2015 Volume 21 Issue 1 Pages 7-11
2015 Volume 21 Issue 1 Pages 7-11
The effect of electricity on the activity of pectin methylesterase (PME) in tomato juice was studied to determine its inactivation mechanism. In this study, the PME in tomato juice was inactivated by applying high electric field alternating current (HEF-AC), raising the temperature to 85° in 0.01 s, and maintaining that temperature for 3.0 s. Such inactivation by HEF-AC was 100 times faster than that by conventional heating at a temperature range of 55°C to 80°C. Whereas the inactivation rate of extracted PME remained low following conventional heating (< 65°C), HEF-AC (> 75°C) increased the inactivation rate of extracted PME to levels similar to that of non-pulp PME. It was assumed that PME attached to the pulp was released by HEF-AC and subsequently denatured at high temperature.
Pectin methylesterase (PME) is an endogenous pectinolytic enzyme found primarily in tomato cell walls. Activated PME causes loss of opalescence in juice during storage (Vercet et al., 1999). In fact, orange juice pasteurization tables are calculated as a function of heat-resistant PME destruction, which requires more energy than that necessary to ensure the microbiological safety of the product. However, heat treatment negatively impacts both the nutritional and organoleptic qualities of orange juice (Balaban et al., 1991; Fourie, 1996; Yeom et al., 2000).
Market opportunities are stimulating the search for new methods of pectinolytic enzyme inactivation that provide a shelf-stable product with nutritional and organoleptic qualities approaching those of freshly squeezed orange juice. Inoue et al. demonstrated that PME inactivation in orange juice was accelerated by high electric field alternating current (HEF-AC) (Inoue et al., 2007a). HEF-AC uses a 20 kHz alternating electric field exceeding 2 kV/cm, causing rapid heating by the joule effect. HEF-AC was studied as a method of inactivating heat-resistant spores in fresh juice (Uemura and Isobe, 2003; Uemura et al., 2009; Uemura et al., 2010).
The objective of the present work was to compare the efficacy of HEF-AC treatment in kinetically inactivating tomato PME with that of conventional heat treatment.
Raw material A fresh eatable tomato (crop 2011 in Kumamoto) was purchased from a local supermarket. The tomato was cut into eight pieces and squeezed using a juicer (Panasonic, MJ-M1, Japan). The resulting tomato juice was stored in a deep freezer at −80°C. Samples were thawed by storing them overnight at 5°C.
HEF-AC setup The HEF-AC system is illustrated in Fig. 1. Tomato juice in a tank was fed to an electrode unit at a constant flow rate (500 mL/min) using a plunger pump (PU718; GL Sciences, Japan). The juice was passed between two electrodes (4 mm wide, 13 mm long, and separated by 2 mm; capacity, 0.1 mL) for 0.012 s. Alternating current (maximum voltage 750 V, maximum current 7 A, frequency 20 kHz) was applied to the electrodes using a joule-heating power supply (JHPW5.5; Frontier Engineering Co., Japan). The maximum electric field was 3.75 kV/cm, and the maximum current density was 13.5 A/cm2.
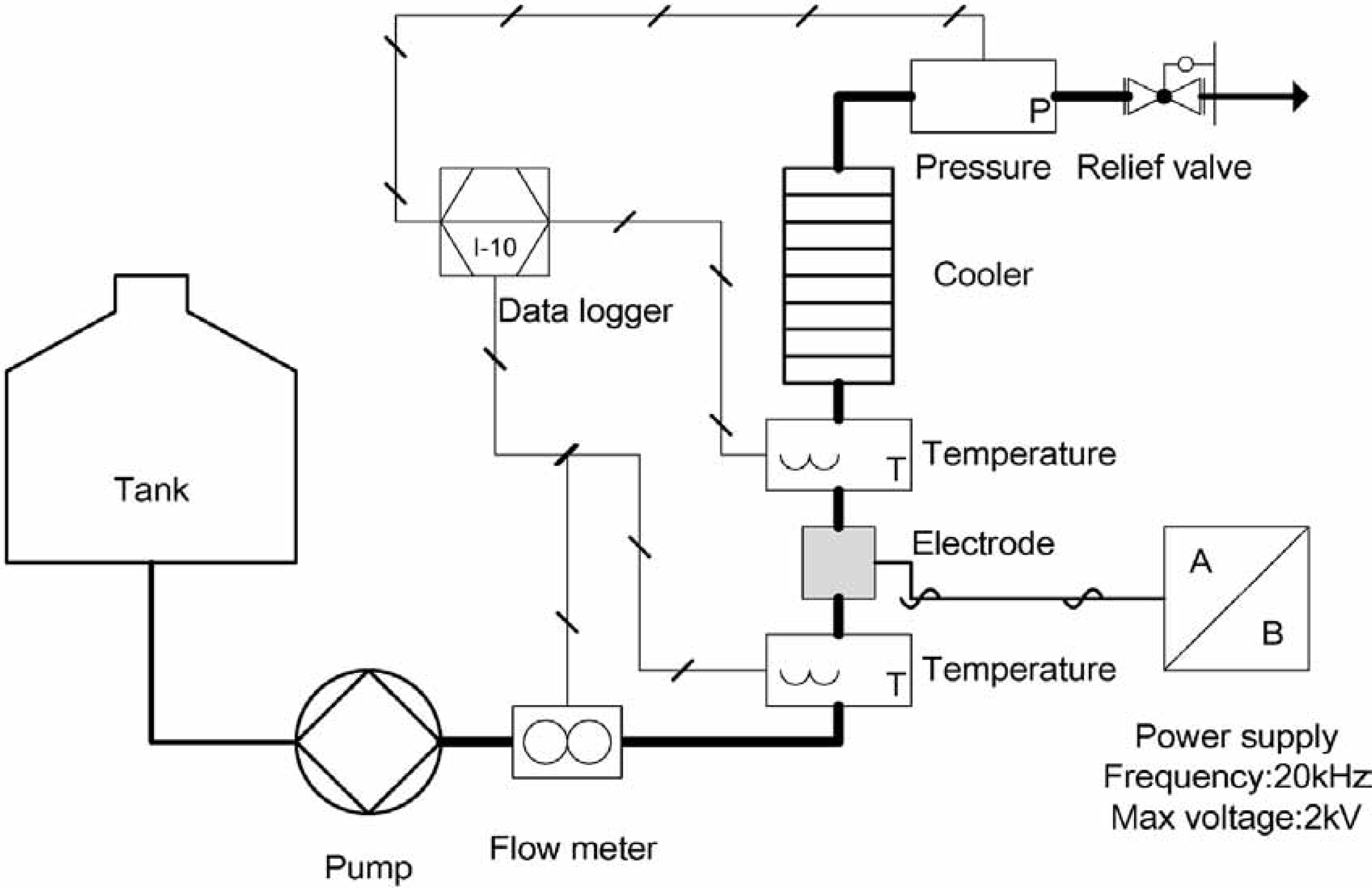
Schematic diagram of the HEF-AC system. The thick line indicates the pressurized section
A thermocouple set in the middle of the pipe 20 mm from the electrode outlet was used to measure the sample temperature every second. The HEF-AC-treated sample was kept at the highest temperature for 1.0 to 3.0 s in a holding pipe and then cooled to 25°C in a heat exchanger for 10 s. The treated sample was removed through a relief valve, which maintained the line pressure at 0.5 MPa.
Conventional heating Capped test tubes containing 10 mL of tomato juice were heated in a water bath controlled at 60, 65, 70, and 75°C for 10 min. For example, the temperature, measured using a thermocouple at the center of the tomato juice, was increased to 70°C in 2 min and then held at 70°C for 8 min.
Step heating Step heating was employed for higher temperatures than conventional heating. First, 2 mL of citric acid buffer in a test tube was preheated in a water bath set to the designated temperature. A 0.5 mL sample was added to the test tube in the water bath, and the temperature was instantaneously raised to the designated temperature. The test tube was immediately transferred to an ice-water bath after the designated time. The 0.5 mL untreated sample and the HEF-AC-treated sample were individually diluted in 2.0 mL of citric acid buffer.
PME assay PME activity was measured using a modified Kimball (1991) method. The carboxylic groups generated by PME during the hydrolysis of a pectin solution at pH 7.0 and 30°C were titrated. Two milliliters of diluted sample was then further diluted with SQ water to 20 mL and adjusted to pH 7.0 with 0.1 N NaOH. A 2 mL tomato juice sample adjusted to pH 7.0 with 0.2 N NaOH was added to 50 mL of 1% citrus pectin (Sigma) solution. The pH was maintained at 7.0 by titrating the reaction mixture with 0.01 N NaOH using an Impulsomat and a Dosumat (665 and 614, respectively; Metrohm, Switzerland). The change in pH was recorded using a pH meter (DELTA320; Mettler Toledo, Switzerland). The consumption of NaOH was recorded for 20 min. The slope dVNaOH/dt was determined in the linear part of the titration curve.
The PME activity of sample A, calculated using Eq. (1) and expressed as microequivalents per min and mL juice, is directly proportional to the slope.
 |
Here, NNaOH is the NaOH concentration, VNaOH is the volume of NaOH used, Vsample is the volume of the sample used, and t is the enzymatic reaction time.
The percentage of residual PME activity (RA) was defined as indicated by Eq. (2).
 |
Here, At is the enzyme activity in the sample after treatment, and A0 is the enzyme activity of the untreated sample.
PME extraction PME is ionically bound to the cell wall, requiring a buffer with high ionic strength (Tris, NaCl) to extract it. Tomato PME was extracted according to the methods of Denes et al. (2000) with modification. Forty milliliters of tomato juice was centrifuged at 5000 rpm for 5 min, and the pellet was separated from the supernatant. Twenty milliliters of 2% (W/V) polyvinylpolypyrrolidone (PVPP) was then added to the pellet and magnetically stirred, followed by centrifugation at 20,000 rpm for 10 min at 4°C. The supernatant was discarded. Forty milliliters of 1 N NaCl (pH 6.0, adjusted with NaOH) was then added to the pellet and magnetically stirred for 3 h. The precipitate containing PME was collected by centrifugation at 20,000 rpm for 10 min at 4°C.
SDS polyacrylamide gel electrophoresis For SDS-PAGE gel electrophoresis, 0.1 µL tomato juice samples were mixed with SDS-PAGE buffer containing 2-mercaptoethanol and applied to a 10% PAGE gel (SuperSep pre-cast gel; Wako, Japan) with Precision Plus molecular weight markers (Bio-Rad Laboratories). Electrophoresis was carried out at a constant current of 20 mA. The gel was fixed and stained with 45% methanol and 10% acetic acid containing 0.2% Coomassie Brilliant Blue, followed by destaining with 7% methanol and 7% acetic acid.
Temperature effect of conventional heating and HEF-AC PME inactivation by conventional heating increased with increasing temperature above 60°C, and total inactivation occurred at temperatures above 75°C. PME inactivation by HEF-AC increased with increasing temperature above 75°C, and 99% of inactivation occurred at temperatures above 85°C (Fig. 2). It was assumed that the temperature difference of 10°C between HEF-AC and conventional heating was due to the difference in heating duration (3 s and 10 min).

PME activity change by conventional heating (C.H., 10 min) and HEF-AC, 3 s at different temperatures
Arrhenius plot of HEF-AC treatment and conventional heating Holding times between the electrodes and the cooling unit varied from 1 to 3 s. An Arrhenius plot was drawn to compare the inactivation at a given holding time for HEF-AC and for conventional heating (Fig. 3). The activation energies for HEF-AC (124 kJ/mol) and conventional heating (119 kJ/mol) were similar; therefore, inactivation by HEF-AC may be attributable mainly to thermal effects. The rate constant difference between HEF-AC and conventional heating was 2 log orders, and inactivation by HEF-AC was 100 times faster than by conventional heating. HEF-AC can inactivate PME in tomato juice with a shorter holding time than conventional heating. This result is similar to the inactivation of Alicyclobacillus acidoterrestris spores in orange juice by HEF-AC (Uemura et al., 2009). Jakób et al. (2010) reported that ohmic heating had a significant entropic effect on the rate of enzyme inactivation. Ohmic heating had no direct effect on enzyme conformation; however, ohmic heating modified enzyme surfaces and the enzyme environment by ionizing solution components and distributing their ions by an applied electric field. Next, we measured alterations in PME activity of extracted tomato juice by conventional heating. PME inactivation rate in extracted tomato juice increased above 75°C using step heating compared to below 65°C. It was assumed that the PME in tomato juice treated by HEF-AC was transitioned to a level of sensitivity that was the same as PME extraction above 75°C by step heating.

Arrhenius plot of PME inactivation using HEF-AC and conventional heating (C.H.) crude tomato juice and extracted tomato juice
▪: HEF-AC crude tomato juice. ♦: C.H. crude tomato juice. ▴: C.H. extracted tomato juice.
SDS electrophoresis of tomato juice Figure 4 presents SDS electrophoretograms of extracted PME in the precipitates of tomato juices treated by HEF-AC at (3) 60°C and (4) 82°C, and by (5) conventional heating at 70°C for 10 min. The observed 35-kDa band represents tomato PME. The majority of PME (94%) in tomato juice (2) was located within the precipitated pulp. HEF-AC decreased the PME within the pulp with increasing temperature, while under conventional heating the PME remained within the pulp. The PME activity in the tomato juice of (3) was 80.6%, that of (4) was 7.1%, and that of (5) was 8.9%. HEF-AC at 82°C and conventional heating at 70°C for 10 min resulted in the same level of PME activity; HEF-AC inactivated PME outside of the pulp, whereas conventional heating inactivated PME within the pulp. Raviyan et al. (2005) reported increased inactivation of PME in tomato juice sonicated by ultrasound compared to thermal treatment alone. The inactivation of PME by HEF-AC was assumed to be analogous to sonication. It was also assumed that crude PME was slowly inactivated within the pulp by conventional heating, whereas HEF-AC forced PME to be released from the pulp, similar to sonication, and it was rapidly inactivated.
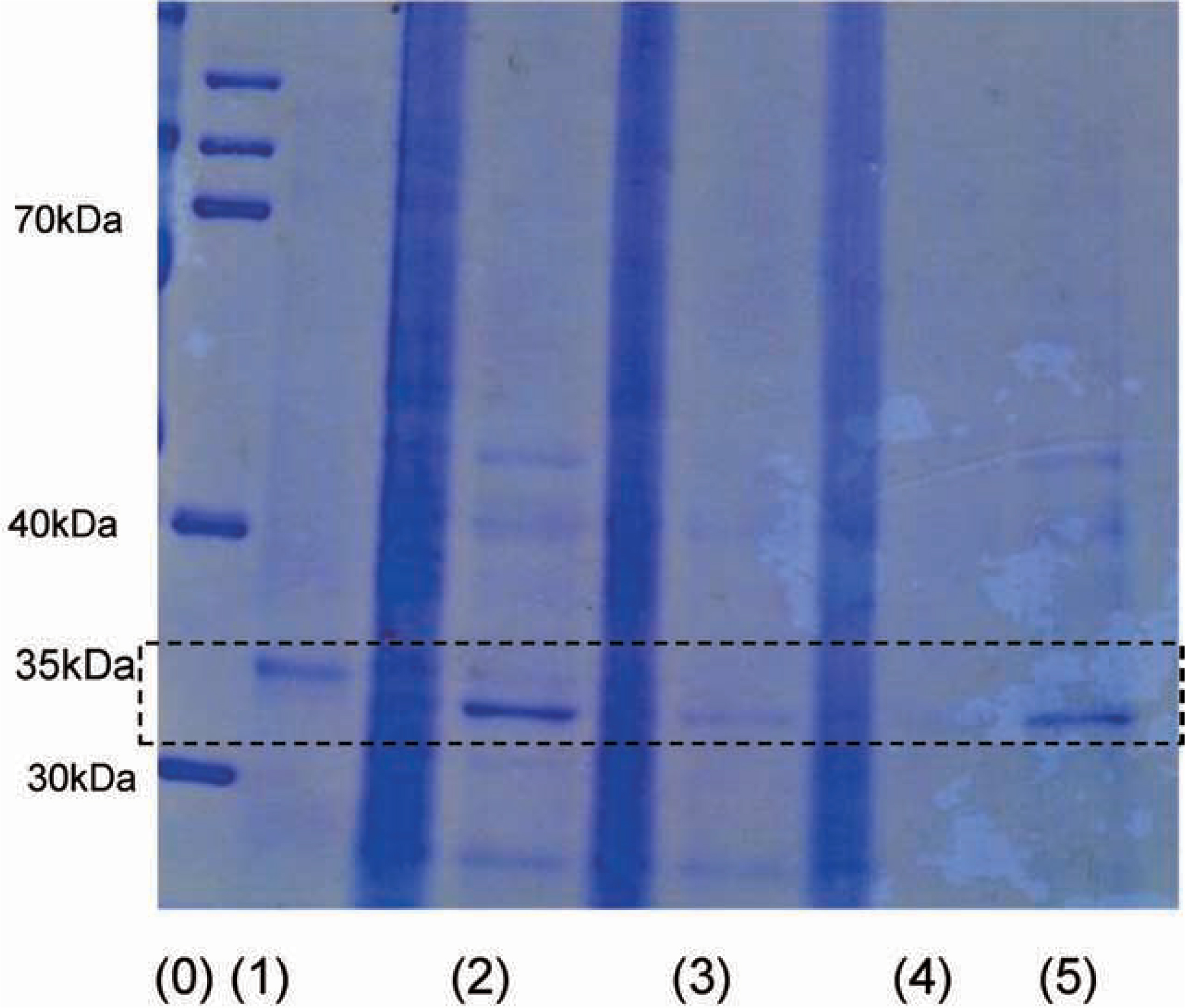
SDS electro-photograms
(0)Marker. (1) PME reagent. (2) Tomato juice. (3) HEF-AC at 60°C. (4) HEF-AC at 82°C. (5) Conventional heating at 70°C for 10 min.
The experiment results indicate that HEF-AC treatment effectively inactivates PME in tomato juice. HEF-AC applied at 85°C for 0.012 s with 13.5 A/cm2 of electric current density released PME from the cell wall; the released PME in the liquid was inactivated within 3.0 s in the holding section. HEF-AC has been developed for use in the sterilization or pasteurization of microorganisms in liquid foods. Moreover, HEF-AC treatment can inactivate heat-resistant spores (Inoue et al., 2007b). A commercial-scale HEF-AC system (maximum capacity 5.0 ton/hour) has been successfully developed by Frontier Engineering Company. In the future, we expect HEF-AC to be used for the rapid inactivation of expendable enzymes in liquid foods.