2014 Volume 20 Issue 6 Pages 1183-1189
2014 Volume 20 Issue 6 Pages 1183-1189
Various charcoals (used in food processing and water treatment) and broiler litter biochar were examined for ability to adsorb water-soluble low-level radioactive cesium (ca. 200 – 250 Bq/kg) extracted from contaminated wheat bran. Among the materials tested, steam activated broiler litter biochar was the most effective sorbent and was able to reduce the concentration of radioactive cesium to less than 10 Bq/kg. Adsorption was observed under neutral and alkaline conditions but not under acidic conditions. Cesium binding to steam activated broiler litter biochar appeared to be stable under alkaline conditions.
The nuclear radiation leakage in March 2011 from the Fukushima Daiichi nuclear power plant, operated by the Tokyo Electric Power Company, caused pollution in soil and water by radioactive materials, resulting in contamination of agricultural products (Hirose, 2012; Tsumune et al., 2012). Agricultural products and drinking water remain under monitoring and regulations; the maximum limits for radioactive cesium (134Cs + 137Cs) in general food products, milk and infant food, and drinking water related to the Great East Japan Earthquake have been established as 100, 50, and 10 Bq/kg, respectively (Ministry of Health, Labor and Welfare, 2013, [i]), and cultivation in heavily polluted areas has been banned (Ministry of Agriculture, Forestry and Fisheries, 2013, [ii]).
Radioactive cesium fallout on soil is commonly contained within the upper 5 cm of the soil layer associated with clay, and only a small portion of radioactive cesium is considered mobile (Facchinelli et al., 2001; Matsunaga et al., 2013; Sanderson et al., 2001). However, radioactive cesium may slowly be redistributed due to uptake of mobile cesium by plants and by repartitioning in the ecosystem through equilibration with non-radioactive cesium (Sanderson et al., 2001; Yoshida et al., 2004). Thus, to minimize contamination and reduce the spread of radioactive cesium in water and agricultural products, immobilization of mobile radioactive cesium is desirable.
Several treatment methods to adsorb aqueous radioactive cesium from contaminated water are known. Zeolites, crystalline silicotitanate, and metal ferrocyanides show selectivity for cesium and have been studied in detail (Borai et al., 2009; Bostick et al., 1997; Faustino et al., 2008; Klasson and Taylor, 2006; Rivera-Utrilla et al., 1984; Verzil et al., 1992; Watari et al., 1988). For example, zeolites have distribution coefficients (Kd values) of 2 – 5 L/g (Borai et al., 2009), and the maximal binding capacity of hexacyanoferrate (Prussian blue) is reported to be 715 mg Cs/g (Faustino et al., 2008). Charcoal has been widely used as an adsorbent of radionuclides in various industries, including food processing, and is reported to adsorb iodine, radon, pertechnetate, thallium, and uranium (Klasson and Taylor, 2006; Rivera-Utrilla et al., 1984; Caccin et al., 2013); limited information about adsorption of radioactive cesium by charcoal exists. In general, these studies show that soluble cesium (radioactive or not) is not effectively removed by charcoal (Caccin et al., 2013; Kosaka et al., 2012; Rivera-Utrilla et al., 1984; Verzil et al., 1992; Liu et al., 2014). However, studies with small-scale filter systems, such as those used by consumers, have yielded conflicting results; in one case, two charcoal-containing filters worked well for cesium, but when loose charcoal (of a third type) was tested by the same investigators, it failed to remove the majority of cesium from water (Sato et al., 2011).
Biochars are a carbon-rich porous material containing a large amount of ash and phosphorus and are produced by pyrolysis of agricultural by-products, wastes, and manure under low oxygen conditions (Beesley et al., 2011; Dias et al., 2007; Uchimiya et al., 2012). Biochars are known to have the ability to adsorb organic and inorganic compounds, including heavy metals, and have been used for remediation of polluted soil (Beesley et al., 2011; Dias et al., 2007; Uchimiya et al., 2012). Adsorption of cesium by agricultural residues (coir pith and walnut shell), which were modified by incorporating nickel hexaxyanoferrate, was reported recently (Parab and Sudersanan, 2010; Ding et al., 2013), but to the best of our knowledge, cesium adsorption by biochar made of broiler litter has not been examined.
In this study, we report on the capability of using various types of charcoal and biochar to adsorb soluble radioactive cesium (ca. 200 – 250 Bq/kg) extracted from wheat bran harvested in 2011 (Kimura et al., 2012). A variety of commercial and experimental materials were used in the studies. The effects of pH and the presence of salts were also examined in one case.
Charcoals and biochars Powdered charcoal for food processing (SD®, BA®, and ZN® brands) and for water treatment (Y-180C®, F-17®) were obtained from Ajinomoto Fine-Techno Co. (Kawasaki, Japan). According to the manufacturer, BA charcoal (median particle diameter (m) = 19.3 µm, standard deviation (S.D.) = 26.7 µm) is a steam activated charcoal made from sawdust and ZN (m = 26.9 µm, S.D. = 29.5 µm) is a zinc chloride activated charcoal also made from sawdust. Y-180C (m = 3.4 µm, S.D. = 4.6 µm) is a steam activated charcoal made from coconut shells. SD (m = 20.7 µm, S.D. = 28.3 µm) activated charcoal is made from sawdust but the activation process used is unknown. The raw material or activation process of F-17 (m = 8.2 µm, S.D. = 15.4 µm) charcoal was not provided by the manufacturer. A third steam activated charcoal based on palm nut shells (PSC, m = 674 µm, S.D. = 543 µm) was obtained from Wako Pure Chemicals Industries (Osaka, Japan). Broiler litter was pyrolyzed under a nitrogen atmosphere at 700°C for 1 h and then activated at 800°C for 45 min with steam (created from 3 mL/min water injected into the furnace), as previously described (Lima et al., 2014), to yield a steam activated broiler biochar (BLB). The BLB biochar was ground to a powder using a Force Mill (Osaka Chemical Co., Osaka, Japan) before use (m=302 µm, S.D.=334 µm). Unactivated biochars from rice hulls and sludge (sold as soil fertilizers) were obtained from Green Tech K. K. (Kanuma, Japan).
The particle size distribution of the charcoals and the BLB biochar was analyzed by using an LS13320 laser diffraction particle size analyzer (Beckman Coulter, Inc, Indianapolis, USA).
Preparation of soluble radioactive cesium sample Wheat bran from a contaminated cultivar (Kimura et al., 2012) was suspended in distilled water and stirred gently overnight at 23°C. The mixture was wrung by hand in a nylon mesh bag (Kidaorimono, Osaka, Japan), and then the water extract was centrifuged to remove debris (10 min, 10,000 × g, 4°C). The supernatant was filtered by using 0.22-µm-pore-size Stericup filter units (Millipore, Billerica, MA, USA) and the filtrate (bran extract) was used as the soluble radioactive cesium sample for experiments. The bran extract prepared contained approximately 200 – 250 Bq/kg of radioactive cesium (134Cs + 137Cs) and 0.7 mg/mL of protein. The bran extract pH was 5.8 – 5.9. When necessary, the pH of the bran extract was adjusted by adding either HCl or NaOH.
Adsorption of radioactive cesium by charcoal Charcoal or biochar (3 g) and the bran extract (30 mL) were mixed in 50-mL Falcon plastic centrifuge tubes (Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA). Tubes were horizontally set on an NR-2 rotary shaker (Taitec, Koshigaya, Japan) and gently shaken (135 rpm) for 20 h at 23°C. The mixture was filtered (Stericup, 0.22 µm) to separate the charcoal from the mixture, and a portion of the filtrate (20 mL) was used for radioactivity measurements.
The distribution coefficient (Kd) for cesium adsorption was determined by equation (1) as an observed equilibrium between the solid phase and the liquid phase:
 |
Recovery of radioactive cesium from BLB For cesium desorption/recovery experiments, BLB was collected after the adsorption experiment and resuspended in 30 mL of acidic (0.1 M HCl), alkaline (0.1 M NaOH), and neutral (0.1 M ammonium acetate, pH 6.8) aqueous solutions. After gentle shaking for 20 h at 23°C , the mixture was filtered and radioactivity in the filtrate was analyzed. The recovery of adsorbed cesium was defined according to the following equation (2):
 |
Radioactivity measurements A 20-mL aliquot of filtered bran extract was transferred to a vial designed for a gamma counter (Model 2480 WIZARD2® Automatic Gamma Counter, PerkinElmer, Waltham, MA, USA). The radioactivity was counted ten times for 1,800 s (total 18,000 s) for each sample and the spectrum obtained was analyzed and converted to Bq/kg by the instrument software. The amount of radioactive cesium was calculated as the sum of 134Cs and 137Cs with standard error. The detection limit was 6.0 Bq/kg. To evaluate the net release of potassium from BLB, a standard electrode coaxial germanium detector system (Model GC2020 detector, 7500SL cryostat, and 2002CSL preamplifier; Canberra Industries, Meriden, CT, USA) coupled to a multichannel analyzer (Model DSA1000, Canberra Industries) was used for measuring 40K. The gamma-ray peak of 1460.8 keV was used to determine 40K concentration (Bq/kg).
Other measurements Protein concentration was determined by a commercial kit (protein assay dye reagent, Bio-Rad Laboratories, Hercules, CA, USA). The color of the bran extract was analyzed by a Color Reader CR-13 (MINOLTA Co., Tokyo, Japan). Color of the bran extract was evaluated by the a* value, indicating a red color. The molar concentration of potassium was calculated from the measured decay and the following equation (3):
 |
Cesium adsorption depends on the type of charcoal and biochar Uptake of mobile radioactive cesium by plants is considered to be involved in the redistribution of cesium to water and agricultural products (Sanderson et al., 2001; Yoshida et al., 2004). Thus, we used a plant material, wheat bran extract, as a matrix for radioactive cesium in this study. Among the activated charcoals and biochars tested, the broiler litter biochar (BLB) and two commercial charcoals for water treatment (Y-180C and F-17) removed more than half of the radioactive cesium from the solution (Fig. 1). The remaining three activated charcoal products (ZN, SD, and BA), made from sawdust and with various activation strategies, did not remove any appreciable cesium (Fig. 1). The palm nut shell-based steam activated charcoal (PSC) also failed to remove cesium (Fig. 1). Thus, it is clear that steam activation of carbon-rich materials is not sufficient to guarantee cesium adsorption. The unactivated biochars made from rice hulls and sludge did not remove any cesium (data not shown), suggesting that activation of char products is necessary for cesium adsorption. Other investigators have shown that surface functional groups (acidic or alkaline) do not promote cesium adsorption, and it was suggested that the low polarizing power of ionic cesium results in weak interaction between the ion and charged surface area groups (Rivera-Utrilla et al., 1984).
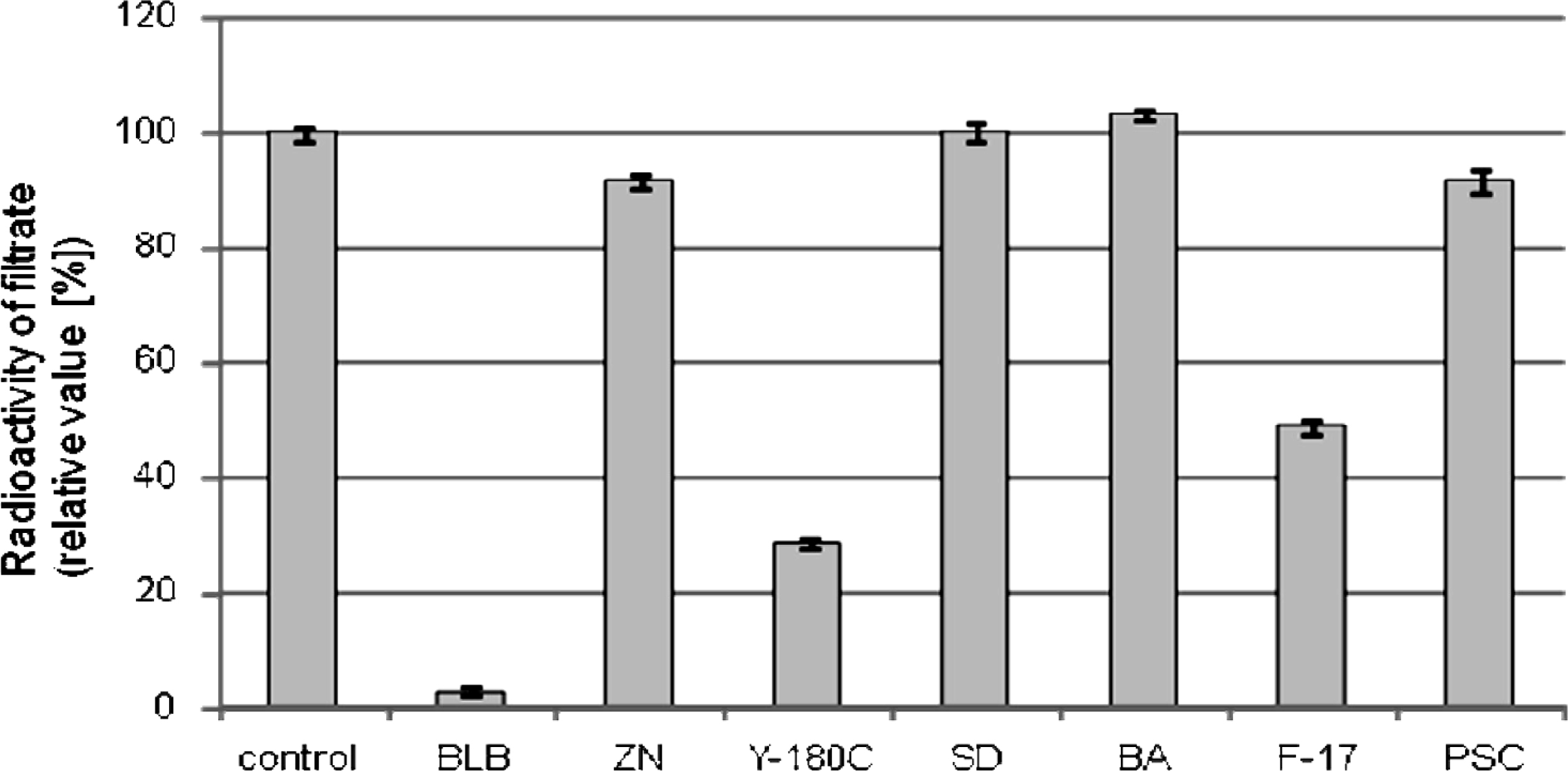
Adsorption of soluble radioactive cesium by various activated charcoals and biochar. Bran extract containing radioactive cesium was mixed with charcoal/biochar (10% [w/v]) for 20 h at 23°C. Percentages of control values (no addition of charcoal/biochar) are shown with standard error bars. pH of the bran extract used was adjusted to 6.8 prior to the experiment.
BLB adsorbs radioactive cesium under alkaline conditions The steam activated broiler litter biochar (BLB) was investigated further, as it exhibited the highest adsorption of radioactive cesium. The adsorption of cesium onto BLB as a function of time is shown in Fig. 2. The absorption rate was slower than reported for zeolite and ferrocyanides (Borai et al., 2009; Faustino et al., 2008), and at 20 hours, adsorption appeared to have reached equilibrium (Fig. 2, left panel). The slow rate suggests an adsorption phenomenon rather than a precipitation effect. Radioactive cesium was adsorbed to BLB dose dependently up to 10% (w/v) (Fig. 2, right panel). As shown in Fig. 3, BLB adsorbed radioactive cesium under neutral and mild alkaline conditions but not under acidic conditions. Below pH 3.0, about 90% of radioactive cesium of the bran extract remained in the filtrate (Fig. 3). Hydronium ion (H3O+) may have competed with Cs+ ion in hypothetical binding pockets created in the BLB, as argued in the case of ferrocyanides (Prussian blue) (Faustino et al., 2008). The Kd values for cesium absorption deduced from the best-fit curve are shown in Fig. 3 (right axis). The value was 1.4 L/g at pH 8.0 and 0.22 L/g at pH 6.6. The Kd value of BLB for low-level radioactive cesium at 23°C was lower than that of zeolite (2 – 5 L/g at 25°C) (Borai et al., 2009) and higher than that of nickel hexacyanoferrate incorporated into walnut shell (0.066 L/g at 27°C) and coir pith (0.17 L/g at 25°C) (Parab and Sudersanan, 2010, Ding et al., 2013). The modified coir pith biosorbent showed higher Kd values of 7.57 and 22.6 at 35°C and 45°C, respectively (Parab and Sudersanan, 2010). We did not perform experiments at 35°C or higher temperatures because the polluted area around Fukushima Prefecture is not a tropical area such as India, where the coir pith biosorbent was examined.

Time course (left panel) and dose dependency (right panel) of BLB adsorption of radioactive cesium.
Bran extracts containing radioactive cesium of pH 5.8 (open circles) and pH 6.8 (open triangles) were mixed with BLB (10% [w/v]) at 23°C for the specified time (left panel). The adsorption of radioactive cesium from bran extract (pH 6.8) was examined with various amounts of BLB (0.1% to 10%) at equilibration (contact time = 20 h). Percentage of control values (no addition of BLB) is shown with standard error bars.

pH dependency of radioactive cesium adsorption by BLB and distribution coefficient (Kd). Bran extract containing radioactive cesium was mixed with BLB (10% [w/v]) for 20 h at 23°C and different pH. Percentage of control values (no addition of charcoal) is shown with standard error bars (left axis). Kd values for cesium adsorption at pH tested are shown (open circles, right axis).
The presence of several cations reduces radioactive cesium adsorption Radioactive cesium adsorption on BLB was also examined in the presence of NaCl, KCl, CaCl2, NH4Cl, and EDTA. While the presence of CaCl2 (10 mM and greater) and NaCl and NH4Cl (100 mM) reduced the adsorption of cesium, KCl had no effect at the concentration tested (Fig. 4). The potassium content of wheat bran (9.3 – 11.3 mg/g) is much higher than that of other minerals (Peterson et al., 1984) and BLB itself also has a significant amount of potassium, as described in the next section, which may explain the lower effect of potassium addition. The presence of EDTA (10 mM) improved the adsorption (Fig. 4), suggesting that the organic fraction of the EDTA-Cs complex (Seliman et al., 2010) may adsorb to the biochar, in addition to free Cs+. The impact of other chelating agents such as natural organic matter has previously been noted by Uchimiya et al., who showed improved removal of some metals from solution by broiler litter biochar when these chelating agents were present (Uchimiya et al., 2010).

Effect of salts and EDTA on the adsorption of radioactive cesium onto BLB. Bran extract containing radioactive cesium mixed with BLB (10% [w/v]) for 20 h at 23 °C, with or without different salts. Percentage of control values (no addition of charcoal) are shown with standard error bars. For NaCl, KCl, CaCl2, and NH4Cl, closed bars mean salt concentration: 1, 10, and 100 mM (left to right). EDTA was examined at 1 (left bar) and 10 mM (right bar).
There is a net release of 40K from BLB The filtered bran extract contained 61.8 ± 12 Bq/kg of natural 40K before treatment with BLB. After BLB treatment, the filtrate contained 172 ± 18 Bq/kg of 40K, suggesting a net release of potassium from this biochar into the bran extract filtrate. Previous studies have shown that broiler litter and activated broiler litter biochar contain significant amounts of potassium (42 and 87 mg/kg, respectively) and that a significant part of this potassium is soluble in water and weak acid (Lima et al., 2014). Based on the measured release of 40K, we estimate that the potassium leached from 1 g BLB during incubation was 36 mg.
Activated charcoal and biochar decolorize bran extract but are not linked to cesium adsorption Decoloration is one of the major uses of charcoals in the food industry. The bran extract used in this study had a brown color (a* value = 10.0). While bran extract treated with BLB still had visible color (a* value = 2.6), bran extract treated by activated charcoal (PSC) lost most visible color (*a value = 0.5). Thus, the capability to adsorb colored substances and cesium ions from bran extract could not be correlated.
Radioactive cesium loaded on BLB is relatively immobile under neutral and alkaline conditions Waste materials that contain more than 8,000 Bq/kg of radioactive cesium are governed under regulation by the Japanese Ministry of Environment (Ministry of Environment, 2013, [iii]), which prompted us to examine desorption of adsorbed radioactive cesium from BLB. More than half of the radioactive cesium was released from BLB by washing it in 0.1 M HCl (Fig. 5). However, the bound radioactive cesium was stable under alkaline and neutral conditions (Fig. 5). For long-term storage of BLB carrying radioactive cesium, pH control would be important to avoid relocation of the adsorbed radioactive cesium and secondary contamination.

Desorption of radioactive cesium from used BLB. Radioactive cesium contained in bran extract was adsorbed to BLB (10% [w/v]) at 23°C for 20 h. The mixture was filtered and the BLB was resuspended in 30 mL of 0.1 M HCl, 0.1 M NaOH, or 0.1 M ammonium acetate for 20 h at 23°C. Percentages of the amount of the initial bound radioactive cesium are shown with standard error bars. pH of the bran extract used was adjusted to 6.8 prior to adsorption.
In conclusion, the capability of charcoal to adsorb radioactive cesium appears to depend on the source material and its activation conditions. BLB is potentially applicable for removing low levels of aqueous radioactive cesium under alkaline conditions. Improvement of the decoloration ability of BLB is required for food processing use, or combination of BLB with a traditional activated charcoal may be used to achieve both cesium and color removal.
Acknowledgments This work was partially supported by a grant from the Skylark Food Science Institute (Tokyo, Japan). The mention of firm names or trade products does not imply that they are endorsed or recommended by the U.S. Department of Agriculture over other firms or similar products not mentioned. USDA is an equal opportunity provider and employer. We are grateful to Dr. Itaru Sotome for his help in analyzing particle size distribution.