2014 年 20 巻 5 号 p. 955-960
2014 年 20 巻 5 号 p. 955-960
BACKGROUND: Pen a1 is a major allergen in shrimp. It is a 284 amino acid protein member of the tropomyosin family with a molecular weight of 36 kDa. Irradiation and heat treatments have been previously shown to reduce the immunoreactivity of Pen a1, but since anaphylaxis is not induced by the whole protein but by epitopes within the protein, studying of the influence of these treatments on the epitopes of Pen a1 is helpful for understanding the sensitization mechanism. In this study, the immunoreactivity of the five epitopes of Pen a1 exposed to heat and irradiation treatments was detected by Ci-ELISA and western blot, using specific anti-sera generated from 5 synthesized epitope peptides of Pen a1. We examined the function of each epitope in the sensitization process by analyzing the variations of their immunoreactivity following irradiation, heat treatment, or a combination of both.
RESULTS: Pen a1 was treated by gamma irradiation, heat at 100°C or a combination of irradiation and heat. The IC50 value for each of the five epitopes was detected. The IC50 value of epitopes 1, 2, and 4 decreased under all treatments, especially epitope 4; its IC50 value decreased sharply from 15.88 to 0.11 follwing 7 kGy irradiation and to 0.03 – 0.04 under 10 kGy or 7 kGy irradiation and heat. The IC50 value of epitope 3 increased from 1.49 to 5.32 under 7 kGy irradiation and to 10.03 under 7kGy and heat, but mildly decreased after 10 kGy irradiation. In contrast, the IC50 value of epitope 5 hardly changed following any treatments.
CONCLUSION: The variation in the IC50 values of epitope 3 was consistent with the IC50 values measured from the whole Pen a1 protein, while epitope 5 was stable following irradiation and heat treatments. Since the variation of immunoreactivity of epitope 3 was consistent with Pen a1, this suggests that epitope 3 is the determinative epitope in the allergenicity of Pen a1; epitope 5 was stable with all treatment conditions, suggesting that it could be used in immunoassay kits for the detection of treated shrimp.
Shrimp are one of the most important foodborne allergens in the world. It is estimated that about 20% of patients treated for allergies are allergic to shrimp, and the pediatric rate for shrimp allergies is as high as 60% in China (Hu, 2013). The major allergen of shrimp is a tropomyosin family member with a molecular weight of 36 kDa. This protein, named Pen a1 by Daul (Daul et al., 1994), is an acid glycoprotein with 4.0% glycosyl content and is recognized to be antigenic in over 80% of shrimp allergy patients.
At present, there are no effective medical treatments available for eliminating allergens; the best therapy for patients diagnosed with food allergies is avoidance of the offending foods (Lehrer, 2003). Therefore, finding an effective method to remove food allergens is important for the health benefits of allergic individuals. Currently, the main processing methods of reducing shrimp allergens are heat, gamma irradiation, high pressure, microwaves, and enzymolysis (Hu, 2013). Among these methods, gamma irradiation is widely studied due to its advantages, including no residues and the ability for mass food processing. It was reported that irradiation of 10 kGy can decrease or abolish the immunoreactivity of the shrimp allergens. In contrast, heat treatment of shrimp alone cannot reduce the allergens, but when combined with irradiation, the immunoreactivity of the shrimp was reduced dramatically (Byun et al., 2002; Li et al., 2007).
Anaphylaxis is not induced by the whole protein, but rather by its epitopes. Based on the frequency and intensity of the IgE reactivities, Ayuso identified five major IgE-binding regions in the Pen a1 protein; all of them were 15 – 38 amino acids long, and some regions contained several epitopes (Ayuso et al., 2002). In addition, the five epitopes examined were all positioned at regular intervals of approximately 42 amino acids (7 heptads). Additionally, Reese observed 8 linear IgE-binding epitopes within the five IgE-binding regions of Pen a1 (Reese et al., 1999ab). Using bioinformatics, researchers constructed a polygenetic tree and found high protein homology among all crustaceans. The amino acid sequences of the Pen a1 epitopes were highly conserved, and some species of shrimp had identical amino acid sequences (Zheng et al., 2011). Most recent research to examine the desensitization of shrimp allergens focused on the whole tropomyosin protein of Pen a1. However, it is still not clear how processing methods alter the function of Pen a1 epitopes leading to reduced immunoreactivity.
In the present paper, the influence of gamma irradiation and heat treatment on Pen a1 was studied. To clarify the mechanism of antigen reduction, the effect of different processing treatments on the five epitopes of Pen a1 were compared using anti-sera specific to each epitope. Variations in the immunoreactivity of the five epitopes following different treatments were also evaluated.
Whole Pen a1 anti-serum and epitope anti-sera (Zhao et al., 2013) Pen a1 anti-serum was generated using Pen a1 protein, extracted and purified from shrimp, as the immunogen in New Zealand white rabbits. Specific epitope anti-sera was generated using five synthetic epitope peptide fragments of Pen a1 as the antigen in New Zealand white rabbits. The five peptides were chosen based on the research of Ayuso (2002) (Table 1), synthesized using the Fmoc method, and then were conjugated to keyhole limpet hemocyanin (KLH) and bovine serum albumin (BSA) respectively, by the glutaric dialdehyde method to get artificial immunes and coating antigens. The specificity of epitope anti-sera was tested using coating antigens and was shown to be specific.
| name | amino acids site | amino acids sequence | length |
|---|---|---|---|
| Peptide1 | 43 – 57 | VHNLQ KRMQQ LENDL-C | 15 |
| Peptide2 | 85 – 105 | VAALN RRIQL LEEDL ERSEE R-C | 21 |
| Peptide3 | 133 – 148 | RSLSD EERMD ALENQ L-C | 16 |
| Peptide4 | 187 – 202 | C-ESKIV ELEEE LRVV GN | 16 |
| Peptide5 | 247 – 284 | C-QKLQK EVDRL EDELV NEKEK YKSIT DELDQ TFSEL SGY | 38 |
Regents All of the regents used for these studies were of analytical grade. Buffers and reagents used for western blotting were as follows: Towbin transfer Buffer, TBS (0.1 mol L−1 phosphate buffer, pH 7.4, 0. 15 mol L−1 NaCl) and PBST (10 mmol L−1 phosphate buffer, pH 7.4, 0. 15 mol L−1 NaCl, 0.05% Tween 20). Buffers and reagents used for competitive inhibition ELISA (Ci-ELISA) were as follows: blocking buffer (0 .01 mol L−1 phosphate buffer, pH 7.4, containing 0.1% BSA, 0. 15 mol L−1 NaCl) and PBST used as a washing buffer. Solid-phase enzyme immunoassays were performed in 96-well microtitre plates (Solarbio, USA) using a Multiscan ELISA reader (I-mark, BIO-RAD, USA).
MethodsPen a1 extraction and purification (Zhao et al., 2013) Shrimp (Penaeus vannamei) were purchased from a local seafood market, the husks were removed, and the shrimp flesh was homogenized. The shrimp homogenate was degreased with precooling acetone at 4°C in a ratio of 1:5 for 30 min, with intermittent stirring. After centrifugation at 12000 × g for 20 min at 4°C, the precipitate was degreased again, dried, and grinded to acetone powder. The powder was dissolved in extraction buffer (1 mol L−1 KCl and 0.05% DTT) in a ratio of 1:4 for 12h at 4°C with constant stirring, then centrifugated, and the supernatant was collected. The precipitate was extracted again, and the supernatant from both extractions were incorporated. Protein extracts were stored at −20°C until further purified. The protein extract was separated and purified by ammonium sulfate and isoelectric precipitation. First, the protein extract was precipitated with 35% ammonium sulfate saturation. The precipitate was dissolved in PBS (0.01 mol L−1, pH 7.4), and then centrifuged. The dissolution of the precipitate was reprecipitated with isoelectric point precipitation at pH 4.74 and centrifuged. The resulting precipitate was dissolved in PBS and dialyzed against distilled water for 48h. The final solution was lyophilized and stored at −20°C. The Bradford assay was used to determine the concentration of the purified Pen a1 protein (Bradford, 1976). The purity of Pen a1 was analyzed by denaturing protein electrophoresis (SDS-PAGE). The purified Pen a1 protein was used for both experiments as well as the immunogen for the production of anti-sera.
Irradiation and heat treatment of Pen a1Gamma irradiation treatment The lyophilized powder was dissolved in PBS (0.01 mol L−1, pH 7.4) at a concentration of 0.3 g mL−1, sealed in tubes, and irradiated by a 60Co source at 0°C at a dose of 7.0 or 10.0 kGy. Each sample was irradiated in the presence of dosimeters. The samples were then subjected to heat treatment.
Heat treatment The irradiated samples and untreated samples were sealed in tubes and heated in a boiling water bath for 5, 15, or 30 min, and then cooled to room temperature. Untreated shrimp protein extract (without treatment of gamma irradiation and heat) was used as control, and all of the samples were stored at −20°C for further study.
Protein electrophoresis (SDS-PAGE) and western-blot We used the method of Laemmli (1970) for denaturing protein electrophoresis. Samples were mixed with loading buffer (1 mol L−1 Tris-HCl, pH 6.8, 0.24 mL; 10% SDS, 0.8 mL; 50% glycerol 1 mL; bromophenol blue, 40 mg; β-mercaptothanol 0.2 mL; distilled water, 7.76 mL) in a ratio of 1:1, boiled in a water bath for 5 min, then electrophoresed in 12% analytical SDS-polyacrylamide gels using a vertical electrophoresis system (AE-6450, ATTO, Japan). Gels were visualized either by staining with Coomassie brilliant blue R-250 or transferred to a nitrocellulose membrane (0.45 nm, Solarbio, USA). For western blotting, Pen a1 protein was transferred electrophoretically from the gels to nitrocellulose membranes by employing a constant current of 96 mA for 1.5 h at 0°C. After transfer, the membranes were stained with ponceau to verify the protein transfer. The membranes were incubated with blocking buffer (skim milk powder, 0.1 mol L−1 TBS, Tween −20, distilled water) overnight at 4°C. Following incubation with blocking buffer, the membranes were incubated with anti-sera (whole Pen a1 anti-serum or five epitope anti-sera, 1:1000 dilution with blocking buffer) for 1 h, washed for 5 – 10 min with TBST (0.01 mol L−1 TBS, 0.1% Tween −20) five times, then incubated for 1 h with a polyclonal goat anti-rabbit IgG (Solarbio, China) secondary antibodies conjugated with horseradish peroxidase (HRP) diluted 1:800 with blocking buffer. After washing with TBST, Diaminobenzidine (DAB) was used in the dark to develop the western blots. Once the bands were visible the membranes were rinsed with TBST, dried, and the membranes and gels were scanned using using Alpha Innotech (AlpH aEase®FC, AlpHa USA).
Competitive indirect enzyme-linked immunosorbent assay (Ci-ELISA) The Ci-ELISAs were performed using the method of Sathe SK (Sathe et al., 2001). We chose Pen a1 protein irradiated at 7 or 10 kGy or irradiated with 7 kGy and heated for 30 min to measure the IC50 values. Ci-ELISAs were performed with a Multiskan ELISA reader (I-mark, BIO-RAD, USA). Microtiter plates with 96 wells (Solarbio, USA) were coated with 100 µL well−1 of Pen a1 protein in carbonate buffer (0.05 mol L−1, pH 9.6) at 4°C overnight. The plates were washed with PBST by applying the Wellwash Versa Microplate Washer (1575, BIO-RAD, USA) five times. After patting dry, the plates were coated with 150µL well−1 of blocking buffer (skimmed milk powder, Tween-20, dissolved in PBS) for 1 h to eliminate non-specific binding. The plates were washed and dried again. We then added 50 µL of treated samples serially diluted in PBST to coat and block the wells, then added 50 µL of anti-sera (either whole Pen a1 antiserum or anti-sera for each of the five epitopes) in an appropriate dilution. Blank wells were coated with 100 µL of PBST as a control for non-specific binding. The plates were incubated for 1.5 h, followed by washing and drying. Then the plates were coated with 100 µL of a 1:10,000 dilution of HRP-conjugated goat anti-rabbit IgE, and incubated for 1 h. The plates were washed with PBST five times and patted dry. Following the wash, 100 µL 0.2% 3,3′,5,5′-tetramethyl benzidine (TMB) in 0.1 mol L−1 phosphate citrate buffer, pH 5.0 with 0.04% hydrogenperoxide (v/v, 30% H2O2) was added to the plates and allowed to develop for 15 min, then 2.0 mol L−1 H2SO4 (50 µL well−1) was added to stop the reaction. The Multiskan FC (I-mark, BIO-RAD, USA) was used to measure the absorbance at 450 nm. All ELISAs were performed in five parallel, and the data obtained were expressed in mean ± SD.
Statistical analysis Appropriate data were analyzed for statistical significance ANOVA (SAS Inc, Cary, NC, USA) and the Fisherman test (P = 0.05).
Molecular weight changes in shrimp protein under heat and irradiation treatment The Pen a1 protein samples that underwent different treatments were compared using SDS-PAGE (Fig.1). All the samples were the same concentration (0.3 mg/mL). All heat-treated samples contained the 36 kDa protein at a similar concentration to the control sample; only new proteins were found when the heating time exceeded 15 min, suggesting that heat treatment alone couldn't substantially change the molecular weight and concentration of the samples. However, Pen a1 treated either with irradiation or irradiation combined with heat demonstrated remarkably lower band concentrations compared with the control. The background color of lanes (5 – 12) all deepened, which indicated that fragmentation, crosslinking and aggregation of the protein might occurred. The combination treatments, especially the 7kGy irradiated combined with heat treatments (lane 6–8) showed an enhanced destructive effect compared to irradiation treatment alone.

SDS-PAGE of Pen a1 under different treatments
Lane 1: Pen a1 untreated; lane 2 – 4: Pen a1 heated at 100°C for 5, 15 and 30 min, respectively; lane 5: Pen a1 irradiated at 7 kGy; lane 6 – 8: Pen a1 irradiated (7 kGy) and then heated for 5, 15, 30 min, respectively; lane 9: Pen a1 irradiated at 10 kGy; lane 10 – 12: Pen a1 irradiated (10 kGy) and then heated for 5, 15, 30 min, respectively
Immunoreactivity change of Pen a1 under different treatments The immunoreactivity of Pen a1 under different treatments was evaluated by western-blot (Fig.2a) and Ci-ELISA tests (Table 1). Pen a 1 protein was detected on the western blots using anti-sera generated from the whole protein. Based on the results from the western analysis, the band intensity of 36kDa protein from the heated samples were almost same, and decreased a little until heated for 30 min, which meant that the immunoreactivity of the heated samples was almost the same with control sample. In contrast, the band intensity of 36 kDa protein from the 7 kGy and 10 kGy irradiated samples as well as all of the samples treated with both irradiation and heat significantly decreased compared with the control sample. And the most significant decrease was observed in samples of 7 kGy irradiated combined with heat treatment and 10 kGy irradiated sample (Fig.2a, lane 6 – 9). The background color of the high molecular weight area (>70kDa) increased in 7 kGy irradiated sample (lane 5). Similar results were demonstrated with Ci-ELISA. Since heat treatments have previously been shown to have little effect on decreasing the allergenicity of shrimp5, only the irradiated and combined treated Pen a1 samples were measured by Ci-ELISA test. The Pen a1 samples were first diluted in a series of concentration gradients, then added to the plates as the competitive antigen. Table 1 showed that the IC50 value of Pen a1 under 7 kGy irradiation treatment decreased slightly (from 19.78 to 18.62), but increased to nearly double (28.93) when the dose was 10 kGy. However, when the Pen a1 was irradiated (7 kGy) and heated (30 min), the IC50 value was 135 fold higher (2717.4) than the control sample.
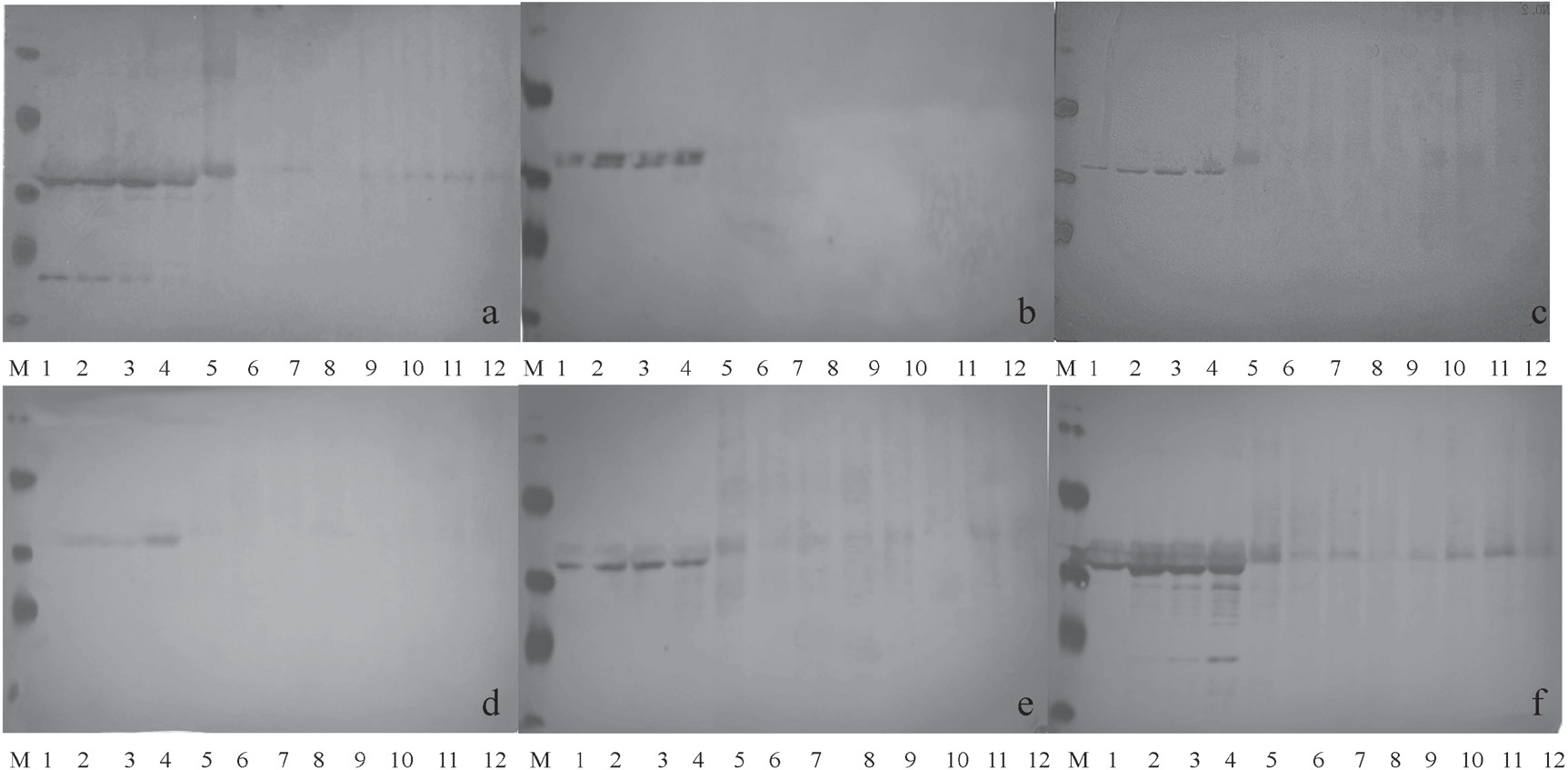
Western blot of Pen a1 under different treatments using antibodies for Pen a1 and specific epitopes of Pen a
a: anti-serum to Pen a1 protein was used as the primary antibody ; b-f: epitope1,epitope 2, epitope 3, epitope 4, epitope 5 anti-sera used as the primary antibodies, respectively.
Lane 1: Pen a1 untreated; lane 2 – 4: Pen a1 heated at 100°C for 5, 15 and 30 min, respectively; lane 5: Pen a1 irradiated (7 kGy); lane 6 – 8: Pen a1 irradiated (7 kGy) and heated for 5, 15, 30 min, respectively; lane 9: Pen a1 irradiated (10 kGy); lane 10 – 12: Pen a1 irradiated (10 kGy) and heated for 5, 15, 30 min, respectively; Lane M: molecular weight marker
Combined, these results indicated that heat treatment alone cannot decrease the immunoreactivity of shrimp. Irradiation alone can decrease the immunoreactivity only when the dose is above 10 kGy, but when combined with heat, the immunoreactivity of Pen a1 significantly decreased.
Immunoreactivity variations of five epitopes under different treatments To examine the effects of different treatments on specific antigenic epitopes of Pen a1, western-blot was performed using five specific anti-sera (Fig. 2b–2f). These anti-sera were generated by immunization of rabbits with five synthetic epitope peptides that were previously described by Ayuso. The results from the western blots with each of the 5 anti-sera were similar to those using whole Pen a1 anti-serum. The band intensity of the five epitopes of Pen a1 from the heated sample was all higher than the control sample. In contrast, the blots showed that the band intensity of 36 kDa decreased significantly in the irradiated samples as well as the irradiated and heat treated samples. The titer and the concentration of used five anti-sera were different, so the image definition of the five western-blots was different. Among the results of five western-blots, the strongest reaction was observed between anti-sera to epitope5 and treated Pen a1. In Fig. 2b and 2d, the bands detected by anti-sera to epitope 1 and 3 almost disappeared in samples that were treated with either irradiation alone or in combination with heat (lane 5 – 12). The immunoreactivity of the five epitopes of Pen a1 under different treatments was also analyzed by Ci-ELISA to determine the IC50 values (Table 2). Compared to Pen a1, each of the five epitopes showed variation in the response to the different treatments. The IC50 values measured with epitope 1, 2, 4 anti-sera decreased with the treatments, suggesting that the immunoreactivity of 3 epitopes increased, particularly epitope 4. IC50 values measured with epitope 3 antiserum increased with irradiation alone (<7 kGy; from 1.49 to 5.32) and irradiation in combination with heat (10.03). This trend in variation was mainly consistent with the variation demonstrated by IC50 values measured with anti-sera against Pen a1 protein. In addition, the IC50 values measured by anti-sera to epitope 5 scarcely changed in any treatment groups, suggesting that epitope 5 of Pen a1 was stable in all treatments.
| Treatment | Anti-serum immunized with Pen a 1 and its 5 Synthetic epitope peptides | |||||
|---|---|---|---|---|---|---|
| Whole Pen a1 | epitope 1 | epitope 2 | epitope 3 | epitope 4 | epitope 5 | |
| Control | 19.78 ± 2.14 | 11.92 ± 1.98 | 9.16 ± 1.54 | 1.49 ± 0.23 | 15.88 ± 2.11 | 0.54 ± 0.11 |
| Irradiated (7kGy) | 18.62 ± 2.09 | 0.03 ± 0.01 | 0.30 ± 0.09 | 5.32 ± 0.87 | 0.11 ± 0.07 | 0.28 ± 0.08 |
| Irradiated (10kGy) | 28.93 ± 3.18 | 0.18 ± 0.07 | 0.18 ± 0.07 | 1.13 ± 0.21 | 0.03 ± 0.01 | 0.10 ± 0.05 |
| Irradiated (7kGy) +heated (30min) | 2717.4 ± 89.45 | 0.22 ± 0.08 | 0.19 ± 0.07 | 10.03 ± 2.01 | 0.04 ± 0.01 | 0.34 ± 0.10 |
Data are expressed as IC50 of processed and unprocessed samples (mean ± SEM).
Our study demonstrated that heat alone cannot reduce the allergenicity of Pen a1. Irradiation at doses below 10 kGy did not decrease, but rather increased the immuoreactivity of Pen a1, while heat combined with irradiation treatment can reduce the immunoreactivity of Pen a1 significantly. These results are consistent with previous studies (Li et al., 2007ab; Hansen et al., 1992; Naqpal et al., 1989; Sancho et al., 2005; Byun et al., 2000). But the impact mechanism of the allergenicity of Pen a1 is still unclear. Our study tried to explain the impact at epitopes level.
To investigate the mechanism of reduction of immunoreactivity of Pen a1, we studied the IC50 values of five epitopes of Pen a1 using Ci-ELISA (Table 1) separately. Our results showed that only the immunoreactivity of epitope 3 decreased consistently with antisera to the entire Pen al protein, while the immunoreactivity of epitope 1, 2 and 4 all increased under the treatment conditions. Only the immunoreactivity of epitope 5 scarcely changed in all treatments. This could be explained by the mode of action of irradiation and heat treatments. Gamma radiation may randomly break the peptide chain and damage the conformation of the protein by forming radicals and heat treatment could enlarge the function, which leaded to protein fragmentation, destroyed epitopes of Pen a1, and reduced immunoreactivity of the whole protein. Besides, irradiation is thought to enhance protein crosslinking, which may lead to protein aggregation and expose hydrophobic groupings on the surface of the protein (Kume et al., 1994). We can see that the background color of the high molecular weight area (>70 kDa) increased in 7 kGy irradiated sample (lane 5), indicated that protein aggregation or crosslinking might happened. This may increased the immunoreactivity, just like the epitopes 1, 2, and 4 in Ci-ELISA. In contrast, since epitope 5 is 38 amino acids long and consists of 3 small epitopes, the treatments may have broken weak bonds which were not in the decisive part of the epitope, and the treatment produced new epitopes while breaking the structure of epitope 5, which made even macroscopically, so epitope 5 remained stable under all treatments. Moreover, epitope 3 might be the determinative epitope of Pen a1, due to its consistency with the results demonstrated by the anti-sera to Pen a1. If epitope 3 was damaged by a change in the sequence of its amino acids, the immunoreactivity of whole protein may decrease.
The results of western-blotting and Ci-ELISA were different from the Figure 2 and Table 2. On the one hand, the western-blot was qualitative and was not as precise as Ci-ELISA, and reactions might be not completely on membranes, which made some immunoblots vague. On the other hand, some damaged peptides with intact epitope were too short to react on the membrane, but still have the immunoreactivity, or because of the low concentration or other reasons, it might not be seen from the immunoblots. And the bands detected by anti-sera to epitope 1 and 3 in Fig.2 almost disappeared in samples that were treated with either irradiation alone or in combination with heat (lane 5 – 12), it indicated that the reaction between anti-sera and the treated protein was weak on the membrane, or the epitope was destroyed, and could hardly react with anti-sera.
To date, the immunologic-based methods for the detection of allergens require kits. The specificity and sensitivity of the ELISAs depend on the antibodies, and the antibodies are usually acquired by immunizing animals. This process is both complicated and time-consuming. These kits also often have the problem of high false positive rates due to the purity of the antibodies. In addition, the treatments, such as heating and baking, may change the conformation of detected proteins, leading to false negative results. So if we use a stable epitope like epitope 5 to immunize animals for anti-sera of the kits, it might be more convenient and economical in the immunologic detection. Even though more work is necessary to explain the impact and structural features of epitopes on Pen a1 immunogenicity, it is concluded that irradiation combined with heat could decrease the immunoreactivity of Pen a1, and the influences on the five Epitopes during the treatments were different, epitope 3 seems to be determinative and epitope 5 was stable to the irradiation and heat treatments.
Acknowledgements This research was supported by the special fund for agro-scientific research in the public interest from the Ministry of Agriculture, China (201103007), 2011–2015