2014 Volume 20 Issue 5 Pages 947-954
2014 Volume 20 Issue 5 Pages 947-954
An imbalance of oxidation and antioxidation is one of the primary causes of atherosclerosis. The use of natural plant compounds with effects has been proven to have clinical relevance. 6-Gingerol, one of the major components of ginger, has diverse pharmacologic effects. In this study, the chemoprotective effect of 6-gingerol against hydrogen peroxide-induced DNA damage in human umbilical vein endothelia cells (HUVECs) was investigated. The comet assay was used to monitor DNA strand breaks. To further elucidate the underlying mechanisms, we tested lysosomal membrane stability, mitochondrial membrane potential, the intracellular generation of reactive oxygen species (ROS) and reduced glutathione (GSH). Our data revealed that 6-gingerol significantly reduced the DNA strand breaks caused by hydrogen peroxide. 6-Gingerol effectively suppressed hydrogen peroxide-induced intracellular ROS formation. The GSH depletion in HUVECs was also attenuated by 6-gingerol pretreatment. Moreover, lysosomal membrane stability was destroyed and mitochondrial membrane potential decreased after treated by hydrogen peroxide. Those effects can be protected by 6-gingerol. These firmly indicate 6-gingerol has a strong protective ability against the DNA damage caused by hydrogen peroxide in HUVECs, and the mechanism may relate to the antioxidant activity. Our data suggest 6-gingerol may be beneficial in the prevention of atherosclerosis.
Naturally occurring phytochemicals, in particular those identified from herbal medicines, are finding therapeutic use (Ho et al., 2012). Those agents show efficacy in treatment of cardiovascular disease, cancer and inflammation, which contribute to their chemoprotective potential.
Ginger rhizome (Zingiber officinale), commonly known as ginger, is widely used as a spice and herbal medicine. It has been used for centuries in many countries. Among all the gingerols, 6-gingerol was the major pharmacologically active component. It has been found to possess diverse interesting pharmacological and physiological effects, such as antioxidant (Chakraborty et al., 2012), anti-inflammation (Guh et al., 1995), antiplatelet aggregation (Liao et al., 2012), and anti-fungal (Wei et al., 2005). As a natural antioxidant, 6-gingerol is recommended for prevention of many diseases.
Coronary heart disease (CHD) is a condition wherein coronary arteries become narrowed or blocked. As a main cause of CHD, atherosclerosis is a slowly progressing, in which the abnormalities of endothelial cells structure and function play an initial role in the development (Liu et al., 2013). However, the underlying mechanisms remain to be determined. Oxidative stress, characterized by an imbalance between pro-oxidants and antioxidants, is a key factor in the pathogenesis of atherosclerosis and other cardiovascular diseases (Sun et al., 2013). Many studies have reported that exposure to reactive oxygen species (ROS) is an important stimulator for endothelial cells dysfunction or damage (Wang et al., 2011). As one of the most important ROS, hydrogen peroxide has been extensively used to induce oxidative stress in vitro models.
Many natural phytochemical have been used to protect the vascular endothelia cells survival from oxidative stress damage. Moreover, these phytochemicals are most appropriate in protecting oxidative stress-related damages due to their tendency to exert better protective effect without any distinct side effect (Donovan et al., 2012). As a naturally occurring plant phenol, 6-gingerol has antioxidant activity. In vitro, it can reduce UVB-induced intracellular ROS levels (Kim et al., 2007). In vivo, our previous study showed that 6-gingerol could prevent patulin-induced DNA damage in HepG2 cells (Yang et al., 2011). The antioxidant properties of the phenolic compound may be related to its abilities to donate electrons and to act as free radical scavengers by the formation of stable phenoxyl radicals (Croft 1999).
The most common outcome of oxidative stress is the increased damage of lipid, DNA and proteins that resulted in the development of different pathologies (Donovan et al., 2012). The aim of this study was to investigate the possibility that 6-gingerol may inhibit the oxidative DNA damage of hydrogen peroxide in human umbilical vein endothelia cells (HUVECs). To ascertain the potential chemoprotective properties of 6-gingerol, the degree of DNA damage was monitored by using the comet assay, which has been proved sensitive and reliable methods to study DNA strand breaks (Kirsch-Volders et al., 1997). To elucidate molecular mechanism involved in the intracellular oxidative defense system, the level of intracellular ROS was detected by the 2, 7-dichlorofluorescein diacetate (DCFH-DA) assay and the depletion of glutathione (GSH) by use of fluorometric methods. Moreover, lysosomal membrane stability and mitochondrial membrane potential were tested using fluorescence spectrophotometer.
Materials 6-Gingerol was purchased from Medicass Biotechnologies, Co., Ltd. (Beijing, China) and dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO present in the experiments was 0.1% (vol/vol), and we had run control experiments with DMSO to exclude confounding effects of the solvent vehicle. Hydrogen peroxide, RNAse A, ethidium bromide (EB), DCFH-DA, o-phthalaldehyde (OPT), N-acetyl cysteine (NAC) were purchased from Sigma (St. Louis, USA). All tissue culture reagents, i.e. Minimum Essential Eagle's Medium (MEM), fetal calf serum (FCS), antibiotics (penicillin and streptomycin) and trypsin-EDTA solution were supplied by Invitrogen (Carlsbad, USA).
Cell culture and treatment The HUVECs were obtained from Peking Union Medical College (Peking, China), and cultured in MEM containing 10% FCS, penicillin (100 IU/mL), streptomycin (100 µg/mL) at 5% CO2 at 37°C. For each experiment, cells were treated with 6-gingerol for 24 h, and then exposed to hydrogen peroxide for 1 h.
Comet assay The comet assay was performed as described by Stephens and Singh (Singh and Stephens 1997) with slight modification. HUVECs were incubated with 6-gingerol (10, 20, 40 µM) at 37°C for 24 h, and NAC (10 mM) was used as a positive control. Then hydrogen peroxide (50 µM) was added for another 1 h. To avoid artifacts resulting from apoptosis and necrosis, Hoechst 33342 (8 µg/mL) and trypan blue (50 µg/mL) were employed to detect the apoptotic cells and cell viability. Cell suspensions without apoptotic cells and with cell viabilities >90% were used for the determination of DNA migration. Twenty microliters of cell suspension was mixed with 1% low-melting agarose and placed on frosted slides prelayered with 1.5% regular agarose. Then the slides were immersed in lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, 1% Triton X-100, pH 10) at 4°C for 1 h. After the lysis, the slides were placed in alkaline solution (1 mM EDTA and 300 mM NaOH, pH 13) for 20 min to allow DNA unwinding, and then they were electrophoresed for 30 min at 200 mA. Finally, the slides were viewed using an Olympus BX-51 fluorescent microscope (excitation filter 549 nm, barrier filter 590 nm). Images of 50 randomly selected cells from each slide were analyzed with Comet Assay Software Project casp-1.2.2 (University of Wroclaw, Poland). Three independent experiments were performed.
Measurement of intracellular ROS The formation of intracellular ROS was measured by use of the DCFH-DA method Sohn et al., 2005). Briefly, cells were pretreated with 6-gingerol (10, 20, 40 µM) at 37°C for 24 h before adding hydrogen peroxide (50 µM) for 1 h, and NAC (10 mM) was used as a positive control. Then cells were washed twice with cold PBS, suspended in PBS at 5×105 cells/mL, and incubated with DCFH-DA at a final concentration of 5 µM for additional 40 min at 37°C in darkness. The relative fluorescence intensity of the cell suspensions was measured at excitation wavelength 485 nm and barrier wavelength 530 nm.
Measurement of intracellular GSH Reduced GSH was measured using a modified method (Hissin and Hilf 1976). Cells were incubated with 6-gingerol (10, 20, 40 µM) at 37°C for 24 h before adding hydrogen peroxide (50 µM) for 1 h, and NAC (10 mM) was used as a positive control. Then cells were washed twice with PBS, and treated with 5% trichloroacetic acid (TCA) at 4°C for 30 min to extract GSH. The reaction was performed by mixing the diluted supernatant, phosphate EDTA buffer (pH 8.3), and OPT (50 µg/mL) solution, and the mixture was incubated at 37°C in darkness for 15 min. The fluorescence was read at an emission wavelength of 420 nm and an excitation wavelength of 350 nm.
Lysosomal membrane stability assay Lysosomal membrane stability was determined with a modified method (Pourahmad et al., 2003). After being treated with 6-gingerol (10, 20, 40 µM) for 24 h, and adding hydrogen peroxide (50 µM) for 1 h, the cells were washed twice with PBS and incubated with acridine orange (AO) at a final concentration of 5 µM at 37°C in the dark for 15 min. NAC (10 mM) was used as a positive control. Cells were washed twice to remove the fluorescent dye from the media. The fluorescent intensity from cell suspensions was measured by fluorescence spectrophotometer (HITACHI 650 - 60, Tokyo, Japan) at excitation wavelength of 495 nm and emission wavelength of 530 nm.
Mitochondrial membrane potential assay Mitochondrial membrane potential was measured using amodified method (Pourahmad et al., 2003). Rhodamine 123 was used for the estimation of mitochondrial membrane potential. The cells were incubated with 6-gingerol (10, 20, 40 µM) for 24 h, and then added hydrogen peroxide (50 µM) for 1 h. NAC (10 mM) was used as a positive control. After being washed twice with PBS, the cell pellet was resuspended in 2 mL of fresh medium containing 1.5 µM rhodamine 123, and incubated at 37°C in a thermostatic bath with gentle shaking for 10 min. The cells were centrifuged, and fluorescence from the supernatant was measured at an emission wavelength of 490 nm and an excitation wavelength of 520 nm.
Statistical analysis All values were presented as means ± standard deviation (S.D.). The data were statistically analyzed by use of one-way analysis of variance (ANOVA) and Student's t-test using SPSS v13.0 software. The level of significance was set at P < 0.05 and P < 0.01 for all statistical analysis.
Effect of 6-gingerol on the DNA strand breaks induced by hydrogen peroxide The effect of 6-gingerol on hydrogen peroxide-induced DNA damage is presented in Table 1. Hydrogen peroxide caused a significant increase of the DNA migration compared to the untreated cells (P < 0.01). The increase of the DNA migration was decreased by NAC (the positive control) (P < 0.01). When the cells were pretreated with 6-gingerol for 24 h, the comet tails were not evident, and the comet tail moment values were significantly decreased compared to that of only hydrogen peroxide -treated cells (P < 0.01). The results also indicated that 6-gingerol did not cause obvious DNA damage in the HUVECs.
| Group | Tail DNA (%) | Tail length (µm) | Tail moment (µm) |
|---|---|---|---|
| control | 6.48 ± 2.79 | 3.86 ± 1.22 | 0.67 ± 0.25 |
| H2O2 (50 µM) | 54.23 ± 9.24** | 39.43 ± 4.28** | 10.86 ± 3.50** |
| 6-gingerol (10 µM) + H2O2 | 45.21 ± 8.18# | 27.70 ± 3.51# | 7.69 ± 2.81# |
| 6-gingerol (20 µM) + H2O2 | 26.36 ± 5.86## | 14.41 ± 2.71## | 5.65 ± 1.34## |
| 6-gingerol (40 µM) + H2O2 | 17.76 ± 2.45## | 13.07 ± 5.17## | 2.24 ± 0.64## |
| NAC (10 mM) | 23.22 ± 7.12## | 15.45 ± 3.56## | 4.98 ± 0.78## |
DNA strand breakages were estimated by Comet assay. HUVECs were pretreated with 6-gingerol (10, 20, 40 µM) for 24 h and then exposed to hydrogen peroxide for 1 h. NAC was the positive control. Results are the mean ± S.D. (n = 3).
Effects of 6-gingerol on ROS formation induced by hydrogen peroxide The generation of ROS in HUVECs was evaluated by the changes in DCF fluorescence intensity (Fig. 1). There was no increase in the intracellular level of ROS after incubation with 6-gingerol compared to that of the untreated sample (data not shown). The DCF fluorescence intensity increased significantly when the HUVECs were treated with 50 µM hydrogen peroxide (P < 0.01). As the positive control, NAC significantly decreased the ROS level (P < 0.01). Pretreatment with 6-gingerol (10, 20, 40 µM) for 24 h produced a significant and dose-dependent reduction of DCF fluorescence in the cells (P < 0.05 or P < 0.01).

Effect of 6-gingerol on the ROS formation induced by hydrogen peroxide. HUVECs were pretreated with 6-gingerol (10, 20, 40 µM) for 24 h and then exposed to hydrogen peroxide for 1 h. NAC was the positive control. Each bar represents mean ± S.D. of three independent experiments (n=3). **P < 0.01, significant difference from control. #P < 0.05 and ##P < 0.01, significant difference from the group treated with hydrogen peroxide only.
Effect of 6-gingerol on the GSH depletion induced by hydrogen peroxide Fig. 2 indicates that a striking decrease of intracellular GSH was observed at the concentrations of hydrogen peroxide (50 µM) (P < 0.01). This reduction was significantly and dose-dependently prevented by the pretreatment with 6-gingerol (10, 20, 40 µM) (P < 0.05 or P < 0.01). As the positive control, NAC significantly increased the GSH level (P < 0.01).

Effect of 6-gingerol on the GSH depletion induced by hydrogen peroxide. HUVECs were pretreated with 6-gingerol (10, 20, 40 µM) for 24 h and then exposed to hydrogen peroxide for 1 h. NAC was the positive control. Each bar represents mean ± S.D. of three independent experiments (n = 3). **P < 0.01, significant difference from control. #P < 0.05 and ##P < 0.01, significant difference from the group treated with hydrogen peroxide only.
Effect of 6-gingerol on lysosomal membrane stability AO was used to detect lysosomal membrane stability. Fig. 3 shows the fluorescence intensity for AO in HUVECs exposed to different groups. A statistically significant increase of AO fluorescence intensity was observed in cells treated with hydrogen peroxide (50 µM) (P < 0.01). Pretreatment with 6-gingerol (10, 20, 40 µM) for 24 h produced a significant and dose-dependent reduction of AO fluorescence in the cells (P < 0.05 or P < 0.01). AO fluorescence was decreased by NAC (P < 0.01).
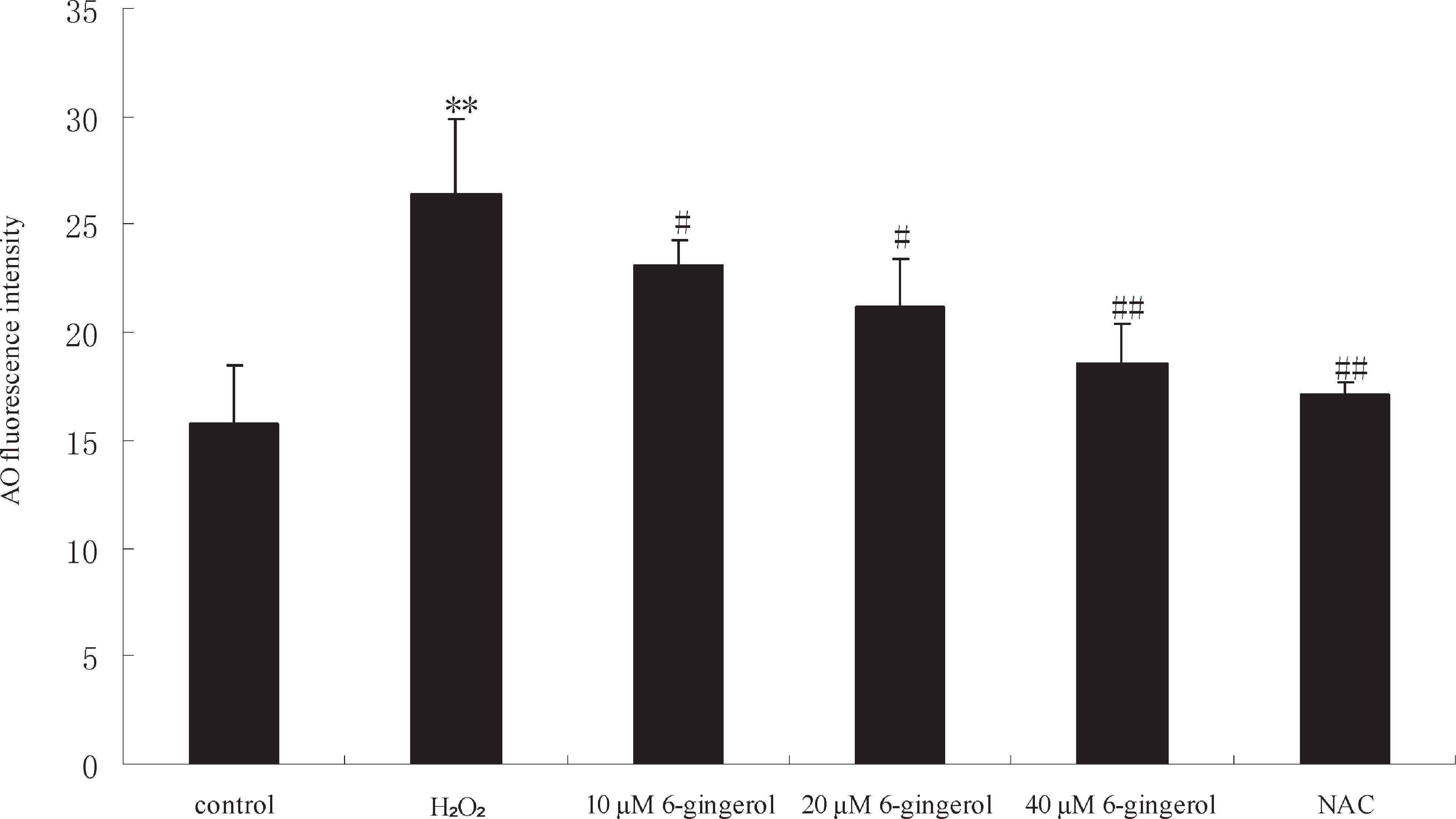
Effect of 6-gingerol on lysosomal membrane stability. HUVECs were pretreated with 6-gingerol (10, 20, 40 µM) for 24 h, then exposed to hydrogen peroxide for 1 h, and stained with AO. NAC was the positive control. Each bar represents mean ± S.D. of three independent experiments (n = 3). **P < 0.01, significant difference from control. #P < 0.05 and ##P < 0.01, significant difference from the group treated with hydrogen peroxide only.
Effect of 6-gingerol on mitochondrial membrane potential depletion induced by hydrogen peroxide The state of the mitochondrial membrane potential was examined by measuring the relative differences in fluorescence of the cationic dye rhodamine 123 between control and hydrogen peroxide, or 6-gingerol treated cells. As presented in Fig. 4, a statistically significant decrease of rhodamine 123 fluorescence intensity was observed in cells treated with hydrogen peroxide (50 µM) for 1 h (P < 0.01). Pretreatment with 6-gingerol (10, 20, 40 µM) for 24 h produced a significant and dose-dependent increase of rhodamine 123 fluorescence in the cells (P < 0.05 or P < 0.01). The positive control, NAC increased the level of rhodamine 123 fluorescence significantly (P < 0.01).

Effect of 6-gingerol on mitochondrial membrane potential. HUVECs were pretreated with 6-gingerol (10, 20, 40 µM) for 24 h, then exposed to hydrogen peroxide for 1 h, and stained with rhodamine 123. NAC was the positive control. Each bar represents mean ± S.D. of three independent experiments (n = 3). **P < 0.01, significant difference from control. #P < 0.05 and ##P < 0.01, significant difference from the group treated with hydrogen peroxide only.
Coronary heart disease represents the primary cause of death with a high incidence on human health and community social costs. The chemopreventive agents particularly phytochemicals are found to be effective in reducing heart disease incidences as they have antioxidative potentials.
This study investigated the protective effect of 6-gingerol against hydrogen peroxide-induced DNA damage in HUVECs and estimated the underlying mechanisms of the oxidative stress in the reaction. DNA is well known for its susceptibility to ROS with oxidative damage (Jha 2008). DNA double strand break (DSB) is one of the occurrences of triggered DNA damage, which is induced by ROS, ultraviolet (UV), and radiation chemicals, that is formed during the course of normal metabolism (Hartlerode and Scully 2009; Hiom 2010). With respect to DNA damage data, hydrogen peroxide caused significant increase of DNA migration in comet assay at 50 µM. Our findings showed that 6-gingerol could exert protection against the toxicity of hydrogen peroxide.
To explore the mechanisms of protection effects, we investigated the level of ROS using DCFH as a fluorescent probe. This study shows HUVECs rapidly generated ROS after hydrogen peroxide treatment. ROS, such as singlet oxygen, superoxide anion and hydroxyl radical are generated from different sources, including normal cell respiration and mitochondria electron flux. ROS play important roles in the pathogenesis of atherosclerotic vascular disease (Kang 2013). It is believed that increased production of ROS and oxidative damage in the vascular endothelium contribute to CHD initiation and progression. The imbalance between ROS production and elimination results increased oxidative stress and tissue injury, and leads to many cardiovascular diseases. ROS can stimulate oxidation of low density lipoprotein (LDL), cholesterol, cholesterol derived species, and protein modifications which can lead to foam cell formation and atherosclerotic plaques (Sauer et al., 2010). Moreover, ROS could react quickly with NO in the cells and forms peroxynitrite which no longer stimulates cGMP production in smooth muscle cells that leads to vascular relaxation. In our experiment, the increase of intracellular ROS formation, induced by hydrogen peroxide treatment, was prevented by 6-gingerol, suggesting that ROS are involved in the inhibitory effect of 6-gingerol on hydrogen peroxide -induced DNA damage. As a potent antioxidant, treatment of cells with 6-gingerol significantly affected the ROS production in many experiments (Lee et al., 2009). The present result suggested that the mechanisms of the preventive effects of 6-gingerol may underlie ROS scavenging.
It has been conformed that antioxidants exert their protective effects not only by scavenging ROS, but also by inducing the antioxidants in the cell. Cells contain numerous antioxidant defenses that can detoxify ROS or reduce their effects. GSH and GSH peroxidases constitute the principal antioxidant defense system in mammalian cells. Depletion of intracellular stores of GSH plays an important role in protecting against oxidative stress by reacting directly with ROS (Elsayed and Omaye 2004; Han et al., 2004). The most important function of GSH is to protect against oxidative damage caused by ROS through enzymatic and nonenzymatic reactions (Meister 1994). In many diseases, including atherosclerosis, cancer, neurodegenerative disease, and aging, GSH can scavenge of free radicals, modulate hydrogen peroxide level and interact with nitric oxide pathways (Armani et al., 2009). There is evidence that 6-gingerol pretreatment significantly enhanced the levels of reduced GSH and activities of superoxide dismutase and catalase against cisplatin-induced oxidative stress and renal dysfunction (Kuhad et al., 2006). In our research, GSH level was significantly increased after 6-gingerol pretreatment, and the ROS generation can be finely controlled. The results suggest GSH is able to quench ROS and protect cells from toxic compounds. In the present study, we noticed that lysosome and mitochondria may be the primary targets for 6-gingerol in HUVECs. Lysosomal and mitochondrial dysfunction can be involved in DNA damage by imposing oxidative stress (Li et al., 2008). Lysosomal destabilization maybe an earlier event, and released lysosomal enzymes can either directly or indirectly attack mitochondria and induce enhanced formation of ROS. It is found that free fatty acids induce lysosomal breakdown, resulting in cathepsin B activation that then triggers mitochondrial dysfunction and increased ROS production (Zhao et al., 2003). Li et al reported 7-oxysterols, the major cytotoxic component found in oxidized low-density lipoprotein, caused apoptosis of HUVECs associated with early accumulation of cellular lipids and activation of oxidative lysosomal pathways, which was followed by cellular oxidative stress, and mitochondrial damage. Mitochondria are primary sites of ROS formation that cause progressive damage to mitochondrial DNA and proteins in parallel to intralysosomal lipofuscin accumulation (Li et al., 2011). Our study demonstrates that 6-gingerol protected hydrogen peroxide-induced DNA damage by preserving lysosomal membrane integrity, increasing mitochondrial potential, and decreasing production of cellular ROS.
Dietary phytochemicals have been widely regarded as antioxidants. In this study, 6-gingerol has a strong protective ability against the DNA damage caused by hydrogen peroxide in HUVECs, and the mechanism may relate to the antioxidant activity. Moreover, the damage of lysosomal and mitochondrial by hydrogen peroxide was also prevented by 6-gingerol. By contrast, there is an increasing body of evidence to suggest the prooxidative effects of phytochemicals (Kelly et al., 2001; Cao et al., 2006). In our previous study, 6-gingerol can apparently act as a prooxidant. It induced genotoxicity probably by oxidative stress in some cancer cells, and lysosomal and mitochondrial damage were observed in 6-gingerol-induced toxicity (Yang et al., 2011). These contradictory results help support the hypothesis that 6-gingerol may play a conflicting dual role in different cells. Such differences in response may be the result of differential sensitivity and antioxidant capacity between different cells (Lambert et al., 2011).
To our knowledge, this is the first study to show the protection effects induced by 6-gingerol in HUVECs. In conclusion, 6-gingerol can protect the DNA strand breaks in HUVECs induced by hydrogen peroxide. These effects are probably contributed to oxidative stress; lysosome and mitochondria may be the primary targets. Hence, 6-gingerol might have potential applications in the chemoprevention of atherosclerosis.
Acknowledgments This work was supported by the National Natural Science Foundation of China (81102135).
The authors have declared that there is no conflict of interest.