2014 Volume 20 Issue 5 Pages 979-985
2014 Volume 20 Issue 5 Pages 979-985
Acrylamide is a toxic compound generated in processed foods prepared at high temperatures. To explore means by which food processing can reduce the acrylamide content in foods, we studied the reaction of acrylamide with lysine and cysteine, which carry nucleophilic functional groups, at temperatures below the initiation of acrylamide generation. The amino acid-mediated reduction of acrylamide content followed first-order reaction kinetics in aqueous solution below 120°C. The profile of acrylamide decrease correlated well with the formation of a 1:1 adduct of acrylamide with the respective amino acid, indicating that these amino acids reacted directly with acrylamide. The reactivity of acrylamide toward both amino acids was pH-dependent. In particular, the reactivity of cysteine toward acrylamide was remarkably enhanced by an increase in pH. These findings suggested the possibility of reducing acrylamide levels during food processing by treating foods with the appropriate amino acid(s) at moderate temperatures below 120°C.
Acrylamide, a water-soluble molecule with a molecular weight of 71, causes hepatotoxicity, neurotoxicity, and probably carcinogenicity in human. Tareke et al. (2002) was the first to report that acrylamide is produced when carbohydrate-rich food materials like potato are processed at temperatures higher than 120°C. Since then, much attention has been paid to the acrylamide content of processed foods, especially in Western countries where potato-based processed foods such as potato chips are widely consumed. Many analyses of the acrylamide levels in processed foods have been reported, as well as studies on methods for reducing acrylamide in processed foods (Becalski et al., 2004; Pedreschi et al., 2004; Palazoğlu et al., 2010). In Japan, the Ministry of Agriculture, Forestry and Fisheries carried out a large-scale survey on the acrylamide content of Japanese food products in 2003, and reported that acrylamide was present in popular processed foods. For example, Japanese rice crackers and roasted tea, which are processed at high-temperatures, as well as food products cooked at high temperatures, were reported to contain significant amounts of acrylamide.
Basically, acrylamide is formed during the Maillard reaction. Heating asparagine at high temperatures in the presence of a reducing sugar leads to the formation of acrylamide. A number of strategies have been proposed to control acrylamide levels in food products, such as choosing food materials containing smaller amounts of precursors for acrylamide formation, i.e., reducing sugars and asparagine (Amrein et al., 2003; Rommens et al., 2008); removing the above-mentioned precursors (Baardseth et al., 2006; Kukurová et al., 2009); processing food materials under conditions that suppress the formation of acrylamide, i.e., at low temperatures (Granda et al., 2004); and adding food ingredients capable of inhibiting the formation of acrylamide (Zheng et al., 2009; Cheng et al., 2010). It was also reported that the addition of certain amino acids other than asparagine reduces acrylamide levels in an aqueous system (Kim et al., 2005). Claeys et al. (2005) kinetically analyzed the effect of amino acids on acrylamide formation/elimination by heating a mixture of asparagine and glucose at temperatures between 140 and 200°C, and reported that the presence of cysteine or lysine significantly reduced acrylamide formation, whereas glutamine promoted the formation of acrylamide. There are also several studies reporting the reaction of acrylamide with amino acids such as glycine, lysine and cysteine at high temperatures (Adams et al., 2010; Liu et al., 2011; Zamora et al., 2010; Hidalgo et al., 2011). The addition of amino acids other than asparagine reduced acrylamide formation in homogenized potatoes heated at 180°C for 20 min, probably due to the competitive consumption of acrylamide precursors and/or elimination of produced acrylamide by nucleophilic components in the amino acids (Rydberg et al., 2003). Functional groups of the amino acids, such as NH2 and SH, serve as nucleophiles to cause a Michael addition reaction with acrylamide. However, the studies mentioned above dealt with reactions over 120°C, where acrylamide is preferentially produced. In contrast, there have been only a few studies of acrylamide formation at temperatures where acrylamide is not readily formed (Adams et al., 2010).
We are interested in practical ways of reducing acrylamide concentrations during food processing. In the present study, the reactions of acrylamide with lysine and cysteine, which have nucleophilic functional groups, were precisely examined at temperatures below 120°C. The results clearly indicated that these amino acids, and especially cysteine, are particularly effective for reducing acrylamide levels in food products.
Chemicals Acrylamide (ultra pure, > 99.9%) was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan) and 13C1-labelled acrylamide (> 98%) for use as an internal standard (IS) was from CDN Isotopes Inc. (Montreal, Quebec, Canada). JIS special grade L(+)-lysine, L-cysteine, ammonium acetate, acetic acid and 25% ammonia solution were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), in addition to LC-MS-grade ultra-pure water, methanol, acetonitrile, and heptafluorobutyric acid (HFBA). Water used for all the reactions was obtained by Auto Pure WT101 UV from Yamato Scientific Co., Ltd. (Tokyo, Japan).
Acrylamide/amino acid reaction One milliliter of acrylamide (0.40 mM) and 1 mL of amino acid (20.0 mM) were added to a 15-mL polypropylene tube, sealed, mixed immediately, and then placed in a thermostated oil bath at 60 – 120°C for 0 – 180 min. After heating, each sample was immediately cooled in ice water for 15 min to stop the reaction. Then, 0.5 mL of each reaction mixture was transferred to 1.5-mL micro tubes and stored in a −80°C freezer until analysis. After thawing, 0.5 mL of 13C1-acrylamide (0.28 mM) as an IS was added to each sample and the sample was passed through a 0.20 µm membrane filter unit prior to LC-MS analysis. Water was used as a control instead of amino acid solution, and the sample blank was prepared by mixing acrylamide and amino acid at room temperature just before LC-MS analysis.
The pH dependence of the thermal reaction between acrylamide and amino acid was examined as follows: 0.5 mL of acrylamide (0.80 mM), 0.5 mL of amino acid (20.0 mM) and 1 mL of ammonium acetate buffer (100 mM) adjusted to a pH in the range 5 – 9 were placed in a 15-mL tube and handled as described above at 80°C for 120 min.
Products of the reaction between acrylamide and amino acid were generated and identified as follows: to 1.0 mL of an equimolar mixture of acrylamide (7.0 mM) and 13C1-acrylamide (7.0 mM), 1.0 mL of amino acid (10 mM) was added in a 15-mL tube and handled as described above at 100°C for 60 min.
Acrylamide quantification by HPLC-MS The reactants were analyzed on a LCMS-2010A (Shimadzu Co., Kyoto, Japan). The samples (injection volume 2 µL) were separated on a reverse-phase C18 column (Gemini C18, 100 × 2 mm i.d., 3 µm, Phenomenex Inc., CA, USA) with a guard column (Security Guard, Phenomenex Inc.) at an oven temperature of 40°C. The mobile phase consisted of 2% methanol in water and the column was eluted at a flow rate of 0.1 mL/min. The eluted acrylamide was ionized using positive electrospray ionization mode (ESI+). The ESI source was operated as follows: nebulizer gas (nitrogen) flow rate, 1.5 mL/min; interface voltage, 4.5 kV; CDL voltage, 25.0 V; CDL temperature, 250°C; block heater temperature, 200°C; detector voltage, 1.8 kV; Q-array DC voltage, 60.0 V; Q-array RF voltage, 150.0 V. Acrylamide and IS were detected and identified in selected ion monitoring (SIM) mode at m/z 72 and m/z 73, respectively. The acrylamide content was quantified using a standard curve prepared from the peak area ratio of acrylamide (0 – 0.20 mM) to IS (0.14 mM). All analyses were performed in triplicate and the results are expressed as means ± SD. The limit of detection and the limit of quantification of acrylamide were 15 ng/mL and 25 ng/mL, respectively.
Determination of acrylamide-amino acid reaction products by LC-MS The reaction products of acrylamide and amino acids were identified using the LC-MS method described above, except that detection was performed in positive ion full scan mode. The sample (0.5 µL) was injected into the LC and separated by gradient elution, with 0.1% HFBA in water (solvent A) and acetonitrile (solvent B) as the mobile phases at a flow rate of 0.2 mL/min. The gradient condition was as follows: 100% A (0 – 5 min), from 100% A to 50% A and B (5 – 10 min), 50% A and B (10 – 15 min), 100% A (15.01 – 30 min). The eluents were analyzed by monitoring the m/z values expected for the reaction products reported elsewhere (Friedman, 2003; Adams et al., 2010) in the range m/z 60 – 300. The reaction products were identified from the isotope abundance ratio.
First, acrylamide and amino acids were directly reacted in an aqueous solution at 100°C without the use of buffer, as it was previously indicated that specific buffer components can affect the acrylamide reaction (Adams et al., 2010). Immediately after mixing, the pH of the reaction solutions containing acrylamide + lysine and acrylamide + cysteine was pH 9.9 and pH 5.3, respectively. After stopping the reaction, the pH of the lysine-reacted mixture shifted to 9.5, and the pH of the cysteine-reacted mixture decreased to 4.3. Figure 1 shows the amino acid concentration-dependent decrease in acrylamide concentration in the reaction solution when increasing concentrations of amino acid (up to 30 mM) were reacted with a defined concentration of acrylamide (0.2 mM) at 100°C. The acrylamide level significantly decreased in the presence of lysine or cysteine, and the rate of acrylamide reduction was clearly dependent on the concentration of each amino acid. Comparison of the effect between lysine and cysteine at the same concentration showed that lysine decreased the acrylamide level more markedly than cysteine.

Dose-dependent decrease in acrylamide after reaction with lysine and cysteine.
Acrylamide (0.2 mM) was reacted with either lysine (Δ) or cysteine (◊) at 100°C, and the residual concentration of acrylamide was quantified by LC-MS with 13C1-acrylamide as an internal standard. Data represent the mean of three experiments ± standard deviation unless otherwise noted.
The temperature dependence of the reaction between acrylamide and lysine or cysteine was further studied by reacting 0.2 mM acrylamide with 10 mM lysine or cysteine at 60, 80, 100 and 120°C (Fig. 2). The reaction rates of acrylamide with lysine or cysteine were determined by following the acrylamide concentration for up to 180 min. At each reaction temperature, the reduction in acrylamide concentration followed first-order kinetics. Lysine showed a greater reaction rate with acrylamide than cysteine at all temperatures examined. The reaction rate constant was calculated from the linear range according to the least squares method, and activation energies (Ea) were determined for both reactions from the Arrhenius plot, as shown in Fig. 3. The Ea values determined were 34.1 kJ/mol and 45.3 kJ/mol for lysine- and cysteine-mediated reactions, respectively.
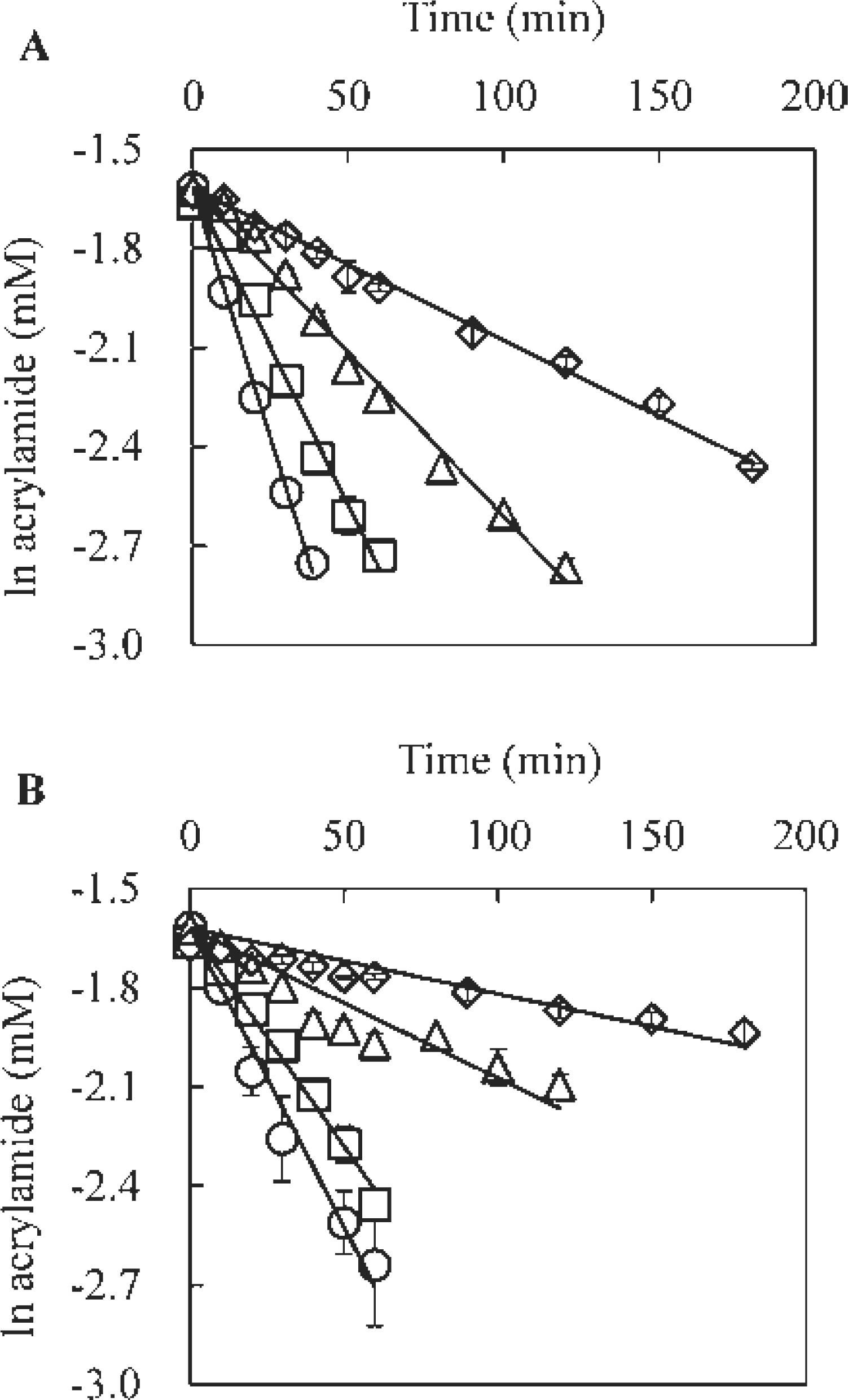
Reaction kinetics of amino acid-mediated acrylamide decrease at different temperatures.
Acrylamide (0.2 mM) was reacted with either 10 mM lysine (A) or cysteine (B) at 120°C (◯), 100°C (□), 80°C (Δ) and 60°C (◊), and the decrease in acrylamide concentration was determined by LC-MS as described in the Materials and Methods.

Arrhenius plots for the reactions of acrylamide with lysine and cysteine.
The reactions were identical to those described in Fig. 2. The calculated activation energies (Ea) from the Arrhenius plots were 34.1 and 45.3 kJ/mol for lysine (Δ) and cysteine (◊), respectively.
To further understand the differential reactivity of these two amino acids, the pH effect was precisely examined to elucidate how pKa differences were reflected in the reactivity of the two amino acids. Using 50 mM ammonium acetate buffer solution, 0.20 mM acrylamide was reacted with 5 mM of each amino acid at pHs ranging from 5 to 9 at 80°C. As shown in Fig. 4, the pH of the reaction solution greatly influenced the reaction of acrylamide with lysine and cysteine. The reactivity of lysine was very low at pH 8 or lower compared to at pH 9.5. Similarly, the reactivity of cysteine increased with increasing pH. Comparison of the reactivity of cysteine at pH 6.5 and 9.0 showed that cysteine significantly decreases acrylamide concentration even at room temperature.

Effect of pH on the reactions of acrylamide with lysine and cysteine.
Acrylamide (0.2 mM) was reacted with 5 mM lysine or cysteine in ammonium acetate buffer at defined pHs at room temperature (open symbols) or 80°C (closed symbols) for 120 min, and then the residual acrylamide concentration was determined as described in Fig. 1. Lysine (Δ, ▲) and cysteine (◊, ◆).
The kinetic profile of acrylamide suppression shows that acrylamide reacts directly with both lysine and cysteine. To identify the reaction product(s) produced from the acrylamide and amino acid reactions, a 1:1 mixture of acrylamide and 13C1-acrylamide was reacted with 5 mM of each amino acid at 100°C and the reaction products were analyzed by LC-MS. Figure 5 shows the mass chromatograms of the reactants of acrylamide and the amino acids. The peaks corresponding to lysine and cysteine were identified from the retention time of the respective authentic sample, as well as from the m/z values. The reaction product(s) of acrylamide reacted with each amino acid was detected from the peaks appearing on the mass chromatogram: specifically, products originating from acrylamide were identified from the presence of a characteristic M+1 peak due to 13C1-labeled acrylamide. The m/z 218 and m/z 193 components were determined as the major products produced from the reaction of acrylamide with lysine and cysteine, respectively, as reported previously (Friedman, 2003; Adams et al., 2010). The molecular weight determinations were consistent with those of 1:1 adducts of acrylamide and lysine or cysteine, respectively. Another reaction product, with a molecular weight of 288, was also determined following the reaction of acrylamide and lysine, although the yield was as low as 20%. This molecular weight agreed well with the 2:1 adduct of acrylamide:lysine. The retention time of this 2:1 adduct was almost identical to that of the major 1:1 adduct with a molecular weight of 217 Da.

Analysis of reaction products in acrylamide-amino acid reactions.
A 1:1 mixture of acrylamide and 13C1-acrylamide was reacted with lysine (A) or cysteine (B) for 60 min at 100°C and the reaction products were analyzed by LC-MS as described in the Materials and Methods. Proposed structures of the acrylamide adducts; 1 and 2, acrylamide - lysine (1:1) products; 3, acrylamide - lysine (2:1) product; and 4, acrylamide - cysteine (1:1) product.
To further investigate the results of Fig. 5, 0.20 mM of acrylamide was reacted with 10 mM of either lysine or cysteine and then the rate of acrylamide decrease was compared with that of 1:1 adduct formation. The results indicated that acrylamide reduction and the formation of the 1:1 reaction product occurred concomitantly as the reaction progressed, indicating that acrylamide reduction results from the direct reaction of acrylamide with lysine (Fig. 6A) or cysteine (Fig. 6B). Under this condition, the formation of 2:1 adduct of acrylamide and lysine was negligible.

Kinetic comparison of decrease in acrylamide concentration and amino acid adduct formation. Acrylamide (0.2 mM) was reacted with either 10 mM lysine (A) or cysteine (B) at 100°C. Acrylamide level (●); acrylamide - lysine (1:1) product (Δ); acrylamide - cysteine (1:1) product (◊).
Acrylamide formation during high-temperature processing of certain food materials is of great concern from the viewpoint of food safety. Therefore, many studies have investigated the mechanism of acrylamide formation, as well as various methods for suppressing acrylamide formation (Becalski et al., 2004; Ishihara et al., 2005; Pedreschi et al., 2004; Palazoğlu et al., 2010; Zyzak et al., 2003). Generally, the formation of some acrylamide is unavoidable during food processing. In the present study, we investigated methods to reduce acrylamide levels in high-temperature processed food products at temperatures below 120°C. The conjugated vinyl group of acrylamide is subject to nucleophilic addition reaction; therefore, lysine and cysteine, which contain nucleophilic functional groups, were chosen as the reactants. Examination of the reaction of acrylamide with lysine or cysteine in aqueous medium at 100°C showed that the decrease in acrylamide concentration was dependent on the concentration of amino acid. The reaction between acrylamide and these amino acids followed first-order kinetics between 60 and 120°C. Furthermore, lysine had a faster reaction rate than cysteine at all reaction temperatures. According to Friedman (2003), the electrophilic double bond in acrylamide can participate in nucleophilic reactions with active-hydrogen-bearing functional groups both in vitro and in vivo. These include the SH groups of cysteine, homocysteine and glutathione, the α-NH2 groups of free amino acids, the N-terminal amino acid residues of proteins, the ε-NH2 group of lysine, and the ring NH group of histidine. It was also reported that SH groups were 100 – 300 times more reactive with conjugated vinyl compounds than NH2 groups. Furthermore, the reaction rates of amino acids with conjugated vinyl compounds such as acrylamide vary among different amino acids and increase rapidly as the pH approaches the pK of the amino group (Friedman and Levin, 2008). Similarly, the pK value of SH governs the dissociation equilibrium (−SH ↔ −S−+H+), and thus the rate of reaction increases rapidly as the pH approaches the pK value of the SH group (Friedman, 2003). The pKs of the α-NH2 group and the lysine side chain are 9.06 and 10.54, respectively, and the pKs of the α-NH2 group and SH group in cysteine are 10.70 and 8.37, respectively. The pH for reaction with acrylamide was 9.9 for lysine and 5.3 for cysteine in the present study. Taken together, our observation that lysine showed higher reactivity than cysteine is due to the pH effect. This conclusion was confirmed by precisely examining the effect of pH on adduct formation (Fig. 4). The reactivity of lysine was considerably lower at pH 8 and below compared to at pH 9.9, and the reactivity of cysteine was remarkably enhanced as the pH of the reaction solution was increased. Further, high cysteine reactivity was observed over a wide range of pH, from around 5 to 9. This suggested that acrylamide exhibits higher reactivity with the SH group than that with the NH2 group, in agreement with the result reported by Friedman (Friedman, 2003).
This study aimed to identify processing conditions and acrylamide scavengers for reducing acrylamide in food. Therefore, we tried to determine the Ea of the reaction system as a whole rather than of each functional group of the amino acid. As shown in Fig. 3, the temperature dependence of the reaction rates between each amino acid and acrylamide followed Arrhenius behavior. The Ea of lysine and cysteine reactions with aqueous acrylamide were 34.1 kJ/mol and 45.3 kJ/mol, respectively. Hidalgo et al. (2011) reported that the Ea for the reaction between acrylamide and cysteine was 24.4 kJ/mol. Their reaction was, however, carried out at pH 7.0 using a phosphate buffer and at a water activity of 0.95. Therefore, the Ea difference between our present experiment and Hidalgo's experiment might be attributed to differences in experimental conditions such as pH, buffer composition, and water activity. It has been reported that certain buffer components show catalytic function, lowering the activation energy, i.e., borax acted as a catalyst in the Michael reaction between cysteine and acrylamide (Hussain et al., 2007). More precise study is required to determine how the differential reaction of acrylamide with each reactive group in lysine and cysteine contributes to the observed Ea.
The mirror image profile of the decrease in acrylamide concentration and increase in product concentration indicated that acrylamide reacted directly with lysine or cysteine. Indeed, LC-MS analysis revealed that adducts of acrylamide and amino acid were produced by the reaction of acrylamide with both lysine and cysteine below 120°C. A 1:1 molecular adduct of acrylamide and amino acid was the major product for both lysine and cysteine. Reaction of lysine and acrylamide provided a 1:1 adduct consisting of two components with the same m/z of 218, indicating that acrylamide reacted with both the α- and ε-NH2 of lysine. Further, a 2:1 molecular adduct of acrylamide and lysine was also detected, but at much lower concentration (Fig. 5). The same 1:1 adduct formation was reported previously by Adams et al. (2010) for the reaction of acrylamide and lysine, in that two separated peaks with m/z of 218 were detected. Although cysteine has two different functional groups that can react with acrylamide, in contrast to lysine, the 2:1 adduct was not detected with cysteine. This might be attributable to the different pK values of the functional groups in the two amino acids and the pH of the reaction system, as discussed above. At pH 9.9, both the α- and ε-NH2 groups in lysine will react with acrylamide, since the pK values of both NH2 groups approach the pH of the reaction solution, and thus two types of 1:1 adducts were formed and separated by chromatography. The 2:1 adduct was also reproducibly detected in the present study. In contrast, the pK of the α-NH2 group of cysteine is 10.7, and thus it is less reactive with acrylamide at pH 5.3. Although the reactivity of cysteine was lower than that of lysine at the pH used in the present experiment (Figs. 1 and 2), the fact that one lysine can scavenge two molecules of acrylamide may support the present finding that the rate of acrylamide elimination was faster with lysine than with cysteine at all temperatures examined, suggesting that lysine is a better scavenger of acrylamide than cysteine under alkaline pH conditions.
In many food products, the concentrations of free amino acids are higher than the concentration of acrylamide; it is therefore expected that acrylamide levels will decrease during storage, especially in lysine- and cysteine-rich products. Hoenicke and Gatermann (2005) investigated the stability of acrylamide in foods during storage, and reported that the acrylamide level in coffee and cacao powder decreased during 3-month storage at 10 to 12°C. Given the high reactivity of cysteine and lysine, as shown in our present study, the addition of cysteine or lysine to food products with high acrylamide contents can reduce acrylamide levels during storage. Zamora et al. (2010) reported that acrylamide could not be detected after 14 days storage in the presence of glycine at 60°C. However, a significant amount of acrylamide was detected when these samples were heated again at 180°C for 20 min, indicating thermal decomposition of the previously formed Michael adducts of acrylamide and amino acid. Therefore, identifying an amino acid that forms a more stable Michael adduct is of practical importance.
As discussed above, the reaction rates of lysine and cysteine with acrylamide depend on the amino acid concentration, reaction temperature and pH of the reaction system. The present study revealed that acrylamide levels may decrease in the presence of lysine or cysteine at temperatures where new acrylamide formation is low. Lysine can form 2:1 adducts of acrylamide-lysine and thus may work more effectively as an acrylamide scavenger at alkaline pH, while cysteine is more effective under neutral and acidic pH conditions. Further, the results indicated that 1:1 adducts of acrylamide and amino acid may be used as a comprehensive indicator of acrylamide production during food processing, since the adduct is the major reaction product formed.