2014 年 20 巻 3 号 p. 705-710
2014 年 20 巻 3 号 p. 705-710
Three Allium vegetables, Welsh onion from Okinawa, and Wakegi from Okinawa and Nagasaki were subjected to salinity-stress using seawater. The K+/Na+ ratio of the three Allium vegetables clearly decreased; however, there was no significant effect on growth parameters. Of the three Allium vegetables, 10% seawater treatment of Welsh onion and Wakegi from Nagasaki clearly enhanced both the sugar content and antioxidant activity. Therefore, seawater treatment may be potentially useful for the development of value-added Allium vegetables, enhancing the palatability and food functionality.
Salinity is a major environmental factor limiting crop growth and productivity (Rhodales and Loveday, 1990). During the onset and development of salinity-stress within a crop plant, major processes such as photosynthesis, protein synthesis, and energy and lipid metabolism are affected, leading to qualitative and yield losses in most crops (Hasegawa et al., 2000; Hagemann and Erdmann, 1997; Hayashi and Murata 1998). However, there are several crops with an inherent capacity to withstand salinity-stress, which allows for stable vegetable production and significantly contributes to palatability and food functionality (Sato et al., 2006). Thus, there have been several reports on the application of salinity-stress for improving the quality of vegetables such as tomato fruits (Auerswald et al., 1999), spinach (Makabe and Tanii, 2008), and strawberry (Keutgen and Pawelzik, 2000).
Welsh onion (Allium fistulosum L.), a member of the Allium family, is a popular vegetable in Asian countries and a reputed good source of phenol compounds with antioxidant activity, leading to beneficial medicinal effects (Helen et al., 2000; Wang et al., 2006). Hence, we focused our attention on exploiting salinity-stress treatment for the development of value-added Welsh onion with augmented palatability and food functionality. There are relatively few studies on the salt tolerance of Welsh onion. In this study, we investigate the effects of artificial salinity-stress on the growth, relative chlorophyll content, and K+/Na+ ratio of Allium vegetables: Welsh onion and Wakegi (Allium x wakegi A.), and assess the effect of salinity-stress on sugar content, total phenol content, and antioxidant activity.
Cultivation The bulbs of three types of Japanese Allium cultivars, green-leafy Welsh onion (Okinawa) and two different cultivation lines of Wakegi from Okinawa and Nagasaki (Nagasaki-Wakegi) were used in this study. The upper end of each bulb was cut to adjust the overall length to 5 cm before cultivation. Each bulb type was randomly divided into four test groups of 15 plants each. One group was treated with distilled water (control), and the other three groups were treated with different concentrations of seawater (2% seawater: SW 1; 10% seawater; SW 2; 100% seawater: SW 3) to induce salinity-stress. Each type of bulb was cultivated in a Wagner pot (1/5000 a) with three plants per pot (total of five pots per test group; plant cultivation was initiated on November 19, 2012). After two weeks of cultivation, the plant leaves were thoroughly sprayed with seawater at 5 mL of seawater per plant, and the same treatment was repeatedly conducted four times in total every 5 days. The mineral content of the 100% seawater used in this study was as follows (mg/100 mL): Na+: 942.7; K+: 28.68; Ca2+: 38.5; Mg2+: 140.7. Five days after the last treatment, the edible portions of each plant were used in the experiment. In the case of growth parameter measurement, the plants (n = 15) were individually analyzed from five pots. Next, three pots of plants were randomly selected from five pots and promptly freeze-dried for mineral analysis. The remainder was collectively analyzed for sugar content, total phenol content, and antioxidant activity measurements, respectively.
Growth parameters and K+/Na+ ratio measurement The growth parameters (plant height, leaf blade diameter, total weight, number of leaves, number of tillers, number of broken leaves, number of dead leaves, and soil and plant analyzer development (SPAD) value as relative chlorophyll content) were evaluated. The SPAD value was measured using a chlorophyll meter (SPAD-502, Minolta Co., Osaka, Japan) and recorded as the mean of ten measurements for each individual leaf (Cho et al., 2007). The Na+ and K+ contents of the freeze-dried leaves were determined using inductively coupled plasma/atomic emission spectroscopy (Zeinera et al., 2005) at respective wavelengths of 330.232 nm and 797.395 nm, on an ICPE-9000 spectrometer (Shimadzu Co., Kyoto, Japan). The levels were expressed in terms of the K+/Na+ ratio.
Sugar content analysis The free sugar content was determined according to the method described by Miyagi et al. (2011). Briefly, a 5 mm section of each plant (10 g) was refluxed in 100 mL of 85% ethanol (80°C, 60 min). The ethanol extract was filtered and adjusted to 200 mL with 85% ethanol. A 20-mL aliquot of the diluted extract was evaporated to dryness in vacuo, and the residue was dissolved in 50 mL of distilled water. An aliquot of this solution was analyzed for the content of reducing (glucose and fructose) and non-reducing (sucrose) sugars using high-performance liquid chromatography on an anion-exchange column (250 mm × 2.0 mm) coupled with a electrochemical detector (Dionex Co., Osaka, Japan). The total sugars were also calculated as the total quantity of each sugar.
Total phenol content analysis Samples for the evaluation of total phenol content were prepared based on the method described by Žitňanova et al. (2006). Briefly, a 5 mm section of each plant (10 g) and distilled water (90 g) were mixed and homogenized in a highspeed blender. The obtained homogenates were centrifuged at 4500 × g for 5 min and the supernatants were assayed for total phenol content of the Allium vegetable samples. The total phenol content of the samples was evaluated using the Folin-Ciocalteu method of Sato et al. (2010). The total phenol content was calculated from a calibration curve of gallic acid (10 – 150 μg/mL) and is expressed as milligrams of gallic acid equivalents (GAE) per 100-grams fresh weight (FW).
Antioxidant activity measurement Samples for the evaluation of antioxidant activity were prepared as for the above total phenol content analysis. The antioxidant activity of the samples was chemically evaluated by determination of the oxygen radical absorbance capacity (ORAC), which has the advantage of utilizing free radical generators, and produces the biologically relevant peroxyl radical (Ishimoto et al., 2012). The ORAC assay was performed according to the method reported by Prior et al. (2003). 2,2′-Azobis(2-amidinopropane)dihydrochloride (Wako Pure Chemical Industries, Osaka, Japan) was used as a peroxyl radical generator, and fluorescein (Sigma-Aldrich, St. Louis, MO, USA) and 6-hydroxy-2,3,7,8-tetramethylchroman-2-carboxylic acid (Trolox; Calbiochem, San Diego, CA, USA) were respectively used as a fluorescent probe (Naguib, 2000) and standard. The ORAC value was calculated by using a quadratic regression equation relating the Trolox or sample concentration and area under the curve (AUC) of the fluorescence, and is expressed as micromoles of Trolox equivalents (TE) per 100-grams FW.
Statistical analysis The data of growth parameters (n = 15) and K+/Na+ ratio (n = 3) of the three Allium vegetables are expressed as mean ± SE. In the case of sugar content, total phenol content and antioxidant activity measurements, each measurement was performed in triplicate and values are expressed as an average. The data of growth parameters and K+/Na+ ratio were analyzed using a one-way analysis of variance with Dunnett's multiple comparison test. Differences were considered significant at p < 0.05 or 0.01.
Effect of salinity-stress on growth parameters and K+/Na+ ratio of Allium vegetables Table 1 shows the growth parameters and K+/Na+ ratio of the three Allium vegetables subjected to salinity-stress using different concentrations of seawater. None of the growth parameters, except for SPAD value, was clearly related to the degree of salinity-stress for the three Allium vegetables. Generally, salinity-stress results in a clear stunting of plants. Wang et al. (2000) reported that a reduction in the rate of leaf surface expansion with increasing salt concentration is an immediate response of salinity-stress. Salinity-stress also results in a considerable decrease in the fresh weights of leaves, stems, and roots (Chartzoulakis and Klapaki, 2000).
| Allium vegetable | Group | Plant height (cm) | Leaf blade dianneter (mnn) | Total weight (g) | Number of leaves) | Number of tillers | Number of broken leaves | Number of dead leaves | SPAD value | K+/Na+ ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| Welsh onion | Control | 28.29 ± 2.5 | 4.37 ± 0.9 | 8.48 ± 1.9 | 8.47 ± 1.6 | 1.80 ± 0.4 | 2.20 ± 1.5 | 2.00 ± 0.7 | 59.69 ± 1.3 | 14.41 ± 3.8 |
| SW1 | 25.51 ± 2.7 | 3.43 ± 0.7* | 5.79 ± 1.5* | 7.53 ± 1.8 | 1.47 ± 0.4 | 1.60 ± 1.3 | 1.80 ± 0.8 | 58.45 ± 1.9 | 12.75 ± 0.8 | |
| SW2 | 29.23 ± 3.2 | 4.00 ± 0.2 | 6.95 ± 1.2 | 7.93 ± 1.9 | 1.60 ± 0.6 | 2.00 ± 0.7 | 1.60 ± 1.1 | 57.15 ± 2.2* | 10.45 ± 2.7 | |
| SW3 | 27.65 ± 2.5 | 3.63 ± 0.2 | 5.82 ± 0.9* | 7.00 ± 1.6 | 1.67 ± 0.5 | 2.60 ± 1.5 | 0.60 ± 0.5* | 57.77 ± 1.7 | 2.53 ± 0.7** | |
| Wakegi(Okinawa) | Control | 33.67 ± 2.8 | 5.57 ± 0.5 | 15.13 ± 1.9 | 11.40 ± 1.1 | 2.00 ± 0.2 | 0.80 ± 1.1 | 3.40 ± 1.5 | 58.27 ± 0.4 | 30.36 ± 2.4 |
| SW1 | 32.35 ± 3.6 | 5.93 ± 0.9 | 13.73 ± 2.5 | 10.93 ± 0.4 | 2.00 ± 0.2 | 1.00 ± 0.7 | 2.20 ± 0.8 | 60.87 ± 1.5 | 26.23 ± 0.5* | |
| SW2 | 36.06 ± 4.9 | 5.10 ± 0.7 | 17.01 ± 5.0 | 13.07 ± 2.6 | 2.27 ± 0.3 | 0.80 ± 0.8 | 1.80 ± 0.8* | 62.59 ± 1.1 | 20.06 ± 3.3** | |
| SW3 | 33.87 ± 3.5 | 4.97 ± 0.6 | 14.91 ± 2.3 | 11.93 ± 1.6 | 2.47 ± 0.2* | 1.00 ± 0.7 | 2.40 ± 0.9 | 61.02 ± 1.4 | 3.83 ± 0.6** | |
| Wakegi(Nagasaki) | Control | 24.63 ± 2.7 | 3.70 ± 0.3 | 9.42 ± 1.9 | 12.47 ± 2.2 | 2.40 ± 0.4 | 0.60 ± 0.5 | 0.00 ± 0.0 | 58.80 ± 2.5 | 11.996 ± 0.5 |
| SW1 | 23.68 ± 3.9 | 3.37 ± 0.5 | 8.22 ± 2.4 | 11.53 ± 2.4 | 2.53 ± 0.3 | 0.40 ± 0.9 | 0.60 ± 0.9 | 57.29 ± 0.9 | 11.168 ± 2.0 | |
| SW2 | 21.75 ± 3.8 | 3.43 ± 0.7 | 7.16 ± 2.5 | 10.13 ± 2.1 | 2.33 ± 0.6 | 0.00 ± 0.0 | 0.60 ± 0.5 | 61.76 ± 1.5* | 7.675 ± 2.5* | |
| SW3 | 24.36 ± 5.9 | 3.50 ± 0.9 | 8.25 ± 2.8 | 10.33 ± 1.2 | 2.40 ± 0.4 | 0.20 ± 0.4 | 2.80 ± 1.9* | 62.35 ± 2.8* | 2.831 ± 0.4** |
The values of growth parameters (n = 15) and K+/Na+ ratio (n = 3) are shown as mean ± SE. The seawater concentrations were 2% (SW 1), 10% (SW 2), and 100% (SW 3). A difference between the control group and each seawater treated group (SW 1, 2, and 3) is considered statistically significant when
Suppression of growth occurs in most plants; however, the tolerance level and rate of growth reduction under salinity-stress vary widely among different plant species or cultivar lines (Paridaa and Das, 2005). Herein, the SPAD values of the leaves of the two types of Wakegi cultivars increased, whereas the SPAD value of the Welsh onion decreased, similar to the general response of most plants (Khavarinejad and Chaparzadeh, 1998). Although the salinity was found to induce little growth suppression in the three Allium vegetables, further investigation into the effects of other seawater loading methods on growth parameters is necessary, as properties, especially plant size, form, and color, also characterize the product value of vegetables.
The K+/Na+ ratio clearly decreased with an increase of seawater concentration up to 100% in all cases. The maintenance of a high K+/Na+ ratio is closely related to salt tolerance in several plants (Gadallah, 1999; Sobahan et al., 2012). For example, salinity-stress decreases the K+/Na+ ratio of typical rice cultivars due in part to increased Na+ uptake, mediated by the apoplastic pathway, in cultivars under salinity-stress (Sobahan et al., 2012). Indeed, the Na+ contents of all of the evaluated Allium vegetables increased with increasing seawater concentration, whereas the K+ content was not significantly affected (data not shown). Therefore, this finding suggests that the K+/Na+ ratio of Welsh onion and Wakegi leaves may serve as a possible index of salinity-stress, at least in case of direct seawater-spray on leaves.
Effect of salinity-stress on sugar content, total phenol content, and antioxidant activity of Allium vegetables Plants exposed to salinity-stress in their environment undergo changes in growth parameters, as well as in the synthesis of active metabolites that control ion and water flux and support scavenging of reactive oxygen species (ROS) (Gadallah, 1999). To elucidate the effects of salinity-stress on active metabolites, the sugar and total phenol contents, as well as the antioxidant activity of Welsh onion and Wakegi were measured. Evaluation of the three plants indicated that the reducing (Fig. 1-a and Fig. 1-b) and non-reducing (Fig. 1-c) sugar contents of the Welsh onion and Wakegi from Nagasaki increased in response to salinity-stress treatment, and the total sugar content increased considerably compared to the respective controls for the 10% seawater concentration (Fig. 1-d). In contrast, the total sugar content was lowered relative to the control only in the case of Wakegi from Okinawa (Fig. 1-d). Salinity-stress increases the reducing and non-reducing sugars in a number of plants due to activation of their osmoprotective, osmotic adjustment, carbon storage, and radical scavenging functions (Kerepesi and Galiba, 2000). Therefore, it was suggested that salt tolerance mechanisms of Wakegi from Okinawa might differ from those of Welsh onion and Wakegi from Nagasaki. Miyagi et al. (2011) conducted sensory evaluation of Welsh onion, and demonstrated a strong positive correlation between free sugar content and palatability. In the case of Welsh onion and Wakegi from Nagasaki, salinity treatment with a seawater concentration of 10% effectively produced a higher content of sugars compared to the control plants not subjected to the treatment, which might lead to good palatability. Figure 2 shows the respective total phenolic contents (a) and ORAC values (b) of the three Allium vegetables in response to salinity-stress. Generally, salinity-stress leads to the formation of ROS in plants, which produces a number of antioxidative phenolic compounds that protect against the potentially cytotoxic activated oxygen species (Gadallah, 1999). Of the two Wakegi cultivars, there was a clear increase in the total phenol content and ORAC value of Wakegi from Nagasaki compared to the control in response to salinity-stress of 2% and 10% seawater, whereas these values did not change markedly in Wakegi from Okinawa. Interestingly, the ORAC value of the Welsh onion was increased notably due to salinity-stress, despite the considerable decrease of its total phenol content.
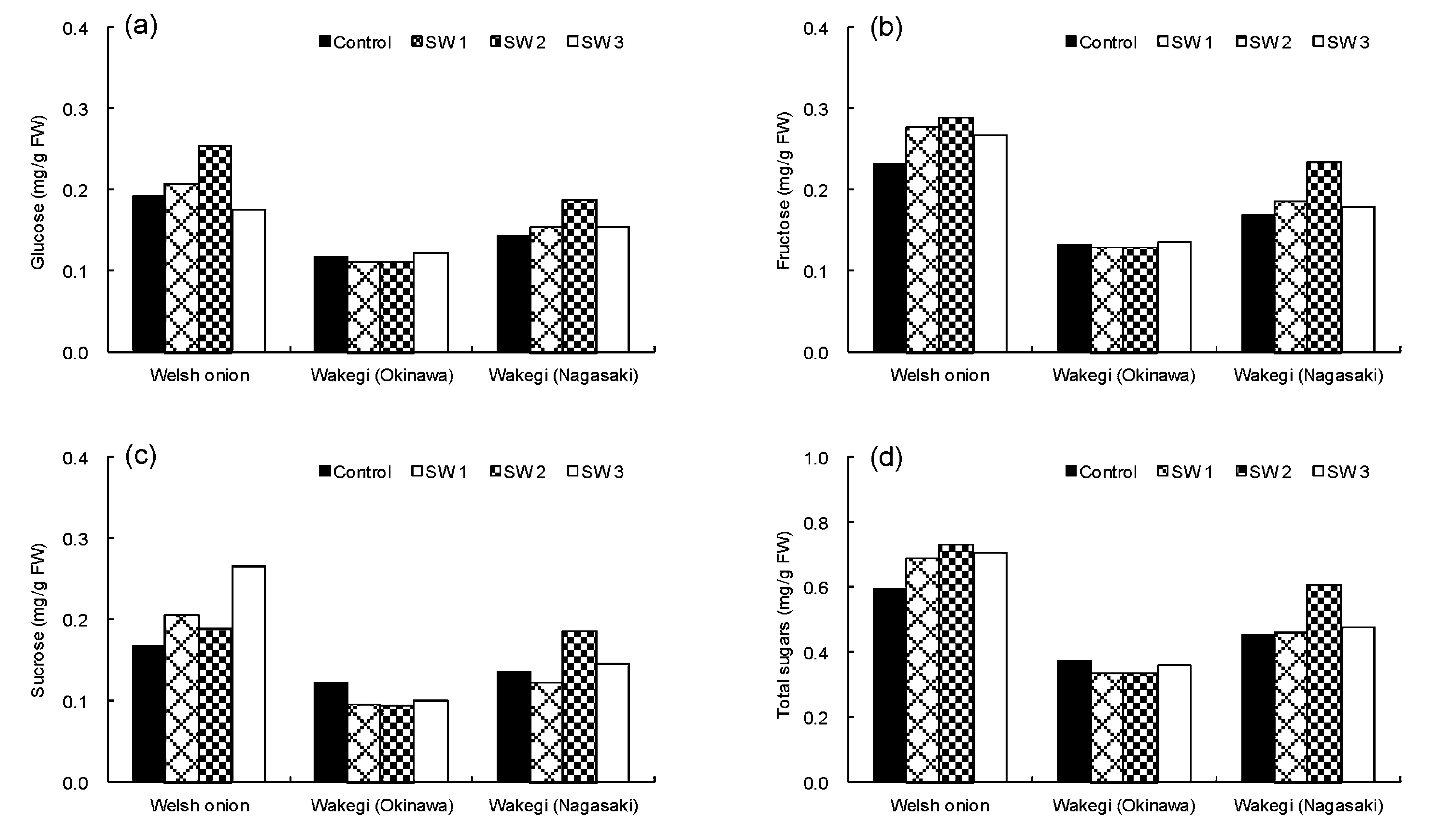
Effect of salinity-stress on the contents of glucose (a), fructose (b), sucrose (c), and total sugars (d) in leaves of the three Allium vegetables with or without salinity-stress treatment for 40 days.
Averaged values are shown. The seawater concentrations were 2% (SW1), 10% (SW2), and 100% (SW3).

Total phenolic content (a) and Oxygen Radical Absorbance Capacity (ORAC) values (b) of the three Allium vegetables with or without salinity-stress treatment for 40 days.
Averaged values are shown. The seawater concentrations were 2% (SW1), 10% (SW2), and 100% (SW3).
Accumulation of antioxidants such as phenol compounds and ascorbic acid (Ishikawa and Shigeoka, 2008), glutathione (Gadallah, 1999), as well as volatile compounds (Zuo et al., 2012) under salinity-stress has been noted in several plants. Indeed, Welsh onion possesses antioxidant compounds such as quercetin and kaempferol as main phenol compounds (Wang et al., 2006), ascorbic acid (Kähkönen and Heinonen, 2003), and volatile sulfur compounds (Wu et al., 2001). Thus, it is possible that the content of other antioxidant compounds besides phenol compounds may be increased in the Welsh onion, thereby increasing the antioxidant activity under salinity-stress. From a functional food perspective, seawater treatment is thus deemed highly advantageous in the case of Welsh onion and Wakegi from Nagasaki. It was found herein that salinity treatment at a seawater concentration of 10% clearly enhanced both the sugar content and antioxidant activity of these species. However, the active component in Welsh onion and Wakegi from Nagasaki that is responsible for the antioxidant activity was not elucidated, and further studies are therefore required.
In conclusion, salinity-stress using seawater may augment the sugar content, antioxidant activity and other growth parameters, allowing for the development of value-added Allium vegetables with enhanced palatability and food functionality.