2014 Volume 20 Issue 4 Pages 841-847
2014 Volume 20 Issue 4 Pages 841-847
Vitamin A acetate is used in fortified food widely. Due to the conjugated double bonds and ester functional group, it is susceptible to light, heat, oxygen and peroxide, resulting in changes in molecular structure and performance. The impact of peroxide on vitamin A acetate in octane solution with the addition of isopropylbenzene hydroperoxide (CHP) in the absence of light and oxygen at 90°C was investigated. By means of HPLC-DAD, GPC and HPLC-MS, it was found that vitamin A acetate was depleted after reaction for 18 days, and the main products were dimers. The reaction mechanism was proposed as follows: CHP homolysed to free radicals, and then abstracted α-H from vitamin A acetate to generate vitamin A acetate free radical, which added to another vitamin A acetate to form dimer free radical, at last dimer free radical abstracted α-H from vitamin A acetate molecule to form dimer product. Furthermore, four most probable dimer product structures were elucidated.
Vitamin A (Fig.1) is a series of compounds with biological activity of retinol and exerts a potent influence on maintaining visual function (Blomhoff R. and Blomhoff H. K., 2006), bone growth and development (Kindmark et al., 1995), cell differentiation and proliferation (Reichrath et al., 2007), immune system (Stephensen, 2001), and anti-cancer (Siddikuzzaman et al., 2011; Tang and Gudas, 2011). So it is commonly used as one of the most important additives for food. Due to the conjugated double bonds and hydroxyl or ester functional group, vitamin A is susceptible to polymerization, oxidation, dissociation, dehydration or decarboxylationreaction under the influence of light, heat, solvent, oxygen and peroxide (An et al., 2013).

Molecular structures of vitamin A.
A lot of efforts have been made to study the instability of vitamin A. By means of supercritical fluid chromatography (SFC), vitamin A esters were found to undergo Diels-Alder reaction under heat condition, losing biological activity, and the products generated were kitols (Runge and Heger, 2000). This conclusion was employed in previous work and it was found that the reaction rate increased with temperature rise (Hong et al., 2013). Through monitoring changes of absorbance at 325 nm with time, it was reported that vitamin A acetate reaction performed in ethanol solution showed a non-zero value of absorbance at 325 nm at the end, indicating an equilibrated reaction, and reaction done in hexane solution did go to completion in terms of the disappearance of vitamin A acetate. Furthermore, the rate constant calculated was dependent on the initial concentration of vitamin A acetate, decreasing with initial concentration rise (Paquette and Kanaan, 1985). When exposed to sunlight, vitamin A acetate was photo-oxidized to 3-ionone, 2-hydroxy-2,6,6-trimethylcyclohexanone, dihydroactinidiolide, desoxyxanthoxin and geronic acid (Crank and Pardijanto, 1995). With the help of ultraviolet spectroscopy monitoring vitamin A retention rate, it was found that transition metal, such as Fe, Cu, Al, made vitamin A decrease more quickly, and the reason might be that transition metal with redox potential could be used as effective pro-oxidant to accelerate the reaction through free radical effect (Junhong and Fan, 2012). Using raman spectroscopy to demonstrate the effects of UVA light and oxygen on vitamin A, Failloux et al. (2004) drew a conclusion that UVA light initiated the breakdown of vitamin A and then oxygen enhanced the photodamage strongly. Through analyzing changes of electronic and infrared spectroscopy, Finkelshtein and Krasnokutskaya (1996) studied the autoxidation of vitamin A acetate, and speculated that oxygen was added to vitamin A acetate molecule to form peroxy radical, but they did not go deeper with further mechanism.
Vitamin A can be uniformly dispersed in oil, and then absorbed by body easily. So oil is expected to be the preferred carrier (Dary and Mora, 2002; Pyka, 2009). But oil is easily auto-oxidized to peroxides (Loveday and Singh, 2008; El-Agamey et al., 2004), which initiate vitamin A to change in molecular structure through free radical reaction (Kozlov et al., 1971). However, there were few reports about the deeper research on the reaction of vitamin A under the influence of peroxide.
Because the reactive terminal functional group is blocked, vitamin A esters are expected to be more stable and used more widely (Cherng et al., 2005; Crank and Pardijanto, 1995). This paper chose vitamin A acetate as reactant, and studied its reaction in octane solution with the addition of CHP in the absence of light and oxygen at 90°C with the analytical methods of high performance liquid chromatography-diode array detector (HPLC-DAD), gel permeation chromatography (GPC) and high performance liquid chromatography-mass spectra (HPLC-MS). Furthermore, the proposed reaction mechanism and four most probable product structures were elucidated.
Chemicals and materials The crystallized all-trans vitamin A acetate (total content > 98%) was supplied by NHU Co., Ltd. (Xinchang, China). CHP (80%) was purchased from Alfa Aesar (Tianjin, China). Chromatographically pure acetonitrile (> 99.8%) and isopropanol (> 99.7%) and analytically pure n-octane (> 99.7%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Sample preparation and experiments Accurately weighed 14.000 g n-octane, 6.000 g vitamin A acetate and 0.075 g CHP were added into a three-neck and round-bottom flask, then aerated by inert nitrogen gas (N2) to purge dissolved oxygen sufficiently. Seal the flask and make it react in oil bath at 90°C. Samples were taken every 24 hours and analyzed. All operations were conducted under dark condition.
HPLC-DAD analysis The HPLC-DAD system (LC-10AT, SHIMADZU) consisted of a LC-20AD pump, a DGU-20A3 degasser, a SIL-20A autosampler, a SPD-M20A DAD detector, a CTO-20A column heater and a work station computer. A reverse-phase C18 column (Kromasil, 250 × 4.6 mm, 5 µm) was used. The separation was performed using a mixture of acetonitrile-isopropanol (6:1, v/v) as the mobile phase, and the flow rate was 1 mL/min. The column was kept at 30°C. All samples (0.040 g each) were weighed and dissolved in 100 mL mobile phase, and 20 µL solution was injected for analysis.
GPC analysis The GPC system (Waters 1525, Waters) was equipped with Waters 1525 pump, Waters 2414 RI detector (Milford, MA) and three PLgel columns (300 × 7.5 mm, 10 µm, 500 Å; 300 × 7.5 mm, 10 µm, 10E3 Å; 300 × 7.5 mm, 10 µm, 10E4 Å) connected in series. It was calibrated by monodisperse polystyrene standards. The flow rate was maintained at 1 mL/min with a column temperature of 30°C. All samples (0.050 g each) were weighed and dissolved in 10 mL tetrahydrofuran, which was used as eluent, and 50 µL solution was injected for analysis.
HPLC-MS analysis Mass spectra were acquired using Agilent mass spectrometer equipped with an APCI source. The following APCI inlet conditions in positive mode were applied. Temperature of the drying gas and APCI heater, 325°C and 350°C respectively. Capillary voltage, 3500 V (+). Sheath gas, 50 psi. Drying gas flow rate, 6 L/min. Discharge current, 4 µA.
HPLC-DAD results In this paper, area percentage at 325 nm was applied to represent relative content of vitamin A acetate. The result of HPLC traces of vitamin A acetate relative content with time is shown in Fig.2.

HPLC traces of relative content of vitamin A acetate with time. Condition: n-octane, 14.000 g; vitamin A acetate, 6.000 g; CHP, 0.075 g; temperature, 90°C. Samples were taken every 24 hours and then analyzed.
Fig.2 shows that vitamin A acetate reacted faster after the 4th day, compared with the first 3 days. Its relative content was below 5% at the 14th day, and then reacted to completion slowly, which was consistent with previous conclusion that vitamin A acetate went to completion in hexane (Paquette and Kanaan, 1985).
HPLC chromatogram of sample after reaction for 9 days is shown in Fig.3(a). The peak at 3.73 min represented unreacted vitamin A acetate. Several weak peaks with retention time between 2.80 – 3.43 min were identified as oxidation products of vitamin A acetate, because their retention time was shorter than that of vitamin A acetate. They should have smaller molecular weight or polar structures. By comparing peak area, it can be found that peaks with retention time between 7.50 – 9.41 min were main products.

HPLC-DAD results. (a) HPLC chromatogram of sample after reaction for 9 days. (b) DAD sscannogram of four products with highest content.
DAD sscannogram of four main peaks was employed to analyze their UV absorption characteristic and the result is shown in Fig.3(b), which demonstrates that their UV maximum absorption wavelength was between 282 nm and 297 nm. According to Woodward-Fieser theory, products were with four conjugated double bonds structures.
GPC results In GPC analysis, monodisperse polystyrenes were used as standard samples to get a standard curve, which showed the relationship between observed average molecular weight (Mw) and retention time of polystyrenes. The observed average Mw of target sample, which was calculated through comparing its retention time and that of the standard curve, was a relative value based on polystyrenes, instead of the frequently-used real Mw based on carbon atom. This could explain why the observed average Mw of pure vitamin A acetate was 366, which was shown in Fig.4(a), instead of 328. The result was also in agreement with previous conclusion that the use of polystyrenes as standards might cause overestimate of Mw (Jayakannan et al., 2001)
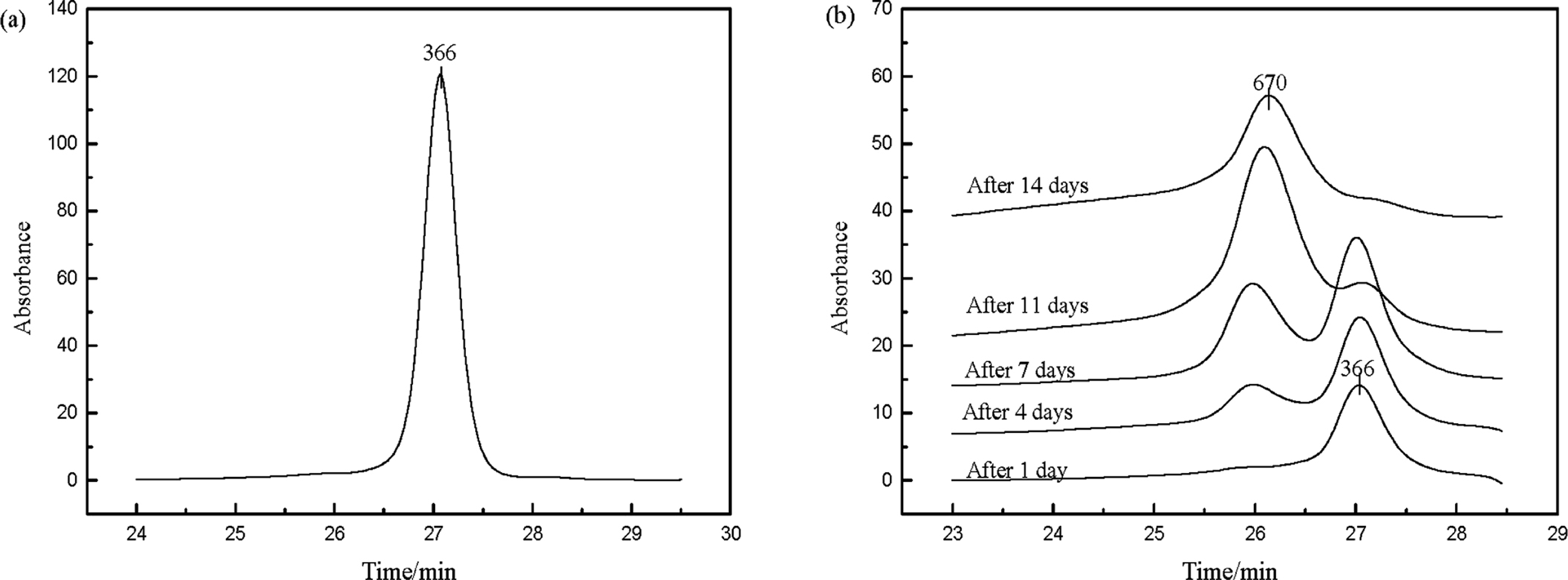
GPC results. (a) GPC result of pure vitamin A acetate. (b) GPC traces of products after reaction for 1 day, 4 days, 7 days, 11 days and 14 days. Condition: n-octane, 14.000 g; vitamin A acetate, 6.000 g; CHP, 0.075 g; temperature, 90°C. Samples were taken every 24 hours and then analyzed.
The GPC result of products after reaction for 1 day, 4 days, 7 days, 11 days and 14 days is shown in Fig.4(b). It illustrates that as reaction time prolonged, the intensity of vitamin A acetate peak weakened and the peak area decreased, which means the content of vitamin A acetate reduced. The peak intensity with observed average Mw of 670 enhanced and the peak area increased. Based on the explanation of the difference between observed average Mw and the real Mw of pure vitamin A acetate in GPC analysis before, the peak with Mw of 670 should be dimer product, whose real Mw was 656.
HPLC-MS results HPLC-MS analysis was used to determine the real Mw of peaks in Fig.3(a). Mass spectrogram extracted from HPLC peak at 3.73 min is shown in Fig.5. Positive ion peak of 269.1 could be speculated as the combination of a molecule, generated by vitamin A acetate removed a acetic acid molecule, and H+, indicating the peak represented unreacted vitamin A acetate.

Mass spectrogram extracted from HPLC peak at 3.73 min.
Four mass spectrograms extracted from HPLC peaks between 7.50 – 9.41 min are shown in Fig.6. Positive ion peak of 657.4 (657.1) in Fig.6(a), (b), (c) could be speculated as the combination of a dimer molecule, whose molecular weight was 656, and H+. Positive ion peak of 597.4 of Fig.6(d) could be speculated as the combination of a molecule, generated by dimer product removed a acetic acid molecule, and H+. So the peaks of HPLC peaks between 7.50 – 9.41 min were isomers of dimers.

HPLC-MS results. (a) Mass spectrogram extracted from HPLC peak between 7.50 – 7.85 min. (b) Mass spectrogram extracted from HPLC peak between 7.85 – 8.17 min. (c) Mass spectrogram extracted from HPLC peak between 8.17 – 8.57 min. (d) Mass spectrogram extracted from HPLC peak between 8.57 – 9.41 min.
Reaction mechanism and elucidation of four products Based on the above conclusions, reaction mechanism was proposed as follows (Fig.7). CHP homolysed to free radicals, and then initiated reaction by capturing α-H from vitamin A acetate to produce vitamin A acetate free radical, which added to another vitamin A acetate molecule to form dimer free radical, at last dimer free radical captured α-H from vitamin A acetate to form dimer product.

Proposed reaction stages of vitamin A acetate initiated by peroxide.
Compared with other α-H atoms, H atom at C-15 (Finkelshtein and Krasnokutskaya, 1996) was much easier to be captured to produce a stable vitamin A acetate free radical, due to weak steric hindrance here and π-π resonance and p-π resonance system of vitamin A acetate free radical. According to resonance theory, this vitamin A acetate free radical had six resonance forms shown in Fig.8.

Proposed reaction mechanism and structures of four most probable dimers
Vitamin A acetate free radical a and f with five conjugated double bonds disagreed with DAD result that dimer structure had four conjugated double bonds at most, so the formation chances of a and f were small and they did not go through further reactions. Vitamin A acetate free radical b and e were both with four conjugated double bonds, but the steric hindrance of b was weaker, so b was more reactive than e, and so was c more reactive than d.
Vitamin A acetate free radical b preferred to attack C-14, compared with other carbon atoms of the double bonds, to produce dimer free radical, which was stable with π-π resonance and p-π resonance system, because the system made electrons delocalize and free radical energy reduce. This dimer free radical had five resonance forms shown in Fig.8. b1 and b5 were both with four conjugated double bonds structures, so the formation chances of b1 and b5 were large and then they captured H atoms at C-15 from another vitamin A acetate to form dimer 1 and dimer 2.
Vitamin A acetate free radical c preferred to attack C-14 to produce dimer free radical, which was also stable with π-π resonance and p-π resonance system. This dimer free radical had five resonance forms shown in Fig.8. c1 and c5 had four conjugated double bonds structures, so the formation chances of c1 and c5 were large and then they captured H atoms at C-15 from vitamin A acetate to form dimer 3 and dimer 4.