and the comparative obvious bands resolving at MW above 200 kDa and about 43 kDa were tentatively identified as myosin heavy chain and actin, respectively. No remarkable decrease in bands of protein was observed at 40°C, which indicated that there was no denaturation of salt-soluble proteins at this temperature. When the core temperature rose up to 50°C, the bands with a MW above 66.4 kDa and less than 29 kDa disappeared, while the actin bands (43 kDa) become lighter significantly. When heated above 80°C, only a slight protein band at MW of 37 – 44 kDa were still visible, which may be relatively heat- resistant troponin-T(37 kDa).

SDS-PAGE pattern of salt-soluble proteins from big head muscle heated at different temperature; M-protein molecular weight marker. (Salt-soluble proteins in muscle)
The content of alkali-soluble proteins markedly increased with increasing temperature (Fig.4), which was mainly due to the denaturation of water-soluble sarcoplasmic proteins and salts- soluble myofibrillar proteins during heat treatment and conversion to alkali-soluble proteins.
On the other hand, the content of alkali-insoluble proteins showed a slight decline (Fig.4). Studies showed that alkali- insoluble proteins were mainly connective tissue, such as collagen. Collagen plays a role of supporting and connecting in vivo. In cooked meat, there is a certain relationship between muscle collagen content and meat texture and tenderness (Lepetit, 2007). Changes in collagen solubility with heating temperature could affect the textural and water-holding properties of the products (Eilert and Mandigo, 1993).
Total collagen content of big head muscle showed a slight fluctuating overall, but no significant changes (Fig.7), which was in accordance with changes in content of alkali-insoluble proteins (Fig.4). At temperatures between 53°C and 63°C the collagen denaturation occurs (Light et al., (1985)), which probably involves first the breakage of hydrogen bonds loosing up the fibrous structure and then the shrinkage of the collagen molecule. If the collagen fibers then are not stabilized by heat-resistant intermolecular bonds, it dissolves and forms gelatin on further heating (Tornberg, 2005).

Effect of heating temperature on the content of total and soluble collagen in big head muscle. Total collagen (Square), Soluble collagen (Up Triangle). (Total and soluble collagen in muscle)
On the contrary, a significant change was observed in the solubility of collagen from big head muscle during heating (Fig.7). The soluble collagen content of samples increased markedly with increasing temperature from 40°C to 70°C, and up to a maximum about 2.5 mg/g, then remained almost unchanged. Light et al. (1985) suggested that collagen began to shrink at 60~70°C with an increased solubility, and was converted to gelatin at 80°C. Although the temperature increases the solubility of collagen, cooking loss were higher than before when the temperature above 70°C (Fig.2), most soluble collagen or gelatin was transferred to the exudates, so the soluble collagen content was not changed too much when temperature above 70°C, as showed in Fig.7.
Changes in composition of exudates at different temperatures As can be seen from Fig.8, the collagen or gelatin content of exudates was increased with the increase of temperature when the temperature under 70°C, which further confirmed that the collagen solubility increased gradually with increasing temperature. No significant changes were found of collagen content when the temperature above 70°C.

Effect of heating temperature on total collagen content in exudates from big head muscle. (Total collagen in exudates)
To further characterize the changes of soluble collagen in muscles, the proteins in exudates at different temperature were analyzed by SDS-PAGE. As shown in Fig.9, the low MW proteins were observed in the exudates of cooked muscle at lower heating temperature and decreased with increased temperatures. In contrast, solubilized collagen (β and α band) was observed with increased temperature and become increasingly apparent especially above 60°C, which was in accordance with the results of Fig.8. On the other hand, the distribution of protein bands with MW less than 97.2 kDa was extremely similar to that of water-soluble proteins (Fig.5), which was largely due to that the exudates were mainly composed of water-soluble components. The protein subunits with MW of 43 kDa disappeared and that between 44.3 kDa and 97.2 kDa decreased gradually when heated to an end-point temperature of 60°C or higher. However, some proteins with MW of 35 kDa and 20.1 kDa showed more heat-stable, since the protein bands were still visible when heated to 98°C. A similar result was reported on the effect of heat treatment on changes in solubility of protein of Thai indigenous chicken muscle (Wattanachant et al., 2005).

SDS-PAGE pattern of total proteins from exudates of big head muscle heated at different temperature; M-protein molecular weight marker. (Total protein in exudates)
Myofibrillar proteins and sarcoplasmic proteins in the big head carp were denatured by different temperature treatments, leading to the decrease of its solubility. This caused changes on the contents of both water-soluble proteins and salt-soluble proteins. Water-soluble proteins reduced, whereas alkali-soluble proteins increased. It can be observed from the SDS-PAGE patterns that most bands of both water-soluble proteins and salt-soluble proteins disappeared with increasing temperature, while the insoluble proteins content gradually increased, which is caused by the thermal denaturation protein and the generation of condensation insoluble in water or salt solution. Moreover, heating caused the great cooking loss, especially along with the significant dissolving of collagen above 60°C according to the soluble collagen content in heated meat and SDS-PAGE patterns of exudates, which might be a major reason for the changes in the tenderness of thermal processed fish meat products.
This research was financially supported by the earmarked fund for China Agriculture Research System (CARS-46), the Fundamental Research Funds for the Central Universities (PCSIRT0627, 111 Project – B07029 and SNG201036). The research was also a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.