2014 Volume 20 Issue 4 Pages 799-807
2014 Volume 20 Issue 4 Pages 799-807
In this paper, we determined appropriate degree of dephosphorization of carp eggs before trypsin hydrolysis according to the degree of hydrolysis and calcium binding activity, and purified a novel oligophosphopeptide with high calcium binding activity from carp eggs hydrolysate. The results showed that dephosphorization treatment can significantly increase the degree of hydrolysis (p < 0.05). However, hydrolysate of DP2 with 30.39% degree of dephosphorization owned the strongest calcium binding ability of 0.67 mmol/g-protein, and excessive dephosphorization was disadvantageous. After ultrafiltration, 88.73% content of hydrolysates was the fraction U1 with MW < 3 kDa which showed better ability to bind calcium. After hydroxyapatite chromatography, H3 eluted with the maximum concentration of phosphate buffer (400 mM) exhibited the highest calcium binding ability of 6.63 mmol/g-protein. Amino acid content analysis showed that the Ser content of H3 is about 2.5 times more than that of U1 but the contents of Thr and Tyr are almost identical. Further purification using size exclusion chromatography and high-performance liquid chromatography, an oligophosphopeptide with high calcium binding ability (7.85 mmol/g-protein) was obtained. Its sequence was identified as (pS)-S-(pS)-A-F-(pS)-(pS)-E-L-A-R through ESI-QTOF tandem mass analysis. It is possible to provide utilization of fish eggs as a novel calcium nutraceutical additive in food industry.
The global fishery production reached 178 million tons in 2011(i), more than 50% of which was discarded as inedible byproducts such as bone, skin, internal organs, head and eggs (Jung et al., 2005). Plentiful research have been implemented on utilizing fishery byproducts (Chakraborty et al., 2011; Duan et al., 2009; Kongsri et al., 2013; Mori et al., 2013). However, the utilization studies on eggs, especially on freshwater fish eggs, are very scarce. Eggs are rich in lipids (especially phospholipids and n-3 polyunsaturated fatty acids), the yolk protein, mineral elements, vitamins, carotenoids, and other nutrients. But in the conventional process, eggs are often treated as waste to be abandoned.
Calcium is an important mineral element to maintain human health, which plays an important role in the formation of bones and teeth, neurotransmitter release, muscle composition, and heart beat regulation. Calcium is mainly ingested through drinks and food, especially milk or cheese products with high calcium content. Influenced by lactose intolerance, dietary habits, and other factors, Asians intake less milk and cheese products and their food mainly focuses on vegetable diet. However, substances such as the phytic acid, oxalic acid, saturated fatty acids, and dietary fiber are easily combined with calcium to form insoluble matter, which is not beneficial to calcium absorption.
Many peptides isolated from animals possessed the ability of binding Ca (Huang et al., 2011; Lee et al., 2009; Jiang et al., 2000; Jung et al., 2007). Casein phosphopeptides (CPPs) were well known for their metal binding abilities because the CPPs contained electronegative phosphoserines (West et al., 1986; Meisel et al., 1990; Yuan et al., 1991; Gerber et al., 1986; Sato et al., 1986). The phosphopeptides obtained from the enzymolysis of fish bone (Jung et al., 2007, 2006, 2005) can also chelate Ca, whose performance is similar to CPPs. Many studies indicated that fish eggs also contain high phosphoprotein and can obtain phosphopeptides by hydrolysis (Catherine et al., 2002; Inoue et al., 1971).
Carp was one of the most productive (over 3.8 million tons) freshwater fish species in the world (ii). In breeding season, the ovarian can weigh 10% to 25.81% of the overall weight. In the present study, we will discuss the appropriate degree of dephosphorization of carp eggs before trypsin hydrolysis and purification of a novel oligophosphopeptide with high calcium binding activity from carp eggs hydrolysate.
Materials Carp eggs were provided by Meijia Co. (Shandong, China). The carp eggs were collected, cleaned and then stored at −70°C. Trypsin (250 U/mg) from bovine pancreas was purchased from Sinopharm Co. Ltd. (Shanghai, China). Other chemical reagents used in this study were of analytical grade and commercially available.
Preparation of carp egg phosphopeptide with Ca-binding activity According to our previous research (Li and Huang, 2011), after defatted with 10 times volume of hexane/ethanol mixed solvent (3:1(V/V)) at 50°C for 6 h and dephosphorylated with 40 times volume of 0.1M NaOH for 2 h, carp eggs with proximate 30.5% dephosphorization degree then were digested with trypsin (enzyme/substrate: 3/100, substrate concentration: 2%, pH 8.0) at 49°C for 12 h. After incubation at 100°C for 5 min to inactivate the enzyme, the carp egg hydrolysate was centrifuged at 10,000 g for 20 min and filtered with 0.45 µm membrane. Degree of hydrolysis (DH) was determined by the o-phthaldialdehyde (OPA) method (Nielsen et al., 2001).
Alkaline dephosphorylation of carp eggs Use certain concentration of NaOH to dephosphorize defatted egg powder for a period of time with the proportion of 40:1 (v/g); adjust the pH with hydrochloric acid into neutral to terminate dephosphorization, then centrifuge it at 8,000 g for 20 min, wash the precipitate with distilled water and centrifuge it twice, collect the precipitate. The phosphorus contents of eggs before and after dephosphorization can be determined according to the method of Chen et al. (1956).
Calcium-binding assay Calcium-binding assay was performed according to the previous method with the following modifications (Sato et al., 1991). After demineralization by a Chelex 100 resin column (Bio-Rad, Richmond, CA, USA), various concentrations of hydrolysates up to 500 mg/L were mixed with 5 mM CaCl2 and 20 mM sodium phosphate buffer (pH 7.8). The mixture was stirred at 22°C for 30 min, and the pH was maintained at 7.8 with a pH meter. Calcium contents of the supernatant after 0.22 µm microfiltration were determined by flame atomic absorption spectrometer (AA-6300C, Shimadzu Co., Japan ). Instrumental conditions were as follows: wavelength = 422.8 nm, slit = 0.8 nm, acetylene flow = 1.65 L/min, air flow = 14.0 L/min. Protein concentration in sample solutions was determined by the method of Lowry et al. (1951) using bovine serum albumin as a standard.
Amino acid analysis The samples were hydrolyzed with 6 N HCl at 110°C for 24 h in vacuum-sealed ampoules. After neutralizing, evaporating and filtering with a glass filter, amino acid composition was determined with amino acid analyzer (Hitachi 835 − 50, Hitachi Co. Ltd., Japan).
Isolation and purification of calcium binding peptides After demineralization by a Chelex 100 resin, the hydrolysate was divided into two fractions by ultrafiltration membrane system (Bioq-MU2010, Huihetang Bioengineering instrument Co., Ltd.) with 3 kDa molecular weight cutoffs. Calcium-binding activity of two fractions (< 3 kDa and > 3 kDa) were determined. The active fractions were concentrated and applied to a hydroxyapatite (HA), (16 × 200 mm Macroprep ceramic HA type 1, Bio-Rad, Richmond, CA, USA) pre-equilibrated with 10 mM potassium phosphate buffer, pH 6.5. The Ca-binding fraction was divided into three fractions by stepwise elution with 100 mM, 200 mM and 400 mM phosphate buffer (pH 6.5) at a flow rate of 3 mL/min and monitored at 220 nm. After desalted through a Sephadex G-15 column (16 × 300 mm), the eluates were tested for calcium-binding activity. The fraction with the highest activity was pooled and isolated further on a Sephadex G-25 column (16 × 600 mm) equilibrated and eluted with distilled water at a flow rate of 1.0 mL/min. All peaks eluted were monitored at 220 nm, and also collected for calcium-binding activity analysis. The fraction showing the highest Ca-binding activity was further purified using a semipreparative RP-HPLC column (Zorbax SB-C18, 9.4 × 250 mm). Elution was performed using a linear gradient of 5 – 25% acetonitrile in water at a flow rate of 0.4 mL/min and monitored at 220 nm. The active fraction from the RP-HPLC column was used for peptide sequencing.
Peptide sequence analysis The accurate molecular weight and amino acid sequence of sample was determined on an ESI-QTOF tandem mass spectrometer to identify molecular mass and amino acid sequence of Ca-binding peptide. All MS/MS experiments were performed on a QTOF tandem mass spectrometer (Micromass Co., Manchester, UK) equipped with a nano-ESI source. The applied voltage to the capillary to produce an electrospray was 1500 eV, and cone voltage was 30 eV. MS/MS spectra were acquired in data dependant MS/MS mode, of which collision energy was step increased to 25, 30, and 35 eV from 10 eV. Argon was introduced as a collision gas at a pressure of 10 psi. Sequencing of active peptide was acquired over the m/z range 50 – 2500 and sequenced by using the PepSeq de nove sequencing algorithm (Micromass Co., Manchester, UK).
Statistical analysis Data were presented as means ± standard deviations of triplicate determinations. A least significant difference (LSD) test was performed using the SPSS software program (SPSS Inc., Chicago, IL, USA) to evaluate the mean differences between the measurements at the 5% confidence level.
Alkaline dephosphorylation of carp eggs Carp eggs contain phosphorus protein, which is similar to phosvitin in hen egg yolk. One kind of phosphorus protein was obtained from Pacific Herring eggs, whose phosphorus content is up to 10.6% and serine accounts for 2/3 of all number of amino acids. The results of an alkali-catalyzed β-elimination reaction suggested that all the phosphorus in the phosphoprotein are present in the form of monoesters linked to the hydroxyl groups of serine (Inoue et al., 1971). Earlier studies have shown that the proteins containing a large number of amino acids modified by phosphorylation are resistant to protease hydrolysis, resulting in low degree of hydrolysis and producing fractions with large molecular weight (Mecham et al., 1949). Hence, dephosphorization treatment to carp eggs can improve the hydrolysis effect of protease. Sodium hydroxide can hydrolyze phosphor ester bond effectively so as to achieve the purpose of dephosphorization. The degree of dephosphorization is used to measure the effect of dephosphorization of carp eggs. From Table 1, we can see that the degree of dephosphorization of carp eggs increased with the raising NaOH concentration and the extending dephosphorization time. By controlling the concentration of NaOH and dephosphorylation time, we can prepare carp eggs with different dephosphorylation rate. Respectively, 0.05, 0.1, 0.2, 0.3 M of NaOH was utilized to conduct dephosphorization for 1, 2, 2, 4 h. Four kinds of dephosphorized carp eggs labeled as DP1, DP2, DP3, and DP4 could be obtained. The degree of dephosphorization of DP1, DP2, DP3, and DP4 was 13.66%, 30.39%, 48.71%, and 83.49% respectively.
| Time(h) | Concentration of NaOH (M) | |||
|---|---|---|---|---|
| 0.05 | 0.1 | 0.2 | 0.3 | |
| 0.5 | 9.15 ± 1.23 | 18.47 ± 2.21 | 21.33 ± 0.76 | 27.69 ± 1.13 |
| 1 | 13.66 ± 2.03 | 24.01 ± 1.32 | 26.67 ± 0.52 | 41.59 ± 2.36 |
| 1.5 | 17.11 ± 1.65 | 26.94 ± 0.96 | 36.25 ± 2.03 | 54.98 ± 2.37 |
| 2 | 21.36 ± 2.25 | 30.39 ± 2.01 | 48.71 ± 1.57 | 65.96 ± 1.03 |
| 4 | 30.12 ± 2.34 | 43.13 ± 2.87 | 78.97 ± 0.82 | 83.49 ± 0.74 |
Enzymatic hydrolysis of carp eggs After hydrolysis with trypsin, the dephosphorized carp eggs as well as the nondephosphorized carp eggs (labeled as DP0) was measured the hydrolysis degree and calcium binding ability of their enzymatic hydrolysates (Fig.1). Dephosphorization treatment can significantly increase the degree of hydrolysis of carp eggs. After hydrolysis for 12 h, the hydrolysis degree of DP1, DP2, DP3 and DP4 was 26.08%, 31.15%, 33.55% and 33.98% respectively, which is higher than that of non-dephosphosphorized carp eggs (22.00%). It is well-known that the phosvitin contains a highly phosphorylated core area possessing 80 serines, where the negatively charged phosphate polynuclear complexes enables the adjacent peptide bonds not easily to be functioned by protease (Goulas et al., 1996; Byrne et al., 1984). During the process of dephosphorization, the changes of structure in the core area make it subject to enzyme (Jiang et al., 2000). This study showed that the carp eggs protein may also contain the highly phosphorylated core area, which is similar to phosvitin.

The degree of hydrolysis (A) and calcium binding activity (B) of carp eggs with different degree of dephosphoration. DP0, carp eggs without dephosphorization. DP1, DP2, DP3, and DP4 represent four kinds of dephosphorized carp eggs with degree of dephosphorization of 13.66%, 30.39%, 48.71%, and 83.49% respectively. Carp eggs were digested with trypsin (enzyme/substrate: 3/100, substrate concentration: 2%, pH 8.0) at 49°C for 12 h. All data were expressed as mean values (mean ± SD, n = 3).
However, the content of phosphorus may be critical to calcium binding ability of the phosphorus peptides. It was verified that the casein phosphopeptides (CPPs) could prevent the formation of insoluble calcium in the small intestine and improve calcium absorption (Sato et al., 1986, 1991). The cause may lie in the fact that it possesses the structure with phosphoserine cluster (Holt et al., 1998). Experimental results showed that a certain degree of dephosphorization could effectively increase the calcium binding ability of hydrolysates. The hydrolysate of DP2 (dephosphorylation rate is 30.39%) owned the strongest calcium binding ability of 0.67 mmol/g-protein (500 mg/L of peptide can bind 13.4 mg/L of calcium). However, excessive dephosphorylation is disadvantageous to the calcium binding ability of the hydrolysates. When dephosphorylation rate was 83.49%, 500 mg/L of peptide could only bind 4.7 mg/L of calcium (0.24 mmol/g-protein). It can be inferred that the ability for the phosphorus peptide to bind calcium has something to do with the phosphorus content and molecular weight of it. The appropriate dephosphorization treatment can produce the phosphorus peptide with appropriate phosphorus content and molecular weight. Therefore, we selected hydrolysate of DP2 to isolate and purify further.
Isolation and purification of calcium binding peptides First, after ultrafiltration classification, two fractions were obtained from hydrolysate of DP2: U1 (< 3 kDa) and U2 (> 3 kDa). The yield and calcium binding ability of U1 are far greater than that of U2 (Table 2), which shows that after hydrolysis, the carp eggs protein has decomposed into small molecule peptides with high calcium binding ability. Many studies have shown that biologically active peptides usually contain 3 – 20 amino acid residues and the molecular weight of discovered peptides with calcium binding capacity are mostly from 1 kDa to 3.5 kDa (Jiang et al., 2000; Jung et al., 2005, 2006, 2007; Lee et al., 2009).
| Fraction | Calcium binding activity (mmol/g-protein) | Content (%) |
|---|---|---|
| U1 (< 3 kDa) | 0.87 ± 0.03 | 88.73 |
| U2 (> 3 kDa) | 0.09 ± 0.01 | 11.27 |
After ultrafiltration, the serine content increased from 6.8% to 8.1% in U1 (Table 3), which showed that after dephosphorization treatment, the section rich in serine in carp eggs protein has been successfully decomposed into peptides of less than 3 kDa by trypsin.
| Amino acid | Content (%, residues/100 residues) | ||
|---|---|---|---|
| Hydrolysates | Fraction U1 | Fraction H3 | |
| Ser | 6.81 | 8.10 | 21.73 |
| Ala | 13.24 | 12.97 | 11.20 |
| Leu | 12.40 | 11.90 | 8.34 |
| Arg | 5.20 | 5.38 | 7.75 |
| Gly | 7.98 | 7.13 | 7.25 |
| Lys | 4.62 | 5.02 | 6.43 |
| Val | 7.57 | 7.13 | 6.30 |
| Phe | 3.78 | 3.97 | 5.10 |
| Asxa | 6.50 | 6.89 | 5.07 |
| Glxb | 6.41 | 6.67 | 4.97 |
| Thr | 4.46 | 4.36 | 3.14 |
| His | 5.34 | 5.02 | 3.14 |
| Tyr | 2.59 | 2.76 | 2.56 |
| Pro | 3.95 | 3.79 | 2.55 |
| Met | 3.83 | 3.68 | 2.42 |
| Ile | 5.33 | 5.24 | 2.03 |
U1 was then applied to hydroxyapatite (HA) chromatography and separated into three fractions marked H1, H2 and H3 by stepwise elution with the concentration of phosphate eluent increasing from 100 mM to 400 mM (Fig. 2). After pooled, desalted and lyophilized, all fractions were adjusted to 50 mg/L of concentration and measured their calcium binding abilities. The results showed that after HA chromatography, the calcium binding activity was significantly increased. Moreover, the activity from H1 to H3 increased gradually, which was corresponded to the characteristics of HA chromatography. The closer the fraction combined the calcium ion, the higher the concentration of phosphate eluent required. H3 was eluted with the maximum concentration of phosphate buffer (400 mM) and exhibited the highest calcium binding ability of 6.63 mmol/g-protein after adjusted to 50 mg/L of protein concentration. The calcium binding abilities of all fractions eluted with phosphate buffer are significantly higher than that of U1, which showed that HA chromatography was very effective to separate peptide with calcium-binding activity.

Elution profile of trypsin hydrolysate by hydroxyapatite chromatography. Hydroxyapatite column (16 × 200 mm) was pre-equilibrated with 10 mM potassium phosphate buffer, pH 6.5. One hundred mM, 200 mM and 400 mM phosphate buffer were adopted to perform stepwise elution at a flow rate of 3 mL/min and monitored at 220 nm. After demineralization the eluates were tested for calcium-binding activity. All data were expressed as mean values (mean ± SD, n = 3).
The protein or peptide with calcium binding activity could identify calcium on the surface of HA (Hoang et al., 2003). By HA chromatography, the peptides and proteins with calcium binding activity have been successfully separated and obtained from fish bones and bullfrogs (Jung, et al., 2005, 2006, 2007; Dohi et al., 1987). Amino acid content analysis showed that the serine content of H3 is 2.5 times more than that of U1, but the threonine and tyrosine contents is almost identical, which proved that the serine in the peptide played a vital role in ability to bind calcium.
H3 was further separated by size exclusion chromatography on Sephadex G-25 column and obtained four fractions marked G1, G2, G3 and G4 according to molecular weight (Fig. 3). Each fraction was pooled, lyophilized and measured for their calcium binding activities after adjusted to 50 mg/L of concentration. The results showed that fraction G2 possessed the highest calcium binding activity of 7.47 mmol/g-protein. Then it was conducted RP-HPLC analysis and fractioned into two major fractions (F1, F2) through semi-preparative C18 column (Fig. 4). After the calcium binding activity assay (50 mg/L of protein concentration), F1 with the highest ability (7.85 mmol/g-protein) was selected for mass spectrometry analysis.
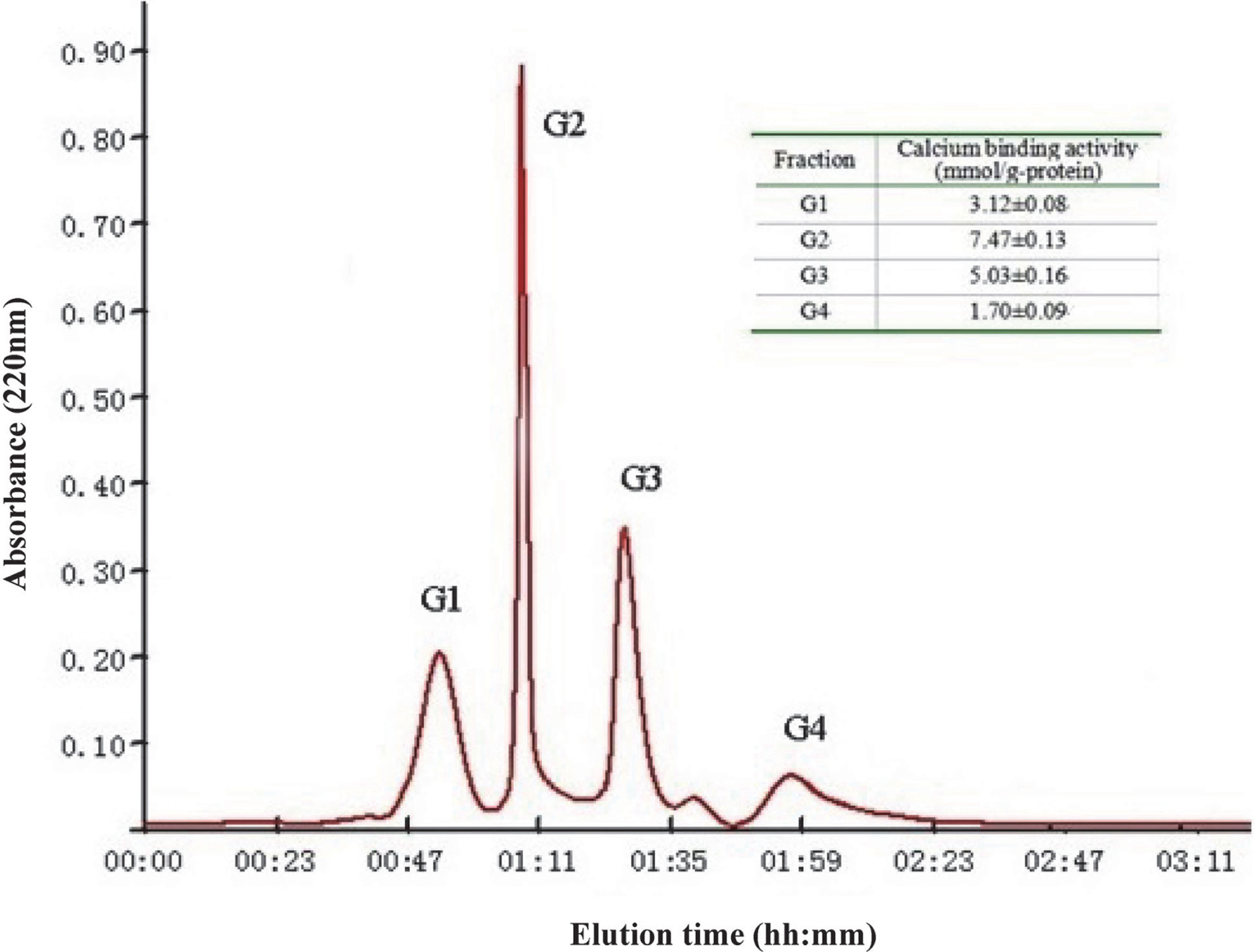
Elution profile of the active fraction (H3) from hydroxyapatite chromatography further purified by Sephadex G-25 size exclusion chromatography. The column (16 × 600 mm) equilibrated and eluted with distilled water at a flow rate of 1.0 mL/min. All peaks eluted were monitored at 220 nm, and also collected for calcium-binding activity analysis. All data were expressed as mean values (mean ± SD, n = 3).

Elution profile of the active fraction (G2) from size exclusion chromatography further purified by RP-HPLC. The semipreparative RP-HPLC column (Zorbax SB-C18, 9.4 × 250 mm) was eluted with a linear gradient of 5 – 25% acetonitrile in water at a flow rate of 4 mL/min and monitored at 220 nm. All peaks eluted were collected for calcium-binding activity analysis. All data were expressed as mean values (mean ± SD, n = 3).
Peptide sequence analysis The amino acid sequence of F1 was identified as follows: (pS)-S-(pS)-A-F-(pS)-(pS)-E-L-A-R (1461Da) (Fig. 5). The peptide contains five serines, among which four are phosphorylated. Juillerat (1989) hydrolyzed whole milk cheese with trypsin and obtained a series of CPPs containing the following fragment: αs1-CN (43-58), 2P; αs1-CN (59-79), 5P; αs2CN (46-70), 4P; β-CN (1-28), 4P; β-CN (2-28), 4P; and β-CN (33–48), 1P. Jung (2006) separated and obtained a calcium binding active peptide with 14 amino acid residues from Alaska Pollack backbone and found it contains abundant Thr, Tyr and Ser. Nishimoto (2003) implemented a purification of carp bone and obtained osteocalcin which contains abundant Thr, Tyr. Jiang (2000) prepared phosphopeptides from the chicken phosvitin, which was composed mainly of Ser and Thr. As we know, Ser, Thr, and Tyr are sites of phosphorylation modification in protein. Thus, phosphorylation modification is probably necessary to the ability for phosphorus peptide to bind calcium. However, there are also some studies suggest that there may be some other calcium binding sites without phosphorylation modification. Huang (2011) discovered a tripeptide containing His from shrimp process byproducts, and His is regarded to play a key role in calcium binding process.

Identification of molecular mass and amino acid sequence of F1. MS/MS experiments were performed on a QTOF tandem mass spectrometer equipped with ESI source. Sequencing of active peptide was acquired over the m/z range 50 – 2,500 and sequenced by using the PepSeq de nove sequencing algorithm (Micromass Co., Manchester, UK).
It was reported that the calcium binding activity of CPP II (Meiji Seika Co., Ltd., Tokyo, Japan) , phosphopeptide from hen egg yolk phosvitin and pollack backbone peptide were 4.0, 5.0 and 7.0 mmol/g-protein respectively when the protein concentration was 50 mg/L (Jiang et al., 2000; Jung et al., 2006). Compared with these peptides above, the peptide derived from carp eggs showed the highest calcium binding activity of 7.85 mmol/g-protein.
Carp eggs contain phosphorus protein, which is similar to phosvitin in hen egg yolk. The calcium binding ability of tryptic hydrolysates of carp eggs was influenced by the content of phosphor. Approximately, carp eggs with 30% dephosphorylation rate can effectively hydrolyzed by trpsin and their hydrolysates exhibited high calcium binding ability. An oligophosphopeptide with high calcium binding ability was purified from tryptic hydrolysates of carp eggs, which contains 5 serines, among which four are modified by phosphorylation. Thus, it is possible to provide a novel nutraceutical with high calcium bioavailability in food industry through further studies on its mechanism of binding calcium and bioavailability in vitro and in vivo assays.
Acknowledgements This work was financially supported by National Natural Science Foundation of China (No. 31101379) and Marine Special Welfare of China (No. 201205027).