2015 Volume 21 Issue 2 Pages 263-269
2015 Volume 21 Issue 2 Pages 263-269
The aim of this study was to analyze four different acidifying strains of Lactobacillus delbrueckii subsp. bulgaricus and their influence on the quality of yoghurt. The results showed L. bulgaricus IM1 and L. bulgaricus IM2 were fast acidifying strains, and L. bulgaricus IM3 and L. bulgaricus IM4 were slow acidifying strains. When the four strains were mixed with Streptococcus thermophilus TA040, the fermentation time of the yoghurt starter culture LS1 (IM1 and TA040) and LS2 (IM2 and TA040) was shorter than that of the yoghurt starter culture LS3 (IM3 and TA040) and LS4 (IM4 and TA040). However, yoghurt samples fermented by LS3 and LS4 had weaker post-acidification, higher viscosity and more exopolysaccharide (EPS). In addition, the highest acetaldehyde and diacetyl contents were observed in yoghurt fermented by the slowest acidifying yoghurt starter culture (LS4). Therefore, selecting slow acidifying L. bulgaricus strain is a considerate design to integrate advantageous performance of strains.
Yoghurt is typically produced using mixed starter cultures comprised of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus and has become a popular fermented dairy product. The increasing consumer demand for yoghurt with favorable flavour and stable texture during shelf life led to considerable interest in researching and exploiting yoghurt starters (Skriver et al., 2003). The development of starter cultures for fermented milk relies on the combination of different lactic acid bacteria (LAB) having superior fermentation performance (Urshev et al., 2006).
The most important fermentation performances of yoghurt starter culture are rapid acidification, texturing capacities, specific flavour compounds, weak post-acidification and health benefits (Ongol et al., 2007; Ruas-Madiedo et al., 2002). Currently, excellent S. thermophilus strains are carefully selected, and their advantageous effects on acidification activity, texture properties and flavour compound of yoghurt have been largely reported. For example, exopolysaccharide (EPS) produced by Streptococcus thermophilus is able to replace chemical stabilizers or milk fat to give considerable rheological effects, mouthfeel, and creaminess to commercial yoghurt (Guzel-Seydim et al., 2005; Purwandari et al., 2007; Robitaille et al., 2009; Wu et al., 2014). L. bulgaricus is also important in producing acid, forming flavour and granting healthy functions (Settachaimongkon et al., 2014; Soomro and Masud, 2012). Some researchers have devoted to investigating the fermentation performance of L. bulgaricus and selecting L. bulgaricus having superior features. Makino et al. (2006) found that the polysaccharides produced by L. bulgaricus had immunomodulatory effects on the human body. Mende et al. (2012) investigated the nutritional requirements for growth and EPS production of L. bulgaricus.
Weak post-acidification property has recently been considered as one of most important factors for yoghurt starter selection because of inadequate chilled-chain distribution especially in developing countries (Han et al., 2014). Ongol et al. (2007) found that the lactic acid was mainly produce by L. bulgaricus during the chilled storage and yoghurt fermented using mutational L. bulgaricus with reduced proton-translocating ATPase (H+-ATPase) activity had markedly reduced post-acidification. Wang et al. (2013) found that the parent L. bulgaricus strain grew and acidified the milk faster than variant strains whose H+-ATPase activity reduced. The result indicated that the acidification ablity of L. bulgaricus was associated with H+-ATPase activity and screening slow acidifying strain might be one of effective approaches of inhibiting yoghurt post-acidification. However, the effect of slow acidifying L. bulgaricus strain on the overall quality of the final yoghurt product was rarely reported.
In this study, four different acidifying L. bulgaricus strains were isolated from Inner Mongolia homemade cheese and co-cultured with S. thermophilus TA040. The influence of four different mixed starter cultures on acidifying rate, post-acidification, viscosity, EPS production and flavour compounds were also investigated.
Microorganisms and culture conditions Four L. bulgaricus strains were isolated and identified from four different kinds of homemade cheese which were made in Horqin, Hohhot, Baotou and Xilingol, Inner Mongolia, China. They were named L. bulgaricus IM1, L. bulgaricus IM2, L. bulgaricus IM3 and L. bulgaricus IM4, respectively. S. thermophilus TA040 (Ziar et al., 2014) was obtained from Danisco (Copenhagen, Denmark). S. thermophilus strain was grown using M17 (Oxoid, Basingstoke, England) supplemented with 0.5% (w/v) lactose (LM17) in Mir-253 (Sanyo, Osaka, Japan) at 42°C overnight. L. bulgaricus strains were grown using MRS (Oxoid, Basingstoke, England) under anaerobic conditions (2.5 L anaerobic jars using AnaeroGEN™ sachets, Oxoid, Basingstoke, England) at 42°C for 18 – 24 h. Before incubating in milk, L. bulgaricus and S. thermophilus were washed with sterile saline to remove residue medium and prepared cell suspensions (OD560nm = 0.95).
Preparation of fermented milk Commercial skim milk powder (protein, 33.4%; fat, 0.8%; Fonterra Ltd, Auckland, New Zealand) was reconstituted in distilled water (reconstituted skim milk, RSM; 12%, w/w) and sterilized at 90 – 95°C for 5 – 10 min. The four L. bulgaricus strains were mixed with S. thermophilus TA040 to prepare two-component yoghurt starters (LS1: IM1 and TA040; LS2: IM2 and TA040; LS3: IM3 and TA040; LS4: IM4 and TA040) according to the ratio 1:1 of S. thermophilus to L. bulgaricus strain. Four L. bulgaricus strains and four yoghurt starter cultures were inoculated in the RSM using cell suspensions at an inoculum of 2% (v/v). The yoghurt samples fermented by starter cultures LS1, LS2, LS3 and LS4 were numbered sample 1, sample 2, sample 3 and sample 4, respectively.
Acidification property Acidification property was measured according to Soomro and Masud (2008) with slight modifications. Four L. bulgaricus strains were respectively incubated in 12% RSM at 42°C for 6 h, and then titratable acidity was determined.
Acidification-graph For Acidification-graph, the France Alliance Cinace acidification monitoring system (Alliance Instruments, Mery-Sur-Oise, France) was used. The pH was automatically recorded every 5 minutes during milk fermentation. The corresponding sample was removed to terminate fermentation until a pH of 4.5 (the pH end point).
Post-acidification When the pH of fermented milk samples reached 4.5, they were stirred by RW20 (IKA, Staufenim, Germany) at 500 rpm for 5 min and stored at 4°C. Titratable acidity of fermented milk sample was determined at 1 day and 14 days cold storage.
Enumeration of LAB Standard plate count was used to enumerate viable counts of LAB at 1 day and 14 days cold storage according to Settachaimongkon et al. (2014). Viable counts of S. thermophilus was determined using LM17 agar under aerobic incubation at 42°C for 24 h. Viable counts of L. bulgaricus was determined using MRS agar under anaerobic incubation at 42°C for 24 h.
Viscosity The viscosity of fermented milk sample was determined at 1 day and 14 days cold storage. It was measured at 20°C using proRheo R180 viscometer (proRheo, Althengstett, Germany) with a spindle No. 2 at 64 rpm.
Isolation and quantification of EPS The isolation of EPS from fermented milk sample was performed according to Prasanna et al. (2012) with slight modifications. The fermented milk sample was mixed with 12% (v/v) of 80% (w/v) trichloracetic acid and stored at 4°C for 24 h to precipitate proteins. The supernatant was mixed with two volumes of absolute ethanol, and then stored at 4°C for 24 h to precipitate EPS. The EPS was obtained by centrifugation at 25000 × g for 30 min at 4°C. The quantification of EPS was determined by the phenol-sulfuric acid assay (Robitaille et al., 2009).
Volatile aroma compounds Acetaldehyde and diacetyl were measured by using AC4000 gas chromatograph-mass spectrometer (Varian Inc., Walnut Creek, CA, USA) according to gas chromatography mass spectrometry analysis combined with the method of standard additions (Condurso et al., 2008).
Statistical analysis All data were reported as the mean or mean ± standard deviation. Physicochemical experiments were carried out in triplicate. Statistical analysis was performed using Statistica 9.2 software (StatSoft, Inc., Tulsa, OK, USA). Comparisons of multiple groups were analyzed by the Tukey test. A p value of less than 0.05 was considered statistically significant.
Characteristics of four L. bulgaricus strains The characteristics of four L. bulgaricus strains are listed in Table 1. When pH reached 4.5, the fermentation time of four strains was recorded by France Alliance Cinace acidification monitoring system. They are shown in the Table 1. In addition, the titratable acidity values of four samples fermented by four strains after 6 h are also shown in the Table 1. According to Raquib et al. (2003) the strains are classified on the basis of their acid production capabilities as low (Titratable acidity < 0.5), moderate (0.5 < titratable acidity < 0.6) and fast (titratable acidity > 0.6) to facilitate the selection, so L. bulgaricus IM1 and L. bulgaricus IM2 were termed as fast acidifying strains and L. bulgaricus IM3 and L. bulgaricus IM4 were termed as slow acidifying strains. The samples fermented by L. bulgaricus IM1 and L. bulgaricus IM2, stored at 4°C, had higher titratable acidity (Table 1, p < 0.05). The result indicated that slow acidifying L. bulgaricus would reduce post-acidification, which was in agreement with the result of Wang et al. (2013).
| Characteristics | L. bulgaricus IM 1 | L. bulgaricus IM 2 | L. bulgaricus IM 3 | L. bulgaricus IM 4 |
|---|---|---|---|---|
| Titratable acidity | 0.80 ± 0.02a | 0.71 ± 0.01b | 0.48 ± 0.03c | 0.40 ± 0.05c |
| Fermentation time (min) | 345 ± 10a | 430 ± 15b | 670 ± 10c | 780 ± 15d |
| Δ Titratable acidity | 0.37 ± 0.04a | 0.32 ± 0.03a | 0.25 ± 0.04b | 0.22 ± 0.02b |
| EPS content (mg L−1) | 37.2 ± 1.1a | 38.9 ± 0.7a | 40.7 ± 0.9b | 46.4 ± 0.5c |
| Viscosity value (Pa·S) | 0.101 ± 0.005a | 0.111 ± 0.006a | 0.124 ± 0.008b | 0.158 ± 0.005c |
| Acetaldehyde content (ppm) | 6.81 ± 0.04a | 7.35 ± 0.03b | 8.29 ± 0.06c | 8.66 ± 0.07d |
| Diacetyl content (ppm) | 2.81 ± 0.03a | 3.01 ± 0.02b | 3.22 ± 0.03c | 3.86 ± 0.05d |
(1) Data are the mean ± standard deviation of three samples each analysed in triplicate. Different superscript in the same line indicates differences between mean values at p < 0.05.
(2) Δ Titratable acidity was the change of titratable acidity after 14 days cold storage.
EPS appear as filaments associated with bacterial cells and the casein network, which can improve the texture properties of yoghurt (Laws and Marshall, 2001). As shown in Table 1, EPS productions of four strains were low, and the difference of their EPS production were significant (p < 0.05). The EPS production of L. bulgaricus IM4 was highest.
As seen in Table 1, there was an increase in the viscosity of fermented milk sample when the EPS production of single strain increased. The viscosity value of fermented milk fermented by L. bulgaricus IM4 was highest, followed by that of L. bulgaricus IM3, L. bulgaricus IM2 and L. bulgaricus IM1 (p < 0.05). The increase of viscosity was most likely due to the interactions between EPS and protein network. It confirmed that EPS was beneficial to enhance the viscosity values of fermented milk.
Acetaldehyde and diacetyl are recognized as major flavor compounds of yoghurt. The highest acetaldehyde and diacetyl content were produced by L. bulgaricus IM4 (p < 0.05).
Acidification activity of yoghurt starter The acidifying rate is a key parameter in modern yoghurt production (Skriver et al., 2003). The acidification graphs of yoghurt samples are shown in Figure 1. When the pH reached 4.5, the fermentation time of four yoghurt samples was 270, 350, 430 and 515 min, respectively. The acidification activity of yoghurt starter LS1 was highest, followed by that of yoghurt starter LS2, LS3 and LS4 (p < 0.05). In addition, the fermentation time was significantly shorter than that of sample fermented by corresponding single strain (p < 0.05). The results indicated that the acidification activities of four yoghurt starter cultures (LS1, LS2, LS3 and LS4) were superior to that of corresponding single L. bulgaricus strain, which is in agreement with the result of Routray and Mishra (2011). The increase of fermentation rate was likely due to synergistic effects between S. thermophilus and L. bulgaricus by exchanging several metabolites (Sieuwerts et al., 2008).

The acidification graphs of four yoghurt samples
Post-acidification performance of yoghurt starter Yoghurt was subjected to the acidity increase during storage at 4°C, which was called post-acidification and explained by the persistent metabolic activity of LAB during cooling and storage (Ongol et al., 2007). The titratable acidity of yoghurt sample during storage is illustrated in Figure 2.

The post-acidification of four yoghurt samples:  the first day;
the first day;  after 14 days at 4°C. Different lowercase letters denote significant differences (p < 0.05).
after 14 days at 4°C. Different lowercase letters denote significant differences (p < 0.05).
The titratable acidity values of all samples increased significantly after 14 days stored at 4°C (p < 0.05). The titratable acidity of sample 4 increased most slowly, followed by that of sample 3, sample 2 and sample 1. The result indicated that using yoghurt starter comprised of slow acidifying L. bulgaricus strain might reduce yoghurt post-acidification during chilled storage. Slow acidifying L. bulgaricus strain displayed weak post-acidification. Thus, selecting slow acidifying L. bulgaricus strain might be one of effective and simple avenues of reducing yoghurt post-acidification during chilled storage.
The viable counts of LAB The viable counts of S. thermophilus and L. bulgaricus are shown in Table 2. The samples fermented by LS3 and LS4 had more viable cells of S. thermophilus than the samples fermented by LS1 and LS2 (p < 0.05). The results indicated that slow acidifying L. bulgaricus give S. thermophilus more time to grow. It was also possible that there was better mutualistic metabiosis between S. thermophilus and slow acidifying L. bulgaricus. However, the viable counts of L. bulgaricus were not significantly different at the same storage time for the four samples (p > 0.05). After 14 days stored in 4°C, the viable counts of LAB decreased significantly (p < 0.05). This may be accounted for acid and hydrogen peroxide accumulation.
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
|---|---|---|---|---|
| Viable counts of L. bulgaricus (log CFU/mL) | ||||
| 1 day | 8.51a | 8.57a | 8.61a | 8.62a |
| 14 days | 7.72b | 7.77b | 7.84b | 7.89b |
| Viable counts of S. thermophilus (log CFU/mL) | ||||
| 1 day | 9.06c | 9.05c | 9.12d | 9.15d |
| 14 days | 8.16e | 8.12e | 8.23f | 8.26f |
(1) Different superscript lowercase letters denote significant differences (p < 0.05).
Effect of yoghurt starter on EPS content and viscosity value of yoghurt Texture of yoghurt is the result of both acid aggregation of casein micelles and production of EPS, which is very important for the quality of the product (Beal et al., 1999). The EPS contents and viscosity values of four yoghurt samples are shown in Figure 3. EPS productions of LS3 and LS4 were higher than that of LS1 and LS2 (p < 0.05). Both L. bulgaricus and S. thermophilus produce EPS, but S. thermophilus is regarded as the main EPS producer generally. The sample 3 and sample 4 had higher viable counts of S. thermophilus than sample 1 and sample 2, which may account for the higher EPS production. In addition, when slow acidifying L. bulgaricus strain was co-cultured with S. thermophilus, it would give S. thermophilus more time to produce EPS.

The EPS contents and viscosity values of four yoghurt samples: (1) the EPS contents of four samples; (2) the viscosity values of four samples;  the first day;
the first day;  after 14 days at 4°C. Different lowercase letters denote significant differences (p < 0.05).
after 14 days at 4°C. Different lowercase letters denote significant differences (p < 0.05).
The physico-chemical properties of EPS determine their viscosifying efficiency (Ruas-Madiedo et al., 2002). As seen in Figure 3, sample 3 and sample 4 fermented by LS3 and LS4 had higher viscosity than other samples (p < 0.05). The highest EPS production corresponded to the highest viscosity and might be related to slow acidification. EPS appears as filaments associated with bacterial cells and the casein network, which is benefit to increase viscosity of yoghurt and avoid gel fracture and syneresis (Tsuda and Miyamoto, 2010). Prasanna et al. (2013) found that porous structure could be formed in yoghurt fermented by EPS-producing LAB. This porous structure could increase the water holding capacity of the gel structure leading to the lower syneresis and resist mechanical treatments without destroying its structure, which is important during mechanical handing in yoghurt production. In addition, viscosity values of sample 3 and sample 4 increased significantly after 14 days stored at 4°C (p < 0.05). This was most likely due to protein rearrangement where more protein-protein contacts were being established during storage (Prasanna et al., 2013).
Effect of yoghurt starter on volatile aroma compounds of yoghurt The major volatile compounds of yoghurt commonly reported are carbonyl compounds such as acetaldehyde, diacetyl, acetone and acetoin. Acetaldehyde is considered as the most prominent compound for the typical yoghurt aroma. L. bulgaricus was generally considered the main acetaldehyde producer (Pourahmad and Assadi, 2005). Diacetyl was another key compound that imparted the yoghurt flavour (Condurso et al., 2008). As seen in Figure 4, the highest acetaldehyde and diacetyl contents were observed in yoghurt sample 4 which was fermented by the slowest acidifying yoghurt starter culture (LS4). This result indicated that acetaldehyde and diacetyl contents were related with the fermentation time. The longer the fermented time was, the higher acetaldehyde and diacetyl contents of yoghurt were. In addition, the viability of S. thermophilus in the sample 4 was highest, which was beneficial to increase the metabolites. It may exert a double positive effect on the production of flavour compounds, on the one hand stimulating L. bulgaricus to produce more acetaldehyde, and on the other hand producing more diacetyl. Acetaldehyde can be formed by decomposition of threonine by the action of threonine aldolase (Wouters et al., 2002). When the acetaldehyde content of yoghurt was higher than 8.0 ppm, the favorable flavour would be generated (Routray and Mishra, 2011). Diacetyl was the product of citrate metabolism and had long been recognized as imparting a distinctive buttery or butterscotch note in fermented milk (Mahajan et al., 2004). However, acetaldehyde and diacetyl contents decreased significantly during storage (p < 0.05). Some researchers have indicated that alcohol dehydrogenase produced by yoghurt cultures converts acetaldehyde to ethanol during storage (Guzel-Seydim et al., 2005).
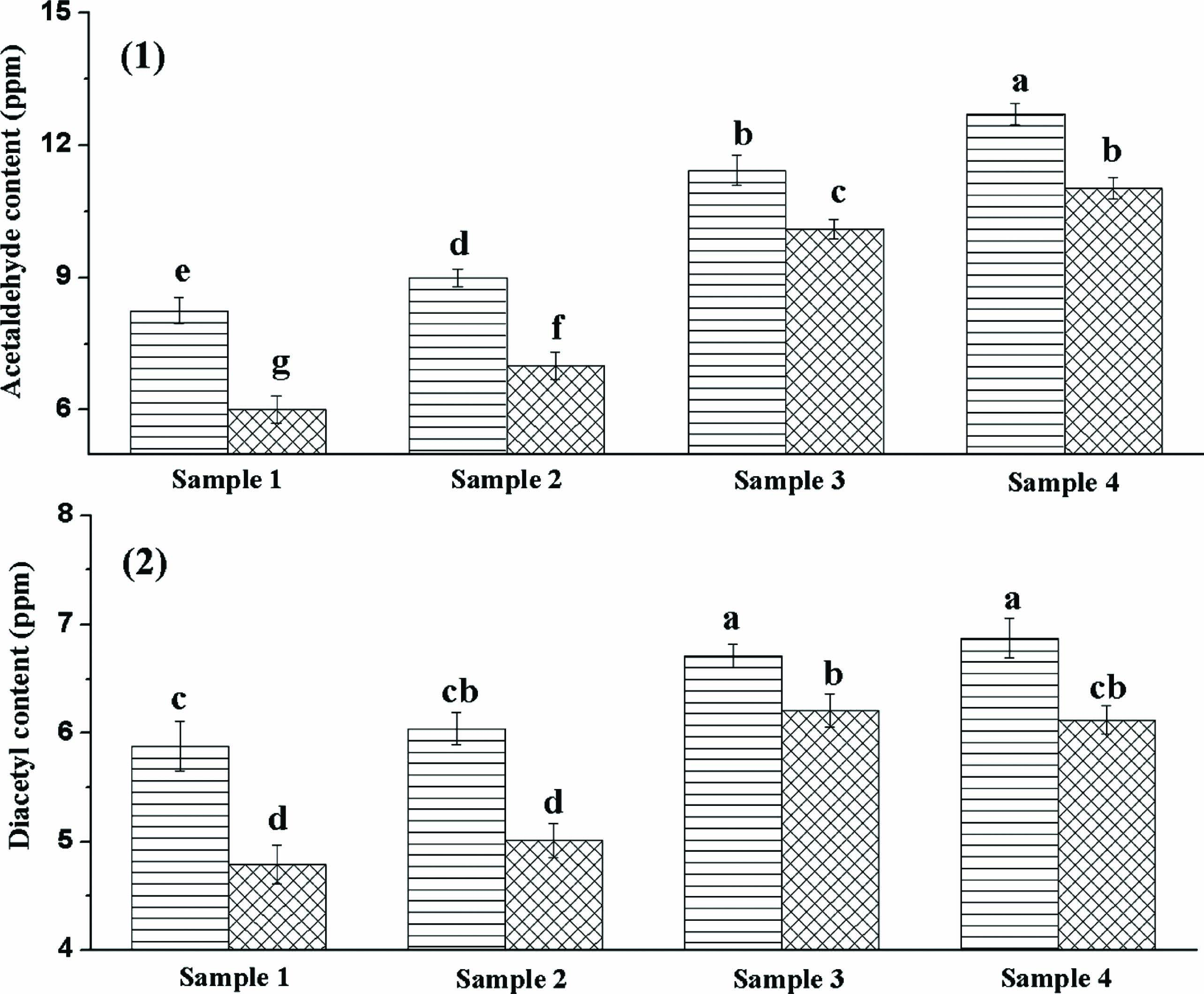
The acetaldehyde and diacetyl contents of four yoghurt samples: (1) the acetaldehyde contents of four samples; (2) the diacetyl contents of four samples;  the first day;
the first day;  after 14 days at 4°C. Different lowercase letters denote significant differences (p < 0.05).
after 14 days at 4°C. Different lowercase letters denote significant differences (p < 0.05).
Four different acidifying strains of L. bulgaricus were co-cultured with S. thermophilus TA040, and some important performances of strains were studied. The fermentation time of the yoghurt starter cultures (LS1 and LS2) containing fast acidifying L. bulgaricus strains was shorter than that of the yoghurt starter cultures (LS3 and LS4) containing slow acidifying L. bulgaricus strains. However, post-acidification of LS1 and LS2 was more serious than that of LS3 and LS4. Moreover, the yoghurt samples fermented by LS3 and LS4 had higher viscosity and EPS production. In addition, the highest acetaldehyde and diacetyl contents were observed in yoghurt sample that was fermented by the slowest acidifying yoghurt starter culture (LS4).
Acknowledgements This work was supported by National High Technology Research and Development Program of China (2011AA100901), and the National Key Technologies Program of China (2013BAD18B01) during the 12th Five-Year Plan Period.