2014 Volume 20 Issue 5 Pages 971-978
2014 Volume 20 Issue 5 Pages 971-978
The ginger rhizome (Zingiber officinale) is known to show a pale yellow color. However, the compounds responsible for the color are not known. In this study, 62 kinds of ginger rhizomes originating from different cultivars or different cultivation locations were collected for analysis of yellow pigment compounds. Ultra-performance liquid chromatography profiles at 420 nm for each sample were used for principal component analysis. Curcumin, demethoxycurcumin, and 6-dehydrogingerdione were identified as the main common compounds contributing to the yellow color, and their average amounts were 2.2 ± 0.1, 1.6 ± 0.1, and 20.0 ± 1.1 mg/100 g fresh weight, respectively. Curcumin and demethoxycurcumin were contained in cv. Kintoki samples at higher levels. However, their variation suggested that the yellow pigment of ginger rhizome is more dependent on the cultivation conditions and less on the cultivar. Comparative analysis of these amounts and the b-value between mature seed rhizomes and immature rhizomes from the same lines suggested that yellow pigments were synthesized during rhizome maturation.
The ginger (Zingiber officinale) rhizome is one of the most popular spices in the world. Its characteristic pungent flavor and fresh citrus-like aroma make it a preferred ingredient in various cuisines and beverages. Especially in Japan, fresh ginger rhizome is frequently treated as a vegetable at the supermarket and is eaten pickled and dipped in seasonings such as Miso paste. Furthermore, it has been used as a garnish in traditional Japanese cuisine for a long time. Therefore, the flavor and appearance of fresh ginger rhizome are more important factors in determining its quality in Japan than in other countries.
Ginger rhizomes show a basically pale yellow color, and the color density depends on the cultivar, cultivation region and maturation status. The rhizome of Curcuma longa, another member of the Zingiberaceae, is well known to contain strong yellow pigments known as curcuminoids. This plant is known as turmeric and is popular as a spice, medicine and colorant. Its main yellow compounds are composed of curcumin, demethoxycurcumin and bis-demethoxycurcumin, and most of the yellow color of turmeric can be explained by the presence of these curcuminoids (Avula et al., 2009; Jin et al., 2010; Kim et al., 2013). The color strength in ginger is less important than in turmeric; however, several reports on color stability during ginger processing (Ahmed, 2004; Tripathi and Nath, 2004; Kim and Lee, 2006; Yamaguchi et al., 2010) have implied that the color of ginger is an important factor in its quality as well as its spiciness. Nevertheless, the constituents responsible for the yellow color of ginger have not been clarified.
The character and application of a ginger rhizome are usually classified by its size (cultivar) and maturation stage. Ginger rhizomes are classified by size into three groups: small, medium and large. In the small group, the dried form of cv. Kintoki is very popular for medicinal uses. On the other hand, the medium and large types are used in a fresh condition for pickles, additives and spices for use in various dishes. Furthermore, mature ginger rhizomes and immature younger ones are differently consumed in Japan. Immature rhizomes are less fibrogenic and have a mild fresh aroma, and moreover, show a pink color when in an acidic condition, e.g., a vinegar solution. Previously, we identified two anthocyanins, cyanidin 3-glucoside and peonidine 3-rutinoside, as compounds responsible for the pink color of the ginger rhizome (Iijima et al., 2003). However, these compounds are unstable and disappear in mature ginger rhizomes. On the other hand, mature ginger rhizomes are fibrogenic, show a stronger yellow color, and contain a strong lemony flavor. Previously, the compositional changes in the aroma constituents during maturation of the ginger rhizome were investigated, and the aroma profile was found to depend on an increase in citral instead of a decrease in geranyl acetate (Sakamura, 1987; Sekiwa-Iijima et al., 2001). However, it remains unclear which compounds contribute to the increase in yellow color. In this study, we identified the compounds responsible for the pale yellow color of the ginger rhizome. Furthermore, comparative analyses of these compounds were performed using various ginger samples (62 varieties) derived from different cultivars, cultivation locations, and maturation stages.
Plant Materials Finger rhizomes harvested in various prefectures of Japan were obtained from supermarkets or ginger product companies (Kimura-Noen Co., Ltd., Aichi, Japan, for various samples of cv. Kintoki, and Shioda Food Co., Ltd., Tochigi, Japan, for rhizomes grown under the same condition). Each analytical sample was prepared by randomly collecting various parts of one rhizome. After the rhizomes were washed and peeled, three sample groups per variety were prepared and frozen at −80°C until use. Immature ginger rhizomes were prepared from mature seed rhizomes by cultivating them for 70 – 90 days in the greenhouse.
Chemicals Standard curcumin, 6-gingerol and formononetin were purchased from Extrasynthese (Genay, France). Demethoxycurcumin was purchased from Tokiwa Phytochemical Co., Ltd. (Chiba, Japan).
Extraction of yellow pigments and quantification Each frozen sample was powdered with a mortar and pestle. Powdered samples (100 mg) were extracted with 300 µL of methanol containing 10 µg/mL formononetin as an internal standard. After samples had been homogenized with a Tissue Lyser II (Qiagen, Hilden, Germany) at 25 Hz/s for 2 min, the homogenates were centrifuged (12,000 g for 10 min at 4°C). After the obtained supernatant was transferred to a new tube, another 300 µL of methanol containing 10 µg/mL formononetin was added to the residue, and the extraction procedure was repeated. The combined supernatant (about 600 µL) was filtered through a 0.2-µm PVDF membrane (Whatman, Brentford, U.K), and the filtrate was used for ultra-performance liquid chromatography (UPLC)-photodiode array analysis (PDA).
UPLC-PDA analysis A Waters UPLC ACQUITY system coupled with a UV-visible diode array detector (Waters Co., Milford, MA) was used for quantification of the yellow pigment compounds. The methanol extracts (5 µL) were applied to an Acquity UPLC BEH-C18 reversed-phase column (2.1 mm I.D. × 100 mm, particle size 1.7 µm, Waters Co., Milford, MA). The column temperature was set at 40°C. Each compound was separated by a gradient consisting of ultra-pure water (A) and acetonitrile (B) at 0.3 mL/min. Total analysis was performed for 16 min with a gradient of 0 min (70% A, 30% B) to 10 min (5% A, 95% B), followed by holding at the last concentration for 3 min. Then, re-equilibration was performed for 3 min. MassLynx software (Waters Co., Milford, MA) was used for quantification. A UV-visible diode array was used in the wavelengths between 210 nm and 600 nm. Yellow pigments were detected at 420 nm; formononetin as an internal standard and 6-gingerol were assessed at 280 nm. Calibration curves were obtained from a standard solution consisting of curcumin, demethoxycurcumin, isolated 6-dehydrogingerdione and 6-gingerol, and used for the quantification of each compound in the ginger rhizomes.
Isolation and identification of 6-dehydrogingerdione Mature ginger rhizomes of cv. Kintoki (about 4 kg) were homogenized in methanol (8 L) and extracted for 1 day under darkness. After filtration, methanol was evaporated from the extract, and a concentrated aqueous solution was obtained. After chloroform was added at the same volume as that of the aqueous solution, the yellow pigment was extracted to chloroform using separating funnels. After evaporation, the obtained concentrate of yellow pigments (about 50 g) was fractionated by silica-gel column chromatography using hexane, diethyl ether and chloroform. Yellow-colored fractions were obtained from the fraction eluted by hexane/diethyl ether = 60:40 and 40:60. Here, elution at hexane/diethyl ether = 40:60 was confirmed to contain curcumin and demethoxycurcumin by comparative analysis with each standard; therefore, the fraction of hexane/diethyl ether = 60:40 was further purified with preparative HPLC using an ODS column. After repeated preparative HPLC under an isocratic condition at 80% methanol and under a gradient condition using water and acetonitrile, 26.4 mg of purified 6-dehydrogingerdione was obtained. An accurate mass spectrometry (MS) value was measured using a Micromass LCT premier mass spectrometer (TOF-MS, Waters Co., Milford, MA) in W mode with a positive mode. Infusion injection after dissolving the sample in methanol was performed. For nuclear magnetic resonance (NMR) analysis, samples were dissolved in CDCl3. 1H-NMR and 13C-NMR were recorded on a JNM-ECX 400P spectrometer (JEOL RESONANCE Inc. Tokyo, Japan). The obtained chemical shift was compared with the reference (Kiuchi et al., 1982; Charles et al., 2000).
Spectral analysis of compound 7 λmax:372 nm; 1H-NMR (ppm, J in Hz, CDCl3): 7.53 (1H, d, J = 15.6, H-1), 7.08 (1H, dd, J = 1.8, 8.2, H-6′), 7.02 (1H, d, J = 1.8, H-2′), 6.92 (1H, d, J = 7.8, H-5′), 6.34 (1H, d, J = 15.6, H-2), 5.63 (2H, s, H-4), 3.94 (3H, s, OMe), 2.38 (2H, t, J = 7.3, H-6), 1.66 (2H, m, H-7), 1.34 (4H, m, H-8, H-9), 0.91 (3H, t, J = 7.3, H-10); 13C-NMR (ppm): 200.2 (C-5),178.1(C-3), 147.7 (C-3′), 146.8 (C-4′), 139.8 (C-1), 127.8 (C-1′), 122.6 (C-6′), 120.6 (C-2), 114.8 (C-5′), 109.6 (C-2′), 100.2 (C-4), 56.0 (OMe), 40.1 (C-6), 31.5 (C-7), 25.4 (C-8), 22.5 (C-9), 14.0 (C-10)
Colorimetry Each powder of immature and mature ginger rhizome (2 g) was added to 2 mL of water and mixed. Each ginger paste was placed onto a glass petri-dish (35 mm in diameter) and its color was measured using a CM-5 spectrophotometer (Konica Minolta, Tokyo, Japan). Color was recorded using the CIE-L*, a*, b* values. Triplicate measurements for each sample were performed.
Statistics The obtained data are shown as the means and standard errors of three replicates for each sample. Correlation co-efficiency was shown using the Pearson coefficient correlation (r). As for principal component analysis (PCA) based on UPLC data, each detected peak area was divided by that of the internal standard to normalize all data, and analysis was performed using Pirouette ver. 4.5 software (InfoMetrix, Bothell, WA).
Profiles of yellow pigment compounds detected at 420 nm Figures 1A and 1B show typical UPLC profiles at 420 nm of extract from a ginger rhizome. As a reference, the profile of extract from Curcuma longa (Aki-ukon) is also shown (Fig. 1C). In the data for ginger, 10 peaks were detected (1 – 10 in Fig. 1, Table 1). While their composition differed among the samples, three peaks detected at 4.56 min (4), 4.73 min (6) and 7.14 min (7) were common main peaks in all samples from ginger rhizomes.
The data from 62 kinds of ginger (186 analytical samples) were subjected to PCA, and the score plot is shown in Fig. 2. The rhizomes from cv. Kintoki are usually used more for medicinal purposes than as spices or food additives. In this study, more than half of all of the samples (35 samples) were from cv. Kintoki; therefore, we compared their profiles with those of other ginger samples (Fig. 2A). Although some exceptional samples were included, cv. Kintoki formed a cluster in the positive region of principal factor 1 and was differentiated from large-type rhizomes. On the other hand, we also attempted to differentiate the samples based on the maturity of the rhizomes, but no clear classification was achieved (Fig. 2B). These results indicate that the profiles of yellow pigments are influenced neither by cultivar nor maturation, but instead appear dependent on the conditions of the cultivation location.
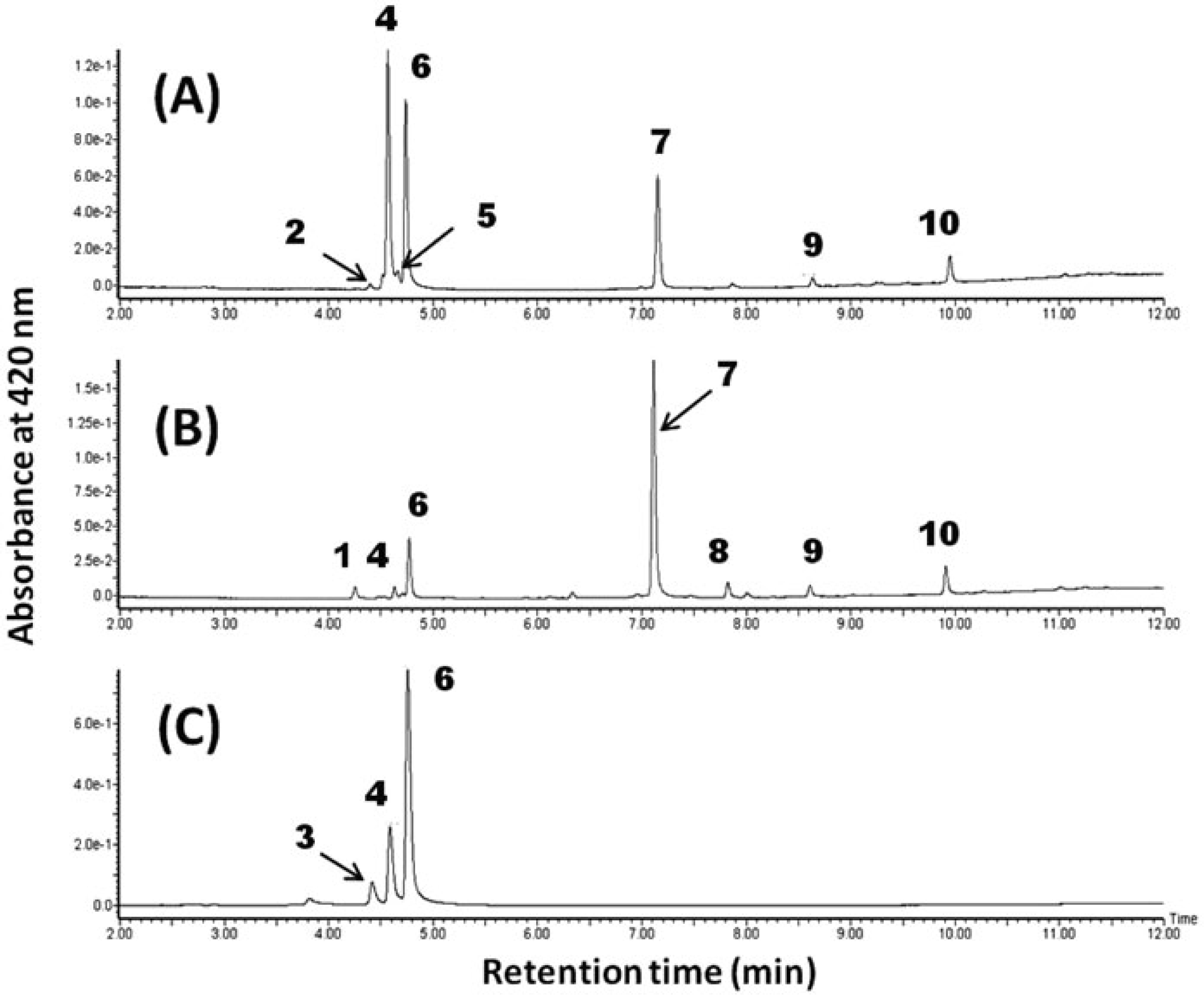
Typical UPLC profiles of extracts of yellow pigments from ginger rhizomes. (A) Typical chromatogram from cv. Kintoki; (B) chromatogram from cv. Ohmi; (C) chromatogram from C. longa (Aki-ukon).
| Peak No. | Retention time (min) | MS value [M + H]+ | Estimated molecular formula | Compound name |
|---|---|---|---|---|
| 1 | 4.28 | not detected | - | - |
| 2 | 4.39 | 341.139 | C20H20O5 | unknown |
| 3 | 4.39 | 309.112 | C19H16O4 | bis-demethoxycurcumin |
| 4 | 4.56 | 339.123 | C20H18O5 | demethoxycurcumin |
| 5 | 4.66 | 329.175 | C20H24O4 | unknown |
| 6 | 4.73 | 369.133 | C21H20O6 | curcumin |
| 7 | 7.14 | 291.159 | C17H22O4 | 6-dehydrogingerdione |
| 8 | 7.85 | not detected | - | - |
| 9 | 8.63 | 319.191 | C19H26O4 | 8-dehydrogingerdione |
| 10 | 9.95 | 347.222 | C21H30O4 | 10-dehydrogingerdione |
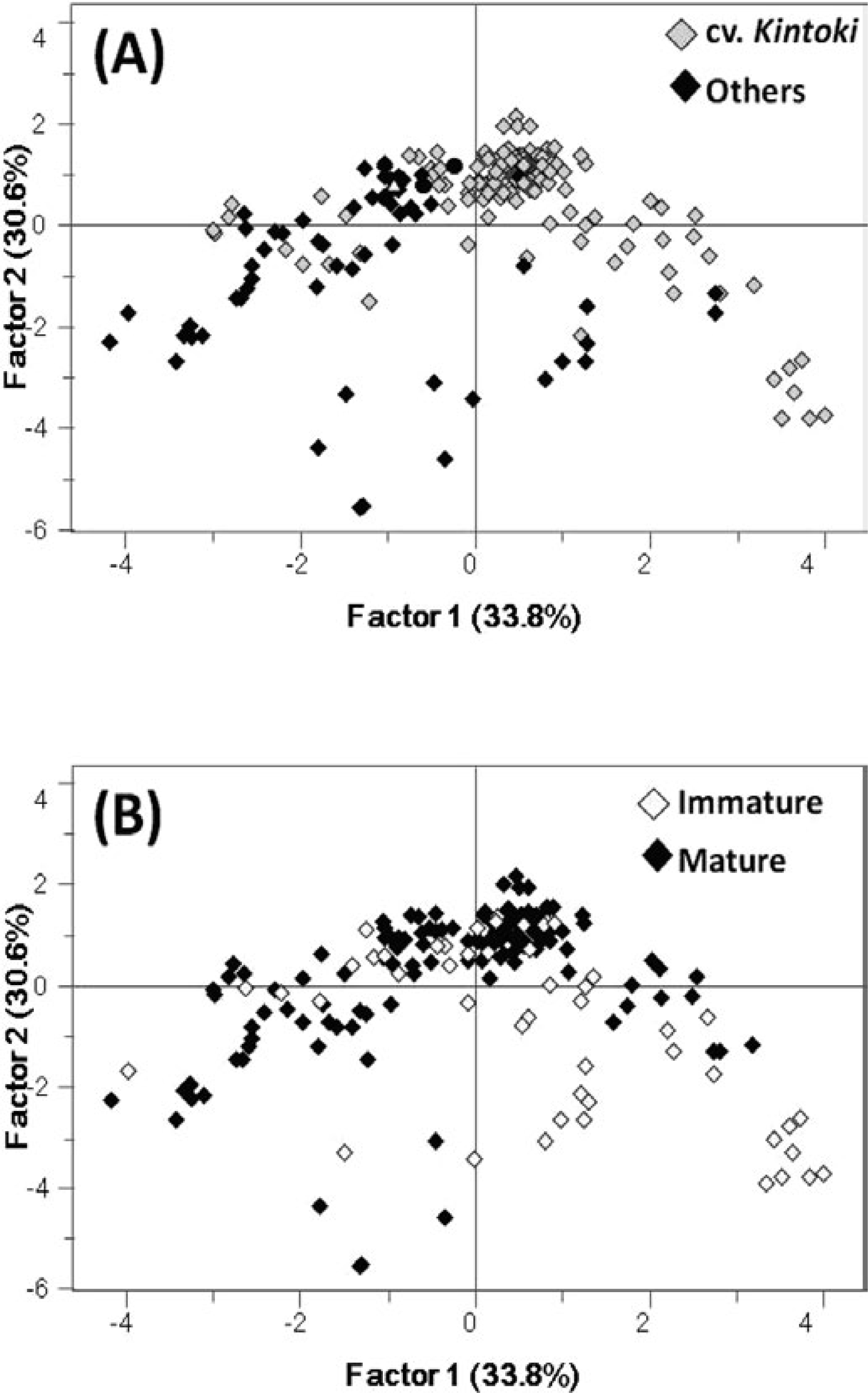
Principal component analysis of various ginger rhizomes using peak area detected at 420 nm by UPLC analysis. All independent data (186 analytical samples) were plotted. (A) cv. Kintoki and other varieties are differentiated; (B) immature and mature samples are differentiated.
Structures of yellow pigment compounds Next, we focused on the three main compounds detected at 4.56 min, 4.73 min and 7.14 min (compounds 4, 6 and 7, respectively, in Fig. 1). Compounds 4 and 6 were identified as curcumin and demethoxycurcumin by comparing their retention time, PDA spectrum and m/z values by LC-MS with those of each standard compound (Figs. 1 and 3, Table 1). The compound at 7.14 min was specifically detected in ginger samples and not in the sample of C. longa (Fig. 1C).

Structures of main yellow compounds (4:demethoxycurcumin; 6: curcumin; 7 6-dehydrogingerdione) and 6-gingerol. Each number indicates the peak number in Fig. 1. Numbers on compound 7 represent the corresponding chemical shift assignments by NMR analysis shown in the relevant section of the Materials & Methods.
Therefore, we isolated this compound and confirmed its structure through accurate MS and NMR analysis. By MS analysis, its chemical formula was estimated to be C17H22O4 ([M + H]+ = 291.1595, delta ppm = 0.36).
NMR analysis indicated that the isolated compound contained an ortho-methoxy phenol, and one double bond (trans form) and two carbonyl groups were contained in the side chain. Comparison with the reference data showed the NMR data of this compound to be consistent with the data for 6-dehydrogingerdione (Fig. 3, (Kiuchi et al., 1982; Charles et al., 2000)). All of these detected compounds, demethoxycurcumin, curcumin and 6-dehydrogingerdione, contained the same partial structures, including feruloylmethane and β-diketone moieties (Fig. 3). The λ max values of demethoxycurcumin, curcumin and 6-dehydrogingerdione were 423 mm, 426 mm, and 372 mm, respectively. This indicates that the color of 6-dehydrogingerdione is a less dense pale yellow than the others, because it contains only a single aromatic ring forming conjugated double bond in the molecule.
Curcumin and demethoxycurcumin are well known yellow pigments contained in the rhizome of C. longa. In C. longa, bis-demethoxycurcumin is also accumulated in addition to these curcuminoids, while it appears in ginger rhizomes at only a trace level. On the other hand, 6-dehydrogingerdione was the specific compound in ginger rhizomes that exhibited a yellow color. Previously, 6-dehydrogingerdione has been reported to be a physiologically active compound exhibiting antioxidative and anti-inflammatory activities (Patro et al., 2002; Li et al., 2012), anti-carcinogenic activity (Chen et al., 2010; Hsu et al., 2010), and a human skin cell regenerative effect (Chen et al., 2013). However, this is the first time its contribution to the yellow color of the ginger rhizome has been recognized. Furthermore, the unknown peaks 9 and 10 in Fig. 1 showed spectral profiles similar to that of 6-dehydrogingerdione. Accurate MS data and fragmentation patterns determined by MS/MS analysis suggested these to be 8-dehydrogingerdione and 10-dehydrogingerdione, respectively (Table 1).
Quantification of yellow pigment compounds in various ginger rhizomes Table 2 shows the contents of the detected yellow pigment compounds, curcumin, demethoxycurcumin, and 6-dehydrogingerdione, in ginger rhizomes. The average amounts of these compounds were 2.2 ± 0.1, 1.6 ± 0.1, and 20.0 ± 1.1 (mean ± S.E.) mg/100 g fresh weight, respectively; however, the contents varied widely among the samples. In all samples, the 6-dehydrogingerdione content was much higher than the contents of the other two compounds. Furthermore, the contents of these compounds, especially curcumin and demethoxycurcumin, were greater in cv. Kintoki than in other varieties. The ranges of curcumin and demethoxycurcumin contents were very broad, with the lowest levels in Tosaichi_2 (0.6 and 0.5 mg/100 g fresh weight, respectively) and the highest in Kintoki_6 (5.0 and 5.2 mg/100 g fresh weight, respectively). In medium and large varieties, the rhizomes of cv. Kogane, which is known as a characteristically yellow cultivar, contained high amounts of 6-dehdyrogingerdione (30.8 – 48.7 mg/100 g fresh weight). To confirm the contribution of these compounds to the yellow color, we prepared the standard mix solution containing the same concentration of curcumin, demethoxycurcumin and 6-dehydrogingerdione as Kintoki_1 extract. Comparison of the absorbance at 420 nm suggested that 92% of the yellow color is dependent on the composition of these three compounds.
| Variety | Size_maturation | Location | Curcumin ± S.E. | Demethoxyurcumin ± S.E. | 6-Dehydrogingerdione ± S.E. |
|---|---|---|---|---|---|
| Kintoki_1 | small_matured | Aichi | 3.6 ± 0. 5 | 4.3 ± 0. 6 | 17.3 ± 2. 3 |
| Kintoki_2 | small_matured | Aichi | 2.2 ± 0. 3 | 2.9 ± 0. 1 | 13.2 ± 1. 8 |
| Kintoki_3 | small_matured | Aichi | 2.4 ± 0. 2 | 3.4 ± 0. 3 | 26.0 ± 2. 2 |
| Kintoki_4 | small_matured | Aichi | 2.7 ± 0. 1 | 2.4 ± 0. 1 | 44.2 ± 1. 7 |
| Kintoki_5 | small_matured | Aichi | 2.9 ± 0. 1 | 3.8 ± 0. 4 | 17.8 ± 4. 8 |
| Kintoki_6 | small_matured | Aichi | 5.0 ± 0. 8 | 5.2 ± 0. 2 | 27.2 ± 5. 5 |
| Kintoki_7 | small_matured | Aichi | 2.8 ± 0. 1 | 1.5 ± 0. 1 | 20.4 ± 2. 8 |
| Kintoki_8 | small_matured | Aichi | 4.2 ± 0. 1 | 3.0 ± 0. 6 | 29.1 ± 0. 6 |
| Kintoki_9 | small_matured | Aichi | 2.5 ± 0. 1 | 1.7 ± 0. 4 | 21. 9 ± 0. 8 |
| Kintoki_10 | small_matured | Aichi | 2.3 ± 0. 1 | 2.1 ± 0. 2 | 22.2 ± 7. 8 |
| Kintoki_11 | small_matured | Aichi | 1.7 ±0.3 | 1.4 ± 0. 1 | 14.8 ± 0. 8 |
| Kintoki_12 | small_matured | Aichi | 2.1 ± 0. 2 | 1.5 ± 0. 1 | 9.6 ± 0. 3 |
| Kintoki_13 | small_matured | Aichi | 2.3 ± 0. 2 | 2.3 ± 0. 2 | 26.0 ± 0. 2 |
| Kintoki_14 | small_matured | Aichi | 3.0 ± 0. 2 | 2.7 ± 0. 1 | 22.9 ± 1. 5 |
| Kintoki_15 | small_matured | Aichi | 2.2 ± 0. 2 | 1.7 ± 0. 3 | 28.3 ± 7. 0 |
| Kintoki_16 | small_matured | Aichi | 2.9 ± 0. 7 | 0.7 ± 0. 1 | 20.3 ± 1. 1 |
| Kintoki_17 | small_matured | Aichi | 1.7 ± 0. 4 | 2.2 ± 0. 2 | 12.6 ± 0. 2 |
| Kintoki_18 | small_matured | Aichi | 2.0 ± 0. 2 | 1.6 ± 0. 1 | 19.2 ± 1. 1 |
| Kintoki_19 | small_matured | Aichi | 1.8 ± 0. 1 | 2.3 ± 0. 3 | 25.2 ±0.3 |
| Kintoki_20 | small_matured | Aichi | 2.6 ± 0. 3 | 1.4 ± 0. 1 | 29.6 ± 1. 2 |
| Kintoki_21 | small_matured | Aichi | 2.4 ± 0. 1 | 1.6 ± 0. 1 | 20.6 ± 2. 6 |
| Kintoki_22 | small_matured | Aichi | 2.3 ± 0. 3 | 1.2 ± 0. 2 | 28.2 ± 3. 3 |
| Kintoki_23 | small_matured | Aichi | 1.7 ± 0. 5 | 1.2 ± 0. 1 | 16.8 ± 2. 1 |
| Kintoki_24 | small_matured | Aichi | 2.0 ± 0. 3 | 2.3 ± 0. 1 | 19.6 ± 2. 0 |
| Kintoki_25 | small_matured | Aichi | 2.2 ± 0. 2 | 1.3 ± 0. 0 | 40.4 ± 3. 6 |
| Kintoki_26 | small_matured | Aichi | 1.7 ± 0. 1 | 1.8 ± 0. 3 | 15.4 ± 2. 1 |
| Kintoki_27 | small_immatured | Aichi | 1.6 ± 0. 3 | 1.1 ± 0. 1 | 24.7 ± 3. 9 |
| Kintoki_28 | small_immatured | Aichi | 2.4 ± 0. 4 | 1.7 ± 0. 1 | 23.2 ± 3. 0 |
| Kintoki_29 | small_immatured | Aichi | 2.4 ± 0. 6 | 2.3 ± 0. 2 | 14.9 ± 0. 4 |
| Kintoki_30 | small_immatured | Aichi | 3.4 ± 0. 2 | 3.6 ± 0. 4 | 15.5 ± 2. 0 |
| Kintoki_31 | small_immatured | Aichi | 2.8 ± 0. 1 | 3.6 ± 0. 3 | 28. 2 ± 5. 9 |
| Kintoki_32 | small_immatured | Aichi | 1.8 ± 0. 0 | 2.4 ± 0. 1 | 17.0 ± 1. 7 |
| Kintoki_33 | small_immatured | Shizuoka | 1.7 ± 0. 3 | 1.3 ± 0. 1 | 23.9 ± 1. 6 |
| Kintoki_34 | small_immatured | Aichi | 1.6 ± 0. 1 | 0.9 ±0.1 | 12.6 ± 1. 8 |
| Kintoki_35 | small_immatured | Mie | 2.2 ± 0. 2 | 1.4 ± 0. 1 | 14.2 ± 0. 7 |
| Sanshu_1 | small_matured | Chiba | 2.6 ± 0. 2 | 0.9 ± 0. 1 | 15.5 ± 0. 6 |
| Sanshu_2 | small_matured | Aichi | 3.0 ± 0. 0 | 0.9 ± 0. 0 | 26.6 ± 4. 3 |
| Yanaka_1 | small_matured | Tochigi | 2.7 ± 0. 3 | 2.3 ± 0. 1 | 13.6 ± 2. 3 |
| Yanaka_2 | small_immatured | Tochigi | 0.9 ± 0. 1 | 1.0 ± 0. 1 | 3.1 ± 0. 5 |
| Boushu_1 | medium_matured | Tochigi | 1.9 ± 0. 6 | 2.0 ± 0. 4 | 10.4 ± 0. 6 |
| Rakuda_1 | medium_matured | Tochigi | 2.5 ± 0. 3 | 0.8 ± 0. 0 | 10.9 ± 0. 6 |
| Rakuda_2 | medium_immatured | Tochigi | 1.7 ± 0. 4 | 0.7 ± 0. 1 | 10.3 ± 0. 3 |
| Kogane_1 | medium_matured | Kochi | 2.6 ± 0. 6 | 0.4 ± 0. 0 | 30.9 ± 3. 5 |
| Kogane_2 | medium_matured | Kagoshima | 0.7 ± 0. 1 | 0.5 ± 0. 0 | 48.7 ± 9. 9 |
| Kogane_3 | medium_matured | Kochi | 1.2 ± 0. 0 | 0.5 ± 0. 0 | 32.6 ± 2. 4 |
| Hachiro_1 | large_matured | Tochigi | 2.6 ± 0. 3 | 0.8 ± 0. 0 | 9.7 ± 2. 1 |
| Hachiro_2 | large_matured | Kumamoto | 0.9 ± 0. 2 | 0.6 ± 0. 0 | 15.4 ± 1. 3 |
| Hachiro_3 | large_matured | Kumamoto | 1.4 ± 0. 1 | 0.5 ± 0. 0 | 15.9 ± 2. 3 |
| Ohmi_1 | large_matured | Nagasaki | 2.9 ± 0. 2 | 0.7 ± 0. 0 | 16.9 ± 0. 5 |
| Ohmi_2 | large_matured | Kochi | 2.5 ± 0. 1 | 0.7 ± 0. 0 | 13.3 ± 1. 9 |
| Ohmi_3 | large_matured | Tochigi | 2.6 ± 0. 5 | 0.9 ± 0. 1 | 11.8 ± 2. 0 |
| Ohmi_4 | large_immatured | Kochi | 2.4 ± 0. 1 | 0.6 ± 0. 1 | 9.5 ± 0. 6 |
| Ohmi_5 | large_immatured | Kochi | 1.6 ± 0. 5 | 0.6 ± 0. 1 | 12.9 ± 1. 2 |
| Ohmi_6 | large_immatured | Kochi | 1.5 ± 0. 5 | 0.5 ± 0. 1 | 17.2 ± 1. 7 |
| Ohmi_7 | large_immatured | Tochigi | 1.2 ± 0. 1 | 0.6 ± 0. 0 | 6.8 ± 1. 7 |
| Otahuku_1 | large_matured | Tochigi | 1.7 ± 0. 3 | 0.7 ± 0. 1 | 11.2 ± 2. 1 |
| Tosaichi_1 | large_matured | Kochi | 1.0 ± 0. 3 | 0.5 ± 0. 0 | 19.5 ± 3. 4 |
| Tosaichi_2 | large_matured | Kochi | 0.6 ± 0. 0 | 0.5 ± 0. 0 | 23.7 ± 9. 5 |
| Unknown_1 | small_matured | China | 1.4 ± 0. 4 | 0.6 ± 0. 0 | 24.0 ± 1. 4 |
| Unknown_2 | large_matured | China | 0.7 ± 0. 0 | 0.6 ± 0. 0 | 20.1 ± 1. 3 |
| Unknown_3 | large_matured | China | 1.0 ± 0. 3 | 0.4 ± 0. 0 | 19.0 ± 1. 1 |
| Unknown_4 | large_immatured | Kanagawa | 2.8 ± 0. 1 | 2.3 ± 0. 2 | 20.0 ± 2. 6 |
Correlation analysis among all samples indicated that the accumulation pattern was similar between curcumin and demethoxycurcumin (r = 0.667, p < 0.01), while the accumulation of 6-dehydrogingerdione occurred independent from them (r = 0.191 and 0.174 to curcumin and demethoxycurcumin, respectively). The content of 6-dehydrogingerdione was previously quantified in ginger tea leaves and ground ginger powder as 2.90 – 19.42 mg/100 g of each product, respectively (Shao et al., 2010). On the other hand, the present study marks the first quantification of curcumin and demethoxycurcumin.
Comparison of mature seed ginger rhizomes and immature ones Immature ginger rhizomes are frequently treated as fresh vegetables in spring and summer. Here, we prepared mature seed rhizomes of three cultivars (cv. Yanaka, Rakuda and Ohmi) and grew them at the same location to produce immature rhizomes. Generally, immature rhizomes are less yellow than mature rhizomes. The mature seed rhizomes and immature rhizomes obtained were used for a comparative analysis of b-values and of the contents of their pigment compounds (Fig. 4). In all cultivars, b-values were commonly higher in mature seed rhizomes than immature rhizomes. The profiles of b-values in cv. Yanaka and cv. Ohmi were similar to those of all three compounds. On the other hand, in cv. Rakuda, the contents of demethoxycurcumin and 6-dehydrogingerdione did not change according to the maturation stage, indicating that the difference in the b-value of cv. Rakuda is hardly affected by their composition. This discrepancy in cv. Rakuda may suggest that other color-contributing compounds are contained, although further detailed investigation is required.

Comparative analysis of yellow pigment compounds and the b-value among immature and mature seed rhizomes. Gray and black bars indicate immature and mature seed rhizomes, respectively.
Previously, differences in metabolite profiles between immature and mature ginger were estimated from volatiles and pungent compounds (Sakamura, 1987; Sekiwa-Iijima et al., 2001; Bailey-Shaw et al., 2008; Kiran et al., 2013). However, differences in the accumulation of yellow pigments have not been clarified. Here, our results suggest that the biosynthesis of curcuminoids is activated during the maturation of ginger rhizomes and thereby increases the yellow color.
Quantitative relationship between 6-dehydrogingerdione and 6-gingerol contents In this study, 6-dehydrogingerdione was identified as a possible contributor to the yellow color in ginger rhizomes; however, a clear positive correlation with the b-value was not shown. Furthermore, the accumulation of 6-dehydrogingerdione is less varied, and is stably contained in all samples. These results suggest that 6-dehydrogingerdione produces the common fundamental pale yellow color in all ginger. In terms of its structure, 6-dehydrogingerdione is an oxidized form of 6-gingerol (Fig. 3), the main pungent compound in ginger rhizomes. This suggests that the biosynthesis of 6-dehydrogingerdione is related to the accumulation of 6-gingerol. 6-Gingerol has been suggested to be biosynthesized by type III polyketide synthase from feruloyl-CoA and malonyl-CoA as well as curcuminoid (Katsuyama et al., 2007; Koo et al., 2013). Although its detailed biosynthesis is not clear, 6-dehydrogingerdione analogs were recently successfully synthesized in E. coli by exploiting a curcuminoid synthase from O. sativa with a β-oxidation pathway from S. cerevisiae (Katsuyama et al., 2010). This suggests that 6-dehydrogingerdione is an intermediate of 6-gingerol. Therefore, the 6-gingerol content in each sample was also measured; correlation analysis of the accumulation of 6-dehydrogingerdione and 6-gingerol showed a high value, r = 0.535 (p < 0.01). This suggests that the ginger samples containing more 6-gingerol would show high accumulations of 6-dehydrogingerdione.
In this study, we identified three main yellow pigment compounds, curcumin, demethoxycurcumin, and 6-dehydrogingerdione, in the ginger rhizome. The profiles differed from those of C. longa, which is also a kind of Zingiberaceae. Their contents varied with each sample, and the cultivars could not be differentiated well based on the involvement of specific compounds that confer their yellow color. However, the contents of these compounds were greater in mature rhizomes than immature rhizomes. These results indicate that the accumulation of these compounds is affected by the cultivation conditions and maturation stage of the ginger rhizome.
Acknowledgements We thank Kimura-Noen Co., Ltd. and Sioda Foods Co., Ltd. for providing the ginger samples. This work was supported in part by a JSPS KAKENHI Grant-in-Aid for Scientific Research (C), no. 24500955.