2015 Volume 21 Issue 2 Pages 145-157
2015 Volume 21 Issue 2 Pages 145-157
Healthy foods such as beans, mushrooms, vegetables, and seafood and healthy dietary patterns such as the Mediterranean diet and Japanese food have higher concentrations of polyamines (spermine and spermidine). The continuous intake of high-polyamine foods has been shown to increase whole blood polyamine levels in mice and humans. In addition, high-polyamine chow inhibited aging-associated pathological changes in Jc1:ICR male mice and extended their lifespan. Aging is accompanied by decreased DNA methyltransferase activities, increased proinflammatory status, and enhanced abnormal gene methylation status, which is considered to be part of the pathogenesis of aging-associated diseases. In vitro and in vivo experiments have shown that polyamine supplementation reversed such changes induced by aging and polyamine-deficiency. In addition, polyamines have many biological activities that may contribute to the inhibition of lifestyle-related diseases such as diabetes, hyperlipemia, and arteriosclerosis. The possible role of dietary polyamines in human health is discussed.
Many epidemiological studies have shown that several foods and dietary patterns have a close association with the inhibition of aging-associated diseases, such as cardiovascular diseases (e.g., myocardial infarction, cerebral infarction) and some types of cancer such as breast and colon cancers. Food preferences and dietary patterns differ widely among countries and regions, emphasizing the role of food components in the inhibition of aging-associated pathologies. The role of antioxidants such as polyphenols (e.g., isoflavone, resveratrol) on human health and longevity has been examined extensively; however, it has not been universally accepted that antioxidants contained in foods help to suppress the occurrence of aging-associated diseases and extend the lifespan (Couzin-Frankel, 2011; Strong et al., 2013).
My colleagues and I have demonstrated that polyamines (i.e., spermine and spermidine) are abundant in healthy foods such as beans, vegetables, fish and shellfish and healthy dietary patterns such as Japanese food and the Mediterranean diet (Soda, 2010b, 2011b). We have also shown that in mice, a lifelong consumption of polyamine-rich chow inhibits aging-associated pathological changes in organs and extends the lifespan (Soda, 2009, 2010a, 2012; Soda, Dobashi et al., 2009; Soda et al., 2013; Soda, Kano et al., 2009).
Compared to lower organisms such as yeast, nematodes and flies, mammals such as mice and humans have far more advanced and complicated neurological, endocrine and immune functions, and longer lifespans. In industrial countries, the occurrence of many aging-associated diseases shorten the human lifespan, including hypertension, diabetes, hyperlipemia and gout as well as cancers such as colorectal and breast cancers. The pathogeneses of these diseases are not alike but their progression and severity can significantly affect the human lifespan. It is thus unlikely that a single gene or only a few genes have pivotal roles in the occurrence and progression of all aging-associated diseases (Burnett et al., 2011; Gierman et al., 2014).
In this review, I describe polyamine-induced biological activities that may help improve health and extend the lifespan of humans, especially the activities promoted as a result of increases in polyamine concentrations, i.e., via a high-polyamine diet.
Polyamines, i.e., spermine and spermidine, are found in almost all living organisms, and thus foods that are comprised of various types of organisms and their products contain polyamines, which can vary widely in concentration (Cipolla et al., 2007; Nishibori et al., 2006; Nishimura et al., 2006; Soda, 2012). Figure 1 shows the pathway of polyamine metabolism and catabolism as well as polyamine transport. Polyamines are synthesized from cellular arginine. Spermidine has three amino groups (−NH2), while spermine has four. The molecular weight of the largest human polyamine, spermine, is approx. 200 g/mol.
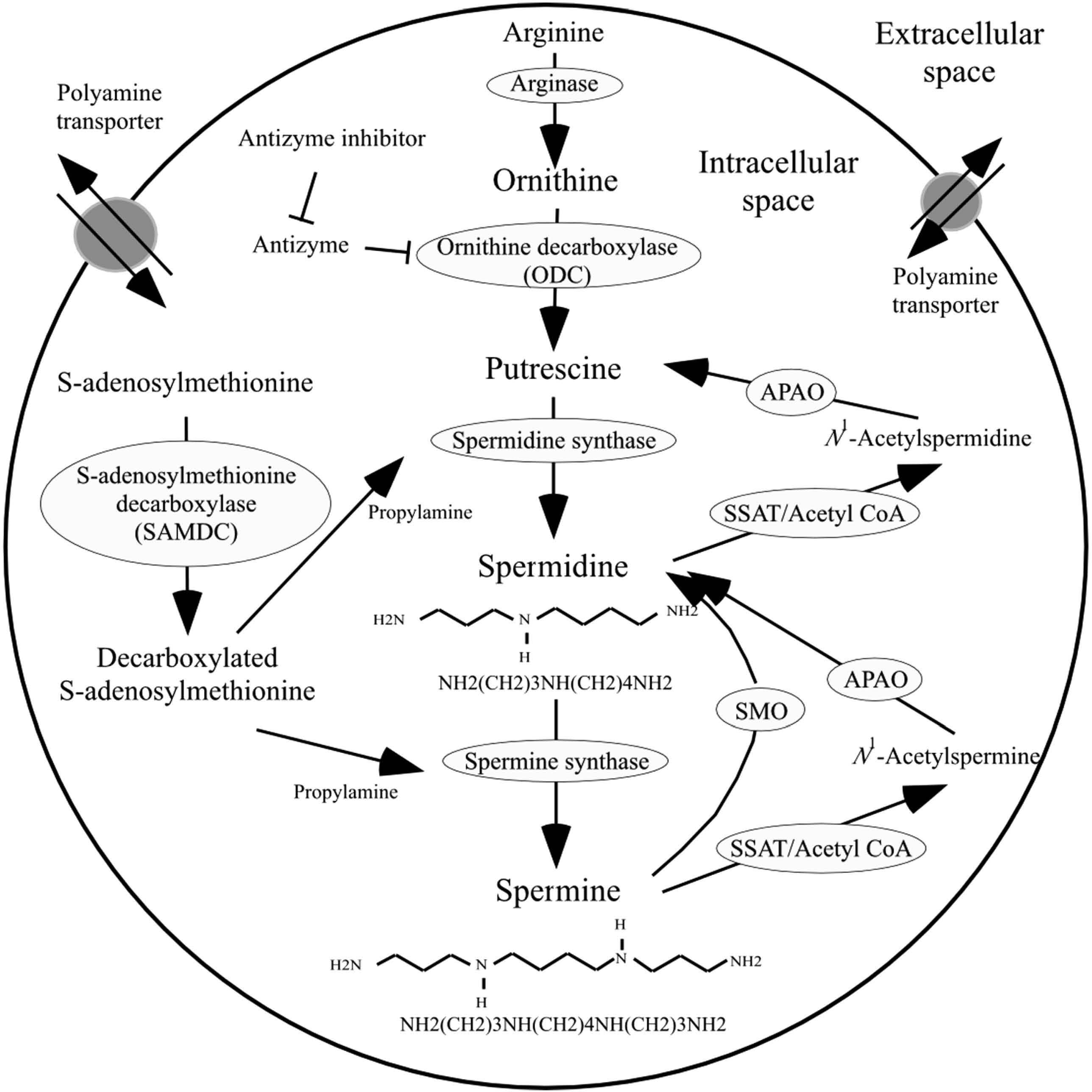
The metabolic pathway of polyamines
Spermine has four amine residues, while spermidine has three amine residues.
APAO: N1-acetylpolyamine oxidase, SSAT: spermine/spermidine-N1-acetyltransferase, SMO: spermine oxidase.
The chemical compound putrescine has two amines and is therefore a diamine, and its biological activities differ from those of polyamines. For example, whereas spermine and spermidine have anti-inflammatory activities and are absorbed quickly from the intestinal lumen, putrescine has no anti-inflammatory properties and is degraded predominantly in the intestinal lumen (Soda, 2009; Soda et al., 2005; Zhang et al., 1997; Bardocz et al., 1990; Bardocz et al., 1995).
The enzymatic activities related to polyamine synthesis, especially those of the enzyme ornithine decarboxylase (ODC), decrease with aging (Ferioli et al., 1976). The activities of ODC, which is a rate-limiting enzyme with a short half-life, can be stimulated by specific stimuli (Ferioli et al., 1976; Janne & Raina, 1969; Russell et al., 1970). Because spermidine synthase and spermine synthase lack a regulatory or rate-limiting role in polyamine synthesis, these enzymes have attracted less attention than ODC, and their properties remain to be fully clarified. However, administration of arginine or ornithine stimulated putrescine levels in elderly people and animals, whereas polyamine synthesis was not necessarily stimulated (Bedford et al., 1988; Schleiffer et al., 2000; Teixeira et al., 2002; Yoshinaga et al., 1993). These findings indicate that the activities of spermine and spermidine synthases decrease gradually with aging and without being revitalized. In animal tissue, an aging-associated decline in ODC activity is observed, with a concomitant gradual decrease in polyamine concentration (Das & Kanungo, 1982; Laitinen et al., 1982). However, when polyamine concentrations in whole blood (mainly in erythrocytes and leukocytes) are measured in adult humans, the aging-associated decline in polyamine concentrations is not remarkable, and large inter-individual differences are found (Elworthy & Hitchcock, 1989; Soda et al., 2005).
Cells can synthesize polyamines intracellularly as well as take up polyamines from the extracellular space through a polyamine transporter on the cell membrane. Major sources of body polyamines in adult humans are thought to be those contained in foods and synthesized by the intestinal microbiota. Polyamines in the intestinal tract are absorbed quickly and distributed to almost all organs and tissues of the body (Bardocz et al., 1990; Bardocz et al., 1995). The exact biological background and mechanisms of the large inter-individual differences in blood polyamine concentrations in humans are not known. However, one of the reasons for the variability is thought to be differences in the amount of polyamines supplied by the intestinal lumen, which may reflect individual food preferences as well as the ability of the intestinal microbiota to synthesize polyamines, likely related to the intestinal bacterial flora composition. In fact, when the polyamine supply from foods as well as from the intestinal microbiota is suppressed, polyamine concentrations in whole blood are decreased (Cipolla et al., 2003; Nishimura et al., 2001), and conversely, when an increased polyamine supply from foods is ongoing, blood polyamine concentrations gradually increase (Soda, Dobashi et al., 2009; Soda, Kano et al., 2009).
We have shown that upon stimulation with lipopolysaccharide and phorbol 12-myristate 13-acetate, polyamines suppress the production of proinflammatory cytokines from immune cells (Zhang et al., 1997). In addition, polyamines decrease the amount of lymphocyte function-associated antigen 1 (LFA-1) on the surface of immune cells (Soda, 2009; Soda et al., 2005) (Fig. 2a). LFA-1, the amount of which increases with aging, is one of the phenomena of immuno-senescence, indicating aging-associated changes in immune functions (Chiricolo et al., 1995; Okumura et al., 1993; Pallis et al., 1993; Powers et al., 1992) (Fig. 2b). LFA-1 on immune cells preferentially binds to intercellular adhesion molecules (ICAMs) on endothelial cells lining the blood vessels. This binding activates immune cells and induces the production of various chemical substances including proinflammatory cytokines. Almost all aging-associated diseases are considered to be induced by chronic (repeated and mild) inflammation, as a result of sustained immune cell activation upon stimulation by degraded cells and endogenous pro-inflammatory substances. Therefore, the increased levels of LFA-1 in the elderly indicates the hypersensitivity of immune cells to such originally inoffensive stimuli, and this hypersensitive condition tends to promote the occurrence of and accelerate the progression of aging-associated diseases.

Polyamine, age, and LFA-1 expression
(a) Relation between serum spermine concentration and LFA-1 (CD11a) expression on human peripheral blood mononuclear cells (PBMCs).
The quantity of LFA-1 on the cell surface of PBMCs (lymphocytes and monocytes) and serum spermine concentrations were examined in 42 healthy male volunteers (age 20–70 yrs). Spermine concentration-dependent decreases in LFA-1 expression were observed irrespective of the age of subjects.
(b) Relation between age and LFA-1 expression.
The quantity (mean fluorescent intensity = MFI) of LFA-1 increased with aging.
LFA-1 was measured by flow cytometry.
Serum spermine concentration was measured by high performance liquid chromatography (HPLC).
The amount of LFA-1 is represented by CD11a MFI.
LFA-1: lymphocyte function-associated antigen 1
Although polyamines suppress the production of proinflammatory cytokines from immune cells upon stimulation and decrease the amount of LFA-1 protein on non-stimulated immune cells, increases in polyamine concentrations enhanced the blastogenic response of immune cells to mitogens such as phytohemagglutinin (PHA) and concanavalin A (Con A) in vitro (Soda et al., 2005). Lymphocyte blast transformation is a method of detecting the potential of immune cell activity. Notably, in the elderly, the blastogenic response of lymphocytes to mitogen is low and the amount of LFA-1 on immune cells is high (Chiricolo et al., 1995; Franceschi et al., 2000; Gillis et al., 1981; Pisciotta et al., 1967; Powers et al., 1992). In addition, it was shown that polyamine extends the lifespan of cultured immune cells (Eisenberg et al., 2009). We have also found that polyamine supplementation inhibits decreases in the natural killer (NK) activities of immune cells obtained from peripheral blood and cultured (Soda, 2009, 2011a). Polyamines also have anti-oxidant, radical scavenger properties and other biological activities that help protect cells and genes from harmful stimuli (Belle et al., 2004; Brune et al., 1991; Chattopadhyay et al., 2003; Chiu & Oleinick, 1998; Douki et al., 2000; Farbiszewski et al., 1996; Fujisawa & Kadoma, 2005; Gaboriau et al., 2005; Goss et al., 1995; Ha, Sirisoma, et al., 1998; Ha, Yager, et al., 1998; Held & Awad, 1991; Khan et al., 1992; Lovaas & Carlin, 1991; Marzabadi & Llvaas, 1996; Newton et al., 1996, 1997; Rajalakshmi et al., 1978; Sava et al., 2006; Soda et al., 2005; Spotheim-Maurizot et al., 1995; Sy et al., 1999; Tadolini, 1988; Tadolini et al., 1984; Warters et al., 1999; Zhang et al., 1997).
A gene is an ‘advanced source of enormous digital information’ comprised of combinations of the four bases adenine, guanine, thymine and cytosine. Gene expression is regulated not only in the ‘digital’ form but also in the ‘analog’ form. An analog regulatory mechanism is the methylation of genes. Gene methylation is a change that arises only in the base cytosine, creating gene information by adding a methyl group to cytosine. Upstream of the gene information, there is a direct repeat of cytosine and guanine called a CpG island. A CpG island is a site of transcription initiation, and in mammals, methylating cytosine within a CpG island can turn the gene off. Conversely, the demethylation of cytosine initiates and enhances transcription, resulting in the increased production of the protein encoded by the gene (Fig. 3). When methylation arises in the CpG islands encoding genes that function to suppress aging-associated disease(s) and/or when demethylation arises in the CpG islands encoding genes that function to provoke aging-associated disease(s), the onset and the progression of aging-associated disease(s) will be accelerated (Ono et al., 1993; White & Parker, 1983).

Analysis of gene methylation status
The genetic code of each gene is composed of the arrangement of the four bases: adenine (A), guanine (G), thymine (T), and cytosine (C).
(Upper row) The iterative array of C and G indicates the existence of gene information in the lower stream (called the CpG island).
(Lower berth) When a methyl group is supplied and cytosine is methylated, the iterative array of CG will become ambiguous. For this reason, the promoter region becomes ill defined, and it becomes difficult for protein synthesis to occur.
There is a close relationship between polyamine metabolism and gene methylation (Fig. 3). When spermidine and spermine synthases act to synthesize spermidine and spermine, propylamine is required. Propylamine is supplied from decarboxylated S-adenosylmethionine (dcSAM), which is converted from S-adenosylmethionine (SAM) by the enzymatic activities of S-adenosylmethionine decarboxylase (SAMDC). The methylation of genes indicates the conversion from cytosine to methyl-cytosine by the addition of a methyl group from SAM due to the action of DNA methyltransferase (Dnmt) (Goll & Bestor, 2005). The increase in SAM enriches the supply of methyl groups to a gene, whereas an increase in dcSAM acts to inhibit Dnmt activities (Tsuji et al., 2001; Yamamoto et al., 2010) (Fig. 4).
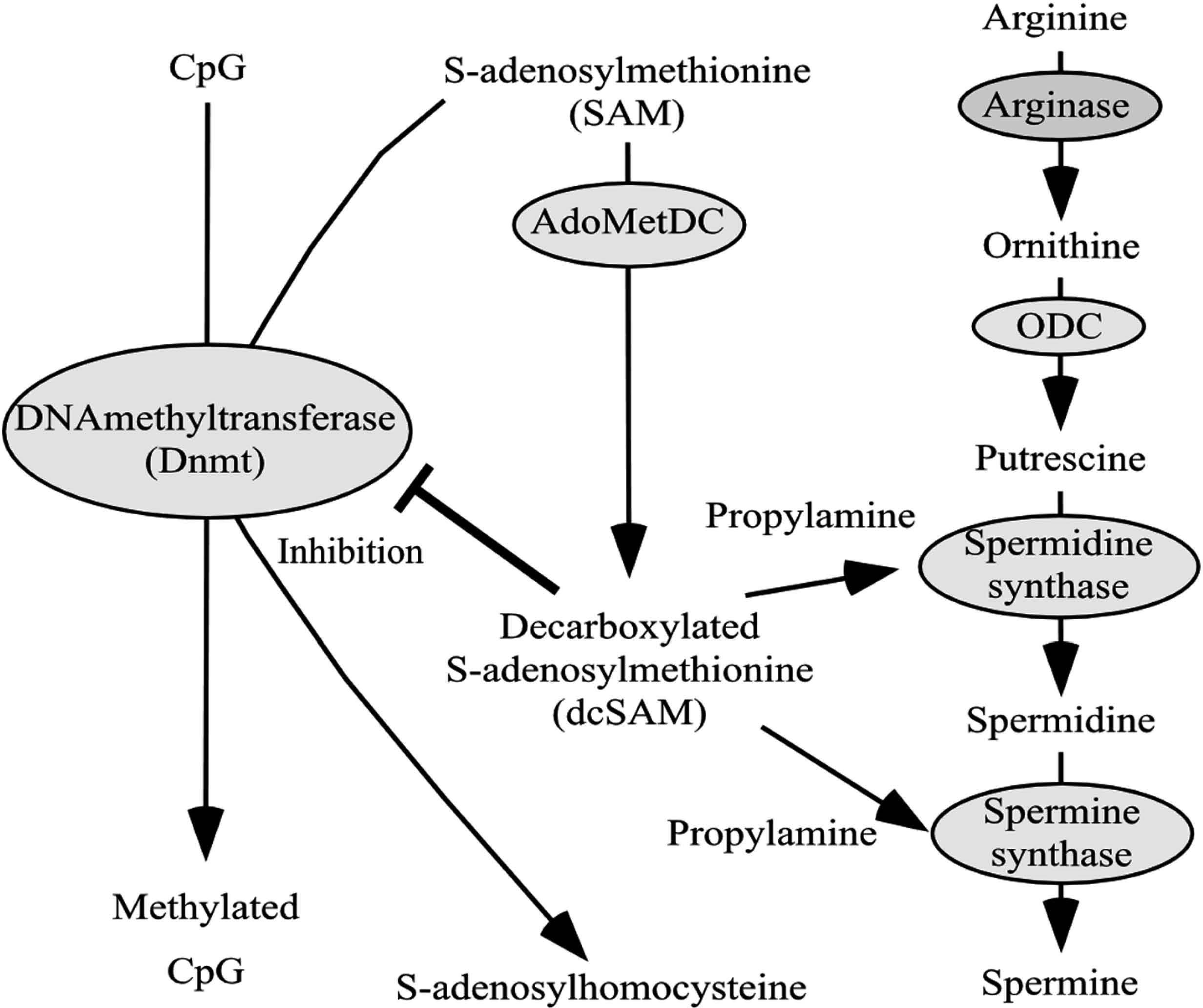
Relation between polyamine metabolism and methylation
Polyamines (spermine and spermidine) are synthesized from arginine. In the process, propylamine is supplied from dcSAM. SAMDC converts SAM into dcSAM. SAM is a methyl group donor, and dcSAM acts to inhibit the activities of Dnmt. Dnmt acts to provide a methyl group to the cytosine of the gene and converts cytosine into methylcytosine.
ODC: Ornithine decarboxylase, SAM: S-adenosylmethionine, dcSAM: Decarboxylated S-adenosylmethionine, SAMDC: S-adenosylmethionine decarboxylase, Dnmt: DNA-methyltransferase.
Enzymatic activities related to polyamine synthesis decrease with aging. To reproduce such a state (of decreased polyamine synthesis) in cultured cells, cells treated with agents that inhibit the activities of ODC or spermine and spermidine synthases or cells deficient in these enzyme activities are used. In cells in which the polyamine concentrations are decreased by overexpressing an antizyme that degrades ODC or by treatment with DL-α-difluoromethylornithine hydrochloride (DFMO), which inhibits ODC activities, or due to a deficit in the activities for spermine synthesis, the intracellular concentrations of dcSAM increase (Frostesjo et al., 1997; Pegg et al., 2011; Shantz et al., 1992; Yamamoto et al., 2010). Simultaneously, such cells have been reported to have enhanced demethylation status of the entire genome (Papazafiri & Osborne, 1987; Tsuji et al., 2001).
We found that Dnmt activities were decreased in cells in which the intracellular polyamine concentrations are decreased by DFMO treatment (Kano et al., 2013). In addition, bisulfite sequencing analyses of the LFA-1 gene revealed significant increases in demethylation of promoter regions, especially those responsible for the expression of LFA-1 on immune cells (Richardson, 2002; Zhang et al., 2002) with concomitant increases in the amount of LFA-1 protein (Kano et al., 2013)(Fig. 5). On the other hand, when polyamines are supplied from an extracellular source, the polyamine concentrations are increased, and their increases provoke negative feedback mechanisms that act to inhibit SAMDC activities (Holm et al., 1988; Mamont et al., 1981). The decreases in SAMDC result in a decreased capability to convert SAM to dcSAM, resulting in increases in SAM and decreases in dcSAM concentrations (Pegg et al., 2011; Yamamoto et al., 2010).

The effects of polyamines on the methylation of the LFA-1 promoter region
The results of bisulfite sequence analyses to determine the influence of polyamines on the LFA-1 promoter region (the domain of the signal of a gene information start) using Jukat cells. Polyamine depletion followed treatment with DFMO, an inhibitor of ODC, and spermine supplementation affected the methylation status of the promoter region. DFMO treatment augmented the demethylation of the region that is closely related to LFA-1 expression on immune cells (arrows in left upper row), and increased the quantity of LFA-1 protein (the lower-berth center bar compared to left bar). Conversely, the methylation status of the region was enhanced in cells with increased polyamine concentration following spermine supplementation (upper row, right), and the amount of LFA-1 was decreased (bar in the right lower-berth compared to center bar).
DFMO: DL-α-difluoromethylornithine
Because dcSAM acts to inhibit Dnmt activities, increased polyamine concentrations from extracellular sources seem to activate Dnmt activities (Bestor et al., 1988; Garcea et al., 1989). When 500 µM of spermine was added to cultures of DFMO-treated Jurkat cells, the intracellular spermine and spermidine concentrations and Dnmt activities were increased (Kano et al., 2013). Moreover, in cells supplemented with spermine, the methylation status of the promoter regions of LFA-1 was enhanced and the amounts of LFA-1 proteins were decreased (Kano et al., 2013) (Fig. 5).
In an evaluation of the effect of polyamines on LFA-1 expression, we found that the majority of membrane molecules that have physiological roles similar to those of LFA-1 were not influenced. LFA-1 suppression by polyamines was observed to be dose- and time-dependent; the decrease in LFA-1 protein was not observed within 24 h but was apparent after 72 h (Soda et al., 2005). Moreover, Ras-proximate-1 (Rap1), which is an intracellular signal involved in LFA-1 expression, was not affected by polyamines (Kano et al., 2013). These results indicate that the suppression of LFA-1 by polyamines is caused by changes in the methylation status of the promoter region of LFA-1 gene (ITGAL).
Although the methylation pattern on the genome, once attached, is generally stably inherited by the next-generation cell (Hashimoto et al., 2010), it has also been reported that the methylation status of some gene regions changes reversibly (Kangaspeska et al., 2008; Kim et al., 2004; Yamamoto et al., 2010). We propose that the promoter region of LFA-1 gene is one such reversible area.
Since polyamine is one of the food ingredients absorbed directly from the intestinal tract, it is of great interest that a food ingredient can affect the methylation status of a gene. It was reported that diet or dietary ingredient(s) other than but related to polyamines exerted changes on the methylation status of a gene; for example, deficiency in the supply of methyl groups in foods resulted in the enhanced demethylation of the global genome. Moreover, it was reported that a diet deficient in methyl groups provoked the demethylation of c-myc, c-fox, H-ras, and p-53 genes (Bhave et al., 1988; Christman et al., 1993; Dizik et al., 1991; Pogribny et al., 1995; Zapisek et al., 1992). In other studies, supplementation of methyl groups affected the methylation status and gene expression of several genes (Garcea et al., 1989; Kano et al., 2013).
The demethylation of genes in salmon, mouse, rat, cow, and in human is enhanced with aging (Golbus et al., 1990; Romanov & Vaniushin, 1980; Vanyushin et al., 1973; Vanyushin et al., 1970; Wilson et al., 1987; Zhang et al., 2002). However, aging-associated increases in the methylation of some genes are also reported (Issa et al., 1994; Issa et al., 1996; Wallace et al., 2010). Generally, ODC (Minois et al., 2011) and Dnmt (Lopatina et al., 2002; Oliveira et al., 2012; Romanenko et al., 1998) activities are decreased, and abnormal methylation status (increases in demethylation and methylation) is increased with aging (Kim et al., 2004; Li et al., 2010; Morgan et al., 2005). Because there is a close relationship between Dnmt activities and the methylation status of LFA-1 promoter regions, and since aging is associated with decreased Dnmt activities as well as increased demethylation of the LFA-1 promoter region (Zhang et al., 2002), the aging-associated enhancement of LFA-1 expression seems to be due to the age-dependent decreases in Dnmt activities. However, as shown in our studies (Kano et al., 2013; Soda et al., 2005), polyamines enhanced Dnmt activities and decreased LFA-1 expression in vitro, suggesting that polyamines counteract aging-associated alterations. In fact, in mice fed high-polyamine chow, the aging-associated increase in LFA-1 (CD11a and CD18) expression -especially increases in the number of bright CD11a cells- was inhibited (Soda et al., 2013).
Polyamines activate Dnmt activities, enhances the methylation of the LFA-1 promoter region, and decreases the amount of LFA-1 protein (Kano et al., 2013) (Fig. 5). However, our studies also showed that the activation of Dnmt did not cause the entire promoter region of LFA-1 to exhibit an increased methylation tendency, but it did enhance the demethylation in some parts of the region (Kano et al., 2013) (Fig. 5). We then investigated the influence of polyamines on the methylation status of the whole genome. The restriction enzyme Not I cleaves Not I sites located throughout the genome, but when cytosine in the Not I site is methylated, Not I fails to cleave it. Depending on the region, the decrease in Dnmt activities induced by a reduction in polyamines, not only provoked increases in the demethylation of some parts of the genome but also reinforced the methylation in other regions. Namely, polyamine deficiency provoked both increased demethylation and increased methylation, resulting in an abnormal methylation status of the whole genome (Soda et al., 2013) (Fig. 6).

Influence of polyamines on the methylation of the whole genome
The influence of spermine on the methylation of the whole gene was examined using Jurkat cells. A restriction enzyme cleaves a specific site containing cytosine, termed Not I site. When cytosine is methylated, the enzyme fails to cleave the gene. Under these conditions, the methylation status of the Not I restriction sites was examined. Each dot indicates the methylation status of the Not I site of each gene fragment compared to that in the control (untreated). A positive value indicates increased demethylation of cytosine at the Not I site compared to the control cells.
(Upper) The methylation status of genes in non-treated cells.
No significant variation in each dot was found, indicating that the methylation status is regulated.
(Middle) The methylation status of cells treated with DFMO and in which the polyamine concentrations were decreased significantly.
Each dot is scattered widely, indicating that DFMO treatment or polyamine deprivation significantly enhances both demethylation and methylation.
(Lower) When spermine was added to cells treated with DFMO, the wide scattering of each dot (observed in the cells treated with DFMO alone) disappeared.
The dysregulation of the methylation of genes observed in cells treated with DFMO seemed to be remedied.
DFMO: DL-α-difluoromethylornithine
The abnormal methylation status was reversed by an increase in polyamine concentration, by the addition of spermine via an extracellular route. Although the Not I site is not necessarily involved in the gene expression, the methylation status of 10% of the gene fragment cleaved at the Not I site was influenced by the changes in polyamine concentration (Soda et al., 2013). That is, in about 5% of the fragment, polyamine supplementation reversed the increase in demethylation induced by the decrease in polyamine concentration, while in about 5% of the fragment, polyamine supplementation reversed the increase in methylation induced by the decrease in polyamine concentration.
Because abnormal gene methylation is associated with aging-associated diseases and aging (Borghini et al., 2013; Maegawa et al., 2014; Ono et al., 1993; Ushijima & Okochi-Takada, 2005; White & Parker, 1983), an elevation in polyamine concentrations by replenishment from foods must regulate the methylation status of various genes relevant to the onset or the inhibition of aging-associated diseases. In addition to the many biological activities that help inhibit damage to cells and genes caused by harmful stimuli, such biological effects on gene methylation have contributed to the lifespan extension of mice (Soda, Dobashi et al., 2009; Soda et al., 2013).
The restriction of dietary polyamines decreases blood polyamine concentrations, whereas long-term increased polyamine intake elevates the concentrations of blood polyamines, especially spermine (B. Cipolla et al., 2003; Nishimura et al., 2001; Soda, Dobashi et al., 2009; Soda, Kano et al., 2009). Polyamines absorbed from the intestinal lumen are transported to the organs and tissue of the body (Bardocz et al., 1990; Bardocz et al., 1995). Moreover, mice fed high-polyamine chow and have increased blood concentrations of polyamines live longer than other mice (Soda, Dobashi et al., 2009; Soda et al., 2013; Soda, Kano et al., 2009). As observed in in vitro studies, the abnormal methylation status observed in aged mice fed normal chow was not observed in age-matched mice fed high-polyamine chow and exhibiting increased blood polyamine concentrations (Soda et al., 2013). Because increased polyamine intake and resultant increases in blood polyamine concentrations were found to be associated with the decreased progression of aging-associated pathologies and with lifespan extension in mice, humans (as a mammal) may also experience beneficial effects from dietary polyamines. Diseases known as lifestyle-related or aging-associated diseases such as diabetes mellitus and arteriosclerosis greatly affect the lifespan of humans. Therefore, in this review section, the food-derived polyamine-mediated inhibitory effects on aging-associated pathologies are introduced.
The administration of streptozotocin (STZ), a naturally occurring chemical that is toxic to insulin-producing beta cells of the pancreas, induces diabetes in animals. In STZ-treated rats, advanced glycation end product (AGE), triglyceride, cholesterol, and low-density lipoprotein (LDL) were increased in the blood. However, when the drinking water of mice was supplemented with spermine, these values fell gradually and the value of high-density protein (HDL) was gradually and significantly elevated compared to the control (Jafarnejad et al., 2008). Moreover, the oral administration of spermine also increased the activities of serum paraoxonase/arylesterase 1 (PON1) and lecithin cholesterol acyltransferase (LCAT) (Jafarnejad et al., 2008). PON1 conjugates circulating HDL and acts to decelerate LDL oxidization (Hine et al., 2012), an initial step in atherosclerosis development.
PON1-knockout mice showed accelerated pathological changes in blood vessels compared to wild-type mice (Shih et al., 1998). LCAT is an enzyme that catalyzes the conversion of free cholesterol to cholesterol ester mainly in HDL, and this conversion accelerates the drawing out of free cholesterol from the cell membrane surface of peripheral tissues. These findings suggest the possibility that an increase in the spermine concentration contributes to the inhibition of arteriosclerosis progression.
Polyamines are involved in the production and secretion of insulin, and insulin mRNA is stabilized by polyamines. It was reported that under a low polyamine concentration condition, the biosynthesis of proinsulin upon increased glucose levels declines (Sjöholm, 1996; Welsh, 1990). Polyamines also protect beta cells from the toxic effects of alloxan, a glucose analogue that preferentially accumulates in pancreatic beta cells. As alloxan is toxic to beta cells, the administration of alloxan provokes glucose intolerance in animals. The increase in glucose levels and triglyceride and cholesterol concentrations after alloxan administration is suppressed by polyamine administration (Mendez & Hernandez Rde, 2005). Polyamines also accelerate the reproduction of pancreatic acinar cells, which secrete digestive enzymes (Mendez & Hernandez Rde, 2005).
When polyamines are supplied from the extracellular space, the intracellular homeostasis that acts to maintain constant intracellular polyamine concentrations is activated. Spermine/spermidine-N1-acetyltransferase (SSAT), which degrades polyamines, is activated upon increases in intracellular polyamine concentrations (Casero & Pegg, 2009; Persson, 2009). The metabolic alteration induced by the activation of SSAT upon increases in polyamine concentrations is shown in Figure 7. When SSAT degrades polyamines, acetyl-CoA is required as a coenzyme. Although acetyl-CoA is generated by glycolysis or beta oxidization of the fatty acid, the activation of SSAT consumes acetyl-CoA, and the resulting increase in SSAT activities accelerate glycolysis and beta oxidization (Kee et al., 2004) (Fig. 7).

Beta oxidization and polyamines
Fatty acids are aerobically metabolized as an energy source (beta oxidization). Long chain fatty acids are converted into acyl-CoA in the cytoplasm. Because the mitochondrial inner membrane does not allow acyl-CoA alone to enter into the mitochondria, carnitine acts as a fatty acyl conveyor to transport fatty acids inside the mitochondria. CPT-1 catalyzes the temporal combination of carnitine and acyl-CoA, and fatty acyl CoA is converted into fatty acid acyl carnitine. Fatty acids undergo beta oxidization within the mitochondria and are decomposed into acetyl-CoA.
A polyamine supply from the extracellular space simulates SSAT, a polyamine-degrading enzyme. Since SSAT needs the coenzyme acetyl-CoA, SSAT activation promotes the consumption of acetyl-CoA. The consumption of acetyl-CoA is related to the reduction in malonyl-CoA content. Malonyl-CoA is the substrate for fatty acid synthesis, and the reduction restricts fatty acid synthesis. Malonyl-CoA also acts to inhibit CPT-1 activities, and a decrease in malonyl-CoA activates CPT-1. The enhanced combination of carnitine and acyl-CoA promoted by increased CPT-1 activities accelerates the beta oxidization of fatty acids.
SSAT: spermine/spermidine-N1-acetyltransferase, ACC: acetyl-CoA carboxylase, CPT-1: carnitine O-palmitoyltransferase 1, APAO: N1-acetylpolyamine oxidase
In mice overexpressing SSAT gene, acetyl-CoA in white adipose tissue is consumed and the amount of malonyl-CoA is decreased (Jell et al., 2007). Malonyl-CoA, formed by carboxylating acetyl-CoA via the enzyme acetyl-CoA carboxylase (ACC), is important for fatty acid biosynthesis. Malonyl-CoA also acts to inhibit the activity of carnitine O-palmitoyltransferase type I (CPT-1). CPT-1 catalyzes the conversion of long-chain acyl-CoA to long-chain acyl-carnitine and allows the fatty acid to be transported to mitochondria, where fatty acid oxidation and degradation occur.
A reduction in malonyl-CoA as a result of SSAT activation thus results in reduced fatty acid synthesis and increased beta oxidization of fatty acids, resulting in reduced fat accumulation. In mice overexpressing SSAT gene, the following were observed: increased oxidization of glucose and palmitic acid, reduced fat storage, increased basal metabolic rate, increased insulin sensitivity, and improved glucose tolerance (Jell et al., 2007). Moreover, in mice overexpressing SSAT, there is a decline in serum cholesterol levels, possibly due to the increase in bile acid synthesis and the inhibition of cholesterol absorption (Pirinen et al., 2010). In addition, mitochondria in adipocytes and hepatocytes increased in number, resulting in improved energy production efficiency in SSAT-overexpressing animals (Koponen et al., 2012).
In contrast, in SSAT-knockout mice, the amounts of acetyl-CoA and malonyl-CoA are increased, the oxidizations of glucose and palmitic acid are decreased, the deposition of fat to adipose tissue is increased, and insulin resistance is increased (Jell et al., 2007; Niiranen et al., 2006). That is, although there is no clear proof at present, the consumption of a high-polyamine diet may promote fat metabolism similar to that induced with exercise, etc., and it may be possible to produce an internal environment that discourages increases in body weight.
A serious problem that arises with aging is the impairment of blood flow due to thrombus formation within arteries. Thrombus formation inhibits the blood supply to tissues and thereby induces the dysfunction of organs, thereby becoming a factor in the development of aging-associated diseases. It has been reported that extracts of soybeans and fermented soybeans, which are considered healthy macrobiotics, inhibit thrombus formation and promote thrombolysis (Potter, 1998; Suzuki et al., 2003; Wilcox & Blumenthal, 1995). However, the underlying mechanisms and substances that elicit such biological activities require clarification. The inducement of inflammation promotes thrombus formation and inhibits the mechanism regulating thrombolysis. When inflammation is inhibited, thrombus formation is inhibited and thrombolysis is promoted.
The inhibition of thrombosis and acceleration of thrombolysis by extracts of soybeans and fermented soybeans may be due to the anti-inflammatory action of these polyamine-rich macrobiotics. Indeed, in mice administered polyamines, thrombus formation was suppressed and thrombolysis was augmented (de la Pena et al., 2000; Pakala, 2003).
The results of many basic and animal studies have indicated the favorable effects of dietary polyamines on human health and longevity. Epidemiological evidence indicating a positive association between a healthy diet and polyamine concentrations provides further support for the role of polyamines in human health. Many epidemiologic surveys investigating the correlation between food preferences and health have been conducted. Among the foods surveyed, legumes such as soybeans, unpolished flour, vegetables, fish, and shellfish have been noted as foods relevant to a healthy and long life. Germ and bran, legumes, vegetables, and shellfish are foods with high polyamine concentrations per calorie (B. G. Cipolla et al., 2007; Nishibori et al., 2006; Nishimura et al., 2006; Soda, 2012). The foods that have a close relationship with an increased incidence of aging-associated diseases include Western desserts made from butter, cream, milk, egg, sugar, and animal fats that contain little polyamines (B. G. Cipolla et al., 2007; Nishibori et al., 2006; Nishimura et al., 2006; Soda, 2012).
To examine the relationship between polyamine content and dietary pattern, the food supply database of 49 western countries of the Food and Agriculture Organization (FAO) of the United Nations was employed. The relationship between the calories of various foods supplied relative to the total calories of supplied foods and the relationship between the amounts of polyamines contained in the supplied foods relative to the total calories in the supplied foods were examined. The study was an ecological investigation, and the data used do not indicate the amount of foods actually consumed; however, as the food supply likely reflects the food demand, the analysis using relative amounts may reflect the food preferences of the people in each country. The study results indicated that the Mediterranean diet preferred in Mediterranean countries is associated with increased polyamine concentrations (Binh et al., 2011; Soda et al., 2012). Notably, although olive oil and wine, two components of the Mediterranean diet, contain no polyamines, people who prefer these ingredients also prefer foods rich in polyamines per calorie (Binh et al., 2011). In contrast, people who prefer animal fats to olive oil and those who prefer spirits and beer to wine prefer foods with low polyamine concentrations (Binh et al., 2011).
In addition, people who prefer cheese (the increased consumption of which is sometimes related to a healthy and long lifespan) also prefer foods rich in polyamines, while people who prefer milk (the increased consumption of which is associated with an increased incidence of aging-associated pathologies such as atherosclerotic diseases) prefer foods low in polyamines (Binh et al., 2011). Moreover, traditional Japanese meals, which comprise a healthy macrobiotic diet, generally consist of many foods with high polyamine concentrations (Binh et al., 2010).
The polyamine concentration in a food may differ depending on the component of the food examined. For example, although the flesh of fishes and shellfish is not as rich in polyamines as beans and vegetables, high levels are found in the internal organs and roe of fish/shellfish. The traditional Japanese diet consists of legumes such as soybeans and azuki beans, small fish with viscera and roe, and shellfish, e.g., fishes and shellfish boiled or stewed in soy sauce and processed roe products made from herring, salmon and cod roes.
It is necessary to identify all genes related to aging-associated diseases, and to determine which genes with abnormal methylation are associated with aging and can be inhibited by polyamines.