2015 Volume 21 Issue 3 Pages 439-444
2015 Volume 21 Issue 3 Pages 439-444
Soymilk was prepared by ohmic heating. Heating time decreased as the applied voltage was increased. Precipitation, viscosity and surface hydrophobicity of protein in the soymilk were increased with the decrease in voltage applied. Voltages of 50 and 100 V resulted in a higher heating rate and consequent formation of a larger amount of protein particles (soluble aggregates), ranging from 40 to 120 nm in size, than the other conditions. Furthermore, soymilk prepared by ohmic heating at 50 or 100 V was converted into firmer tofu curd than that at 30 V. Ohmic heating at 30 V was thought to form larger protein particles than that at 50 and 100 V, and the resultant larger sized aggregates are considered to result in inferior tofu curd texture.
Soymilk, which consists of protein suspension, lipid emulsion and other soluble constituents, is an important soybean product. Not only is it a nutritious beverage, processed soymilk is also used to produce tofu (soybean curd), yuba (soymilk skin), and aburaage (fried soybean curd) (Liu, 1997). Heating is essential in the production of soymilk because of the firm consistency of raw soybean, the low digestibility of native constituents such as globulin proteins, and the large number of bioactive constituents.
Many studies concerning the heating of raw soymilk or raw soy slurry (“namago” in Japanese) have been performed. Raw soymilk consists of an emulsion of lipid droplets covered with globulin proteins (mainly glycinin), most of which are liberated to form protein particles and soluble protein on heating (Ono et al., 1991; Guo et al., 1997). Soybean globulin protein is mainly divided into glycinin and β-conglycinin. Glycinin is a hexamer protein consisting of five different subunits with a total molecular mass of 360 kDa. Each of these glycinin subunits consists of acidic and basic polypeptides, linked by disulfide bonds (Moreira et al., 1979; Staswick et al., 1981). On the other hand, β-conglycinin is a trimer protein made up of a combination of α-, α′- and β-subunits with a total molecular mass of 180 kDa (Thanh and Shibasaki, 1976; 1978). Glycinin and β-conglycinin are denatured at different temperatures: 90 and 70°C, respectively (Shiga et al., 1988; Zhang et al., 2004; Liu et al., 2004). Previous reports noted the differences between glycinin and β-conglycinin, and the effects of two-step heating on tofu making (Wang et al., 2007) and the dispersion stability of soymilk (Shimoyamada et al., 2008).
In our previous reports, the effects of heating conditions on soymilk quality and gelation (Shimoyamada et al., 2000; 2008; 2010) were examined using a boiling water bath and a temperature-controlled water bath. However, the resulting data were insufficient to allow application in a pilot plant, where soymilk is heated by steam injection (unpublished data). Thus, data obtained in laboratories are not universally applicable, even in pilot-scale plants. Thus, further investigation of rapid and precisely controlled methods of heating soymilk are necessary, even when the use of steam is not possible.
In the past, ohmic heating has been applied to various food heating operations. Ohmic heating, also known as Joule heating or electrical resistance heating, is performed by passing an alternating electric current through the food material. Heat is internally generated within a material due to resistance against the applied electrical current (Bengtson et al., 2006; Singh and Heldman, 2009). Few reports have documented the ohmic heating of soymilk. Two-step ohmic heating of raw soymilk has been shown to produce firm tofu curd after mixing with glucono-δ-lactone (GDL) (Liu et al., 2004; Wang et al., 2007); however, there has been no discussion of the heating principles involved. Shen et al. (2009) also used ohmic heating to prepare yuba, addressing electricity consumption but not the effects or the exact conditions.
The aim of this study, therefore, was to characterize soymilk prepared by ohmic heating and assess the effect of voltage and heating rate on the resulting soymilk quality. For this purpose, we created a new apparatus specifically designed for the preparation of ohmically heated soymilk.
Preparation of raw soymilk Soybeans (Glycine max L. var. Miyagishirome, 25 g), purchased at a local market and stored at 4°C until used, were imbibed overnight at 4°C. Imbibed seeds (total weight: 50 g) were milled with 175 mL of water for 8 min at 10,000 rpm in a High Flex Homogenizer (HF93; SMT Co., Tokyo, Japan) and then immediately filtered using a 5-ply polypropylene mesh to provide the raw soymilk.
Heat treatment of raw soymilk Ohmic heating of the raw soymilk was performed as follows: 140 mL of raw soymilk was placed in a polypropylene vessel (52 × 53 × 92 mm, inner dimensions), which was then wrapped in polystyrene foam. The top of the vessel was covered with silicone rubber (55 × 50 × 5 mm) and a pair of titanium electrodes were inserted into the soymilk such that the contact area was 50 × 50 mm and the distance between electrodes was maintained at 50 mm. During heating, the sample was stirred with a magnetic stirrer. A compact alternating current power supply (PCR500M; Kikusui Electronic Co., Yokohama, Japan) was employed, with variable frequencies and voltages applied to the sample. The temperature of the sample soymilk was monitored using a thermocouple (type T) with a data recorder (LR4210; Yokogawa Electric Co., Tokyo, Japan). Upon reaching 95°C, samples were immediately cooled in an ice-water bath. The heated soymilk was then centrifuged at 1,500 × g for 10 min to remove the fine insoluble fraction from the sample (Shimoyamada et al., 2010). For reference, aliquots of the raw soymilk (20 mL each) were poured into a screw-capped plastic tube (50 mL volume) and separately heated in a boiling water bath for 15 min.
Soymilk viscosity Viscosity of the ohmically heated soymilk was measured using a sine-wave vibro viscometer (SV-10; A&D Co. Ltd., Tokyo, Japan). Ten milliliters of sample after centrifugation (1,500 × g, 10 min) was placed in a small sample container, which was then inserted into the apparatus at room temperature.
Protein surface hydrophobicity Surface hydrophobicity of the soymilk protein was measured using 8-anilino-1-naphtalene sulfonic acid (ANS) (Hayakawa and Nakai, 1985). An aliquot (4 mL) of the sample, previously diluted with 0.01 M phosphate buffer (pH 7.0), was mixed with 20 µL of 8.0 × 10−3 M ANS and allowed to stand at room temperature for 20 min. The resulting fluorescence (excitation: 390 nm; emission: 470 nm) was measured using a fluorescence spectrophotometer (F-2500; Hitachi High-Technologies Co., Tokyo, Japan). Fluorescence was divided by the protein content, determined according to Lowry et al. (1951), to give the relative hydrophobicity.
Ultracentrifugation The heated soymilk samples, minus the insoluble fraction previously removed by centrifugation, were centrifuged at 152,000 × g for 30 min using an ultracentrifuge (Optima max; Beckman Coulter, Inc., Brea, CA, USA). The protein content of the supernatant was then determined according to Lowry et al. (1951) to determine the amount of protein aggregates, which were reported as protein particles in previous papers (Ono et al., 1991; Guo et al., 1997; Ono, 2008).
Particle size distribution Particle size distribution of the soymilk was analyzed using a laser diffraction particle size analyzer (LS13 320; Beckman Coulter, Inc., Miami, FL, USA). The refractive indexes used were 1.6 and 1.33 for the sample and solvent (water), respectively. Measurements ranged from 0.04 to 2000 µm, and no signals were observed between 10 to 2000 µm in any of the samples.
Preparation of tofu curd from ohmically heated soymilk Raw soymilk for tofu curd formation was prepared using one part whole soybean and 6 parts water, by weight. Imbibed soybean was milled with the remaining volume of water and filtered using a 5-ply polypropylene mesh to provide raw soymilk. The soymilk was then ohmically heated and cooled in an ice-water bath. The cooled soymilk was placed in a cooled beaker, mixed with GDL to a final concentration of 0.3%, placed in a plastic tube (23 mm i.d.) and heated in a water bath at 80°C for 60 min. The resulting tofu was allowed to stand in a refrigerator overnight. Stress-strain curves of the resulting tofu curd, after warming to room temperature, were measured using a rheometer (RE-3305; Yamaden Co., Ltd., Tokyo, Japan).
Statistical analysis Data were subjected to a one-way analysis of variance (one-way ANOVA). Significant differences among mean values were determined using Bonferroni's multiple comparison test (p < 0.05). All analyses were carried out using EXCEL Statics version 7.0 (ESUMI Co., Ltd, Tokyo, Japan).
Ohmic heating of soymilk The prepared raw soymilk was placed in a plastic vessel and a pair of titanium electrodes were inserted into the soymilk, then an alternating voltage (frequency; 50 Hz) was applied between them. The temperature of the internally heated soymilk via ohmic heating was monitored (Fig. 1). As for the reference, the temperature of the soymilk heated in boiling water increased rapidly at first but soon the rate of increase declined. Initially, the rate was almost equivalent to that of ohmic heating at 100 V; however, when the temperature reached >80°C the rate slowed to less than that at 30 V. In soymilk subjected to alternating voltages of 30, 50, 70 and 100 V, the temperature increased almost linearly to 95°C, with the plot of temperature versus time being only slightly concave. Overall, the average heating rate increased with increasing voltage. The heating rate was much slower at 30 V than at the other voltages tested or with boiling water. On the other hand, the use of 50 V resulted in a heating rate similar to that of boiling water heating, although there was a difference in the volume of the soymilk (boiling water: 20 mL; ohmic heating: 140 mL). A voltage of 100 V gave a much more rapid heating rate; the soymilk reached 95°C after only 2.4 min of alternating voltage application.

Effect of alternating voltage on changes in the temperature of soymilk subjected to ohmic heating.BW, boiling water heating (solid line); 30 V, ohmic heating at 30 V (dotted line); 50 V, ohmic heating at 50 V (dashed line); 70 V, ohmic heating at 70 V (bold line); 100 V, ohmic heating at 100 V (dotted and dashed line).
There were few differences among the tested frequencies of 50 to 500 Hz; the upper limit was 500 Hz because of limitations of the apparatus used in this study (data not shown). Overall, at frequencies of <500 Hz, ohmic heating had an equivalent potency to electrical heating of soymilk. Although problems such as corrosion and dissolution of the electrodes were experienced, a frequency of 50 Hz, which is the industrial frequency, was used to analyze the effect of ohmic heating on the soymilk.
Characterization of soymilk prepared by ohmic heating Soymilk prepared by ohmic heating had a similar appearance to soymilk heated in boiling water. In a previous study (Shimoyamada et al., 2010), the amount of precipitation in soymilk obtained by centrifugation at 1,500 × g changed depending on the heating temperature and milling conditions. The increase in precipitation was thought to be partly related to the formation of insoluble aggregates, which were derived from the heating of proteins. Accordingly, the ohmically heated soymilk was also subjected to centrifugation to determine the amount of precipitation, and the findings were compared among the various voltages. The amount of precipitation in the ohmically heated sample was significantly increased with the decrease in applied voltage (Fig. 2). Slower heating appeared to accelerate the precipitate formation. Precipitation of the soymilk heated by boiling water was equivalent to that ohmically heated at 50 V, where the heating rate was similar to that observed with boiling water heating.

Precipitation of soymilk prepared by ohmic heating. Raw, raw soymilk (unheated); BW, boiling water heating; 100 V, 50 V, 30 V, applied voltages in ohmic heating.
Different letters are significantly different (P < 0.05).
The viscosity of the soymilk prepared by ohmic heating at the various voltages is shown in Fig. 3. The values were found to be comparable with those in previous reports (kinetic viscosity: 2.80 × 10−6 m2/s (Pathomrungsiyounggul et al., 2007) and 3.56 mPa·s (Toda et al., 2007)). On the other hand, they were considerably smaller than those reported by Liu et al. (2004), who employed a more concentrated soymilk. Soymilk viscosity significantly increased with a decrease in voltage, which supports the precipitation result. The viscosity at a voltage of 50 V appeared to be almost equivalent to that with boiling water heating. Liu et al. (2004) reported that the viscosity of soymilk prepared by two-step heating (75 and 95°C) was higher than that after one-step heating at 95°C. In this study, ohmic heating at 30 V resulted in a much lower heating rate (Fig. 1), suggesting that the temperature of soymilk increased slowly from about 70 to 80°C, at which point β-conglycinin but not glycinin was selectively denatured. The slower heating rate at 30 V may act similarly to two-step heating, increasing the viscosity to higher levels than observed at 100 and 50 V. The soymilk ohmically heated at 30 V also showed high precipitation (Fig. 2); therefore, viscosity is expected to correlate with precipitate formation.

Viscosity of soymilk prepared by ohmic heating.
Raw, raw soymilk (unheated); BW, boiling water heating; 100 V, 50 V, 30 V, applied voltages in ohmic heating.
Different letters are significantly different (P < 0.05).
The protein denaturation rate was estimated by surface hydrophobicity using ANS fluorescence intensity. Surface hydrophobicity of soymilk heated in boiling water was equivalent to the sample ohmically heated at 50 V, which concurs with the precipitation and viscosity results. These two samples were heated at similar heating rates, but with different volumes and by different heating principles. These data suggest that the heating rate has an important effect on the quality of soymilk. Soymilk hydrophobicity was also increased when heated at a lower voltage, where the heating rate was slow (Fig. 4). It has been previously reported that the temperature of the hot water bath and two-step heating clearly affected the surface hydrophobicity of heated soymilk protein (Shimoyamada et al., 2008). Thus, a low heating rate or long heating time is considered to accelerate exposure of the hydrophobic region during thermal treatment of soymilk proteins. However, the surface hydrophobicity of proteins may be affected by aggregate formation as described below, and for this reason, a more precise estimation of protein denaturation may be required in the future.

Surface hydrophobicity of the protein fraction in soymilk prepared by ohmic heating.
Raw, raw soymilk (unheated); BW, boiling water heating; 100 V, 50 V, 30 V, applied voltages in ohmic heating.
Different letters are significantly different (P < 0.05).
Protein aggregate formation in soymilk prepared by ohmic heating Size distributions of the protein suspension and oil droplets (emulsion) in the ohmically heated soymilk were also determined. Soymilk is known to contain soluble proteins, protein particles (diameter: 40 – 100 nm; Ono et al., 1991; Guo et al., 1997), and emulsion droplets (diameter: ca. 300 nm), which consist of lipid surrounded by a specific protein (oleosin) (Tzen et al., 1990). Soymilk heated in a boiling water bath showed an almost unimodal distribution ranging from 80 to 800 nm (Fig. 5). The distribution was assigned to oil droplets, that is, the oil body-like particles (Ono, 2008; Toda et al., 2007).
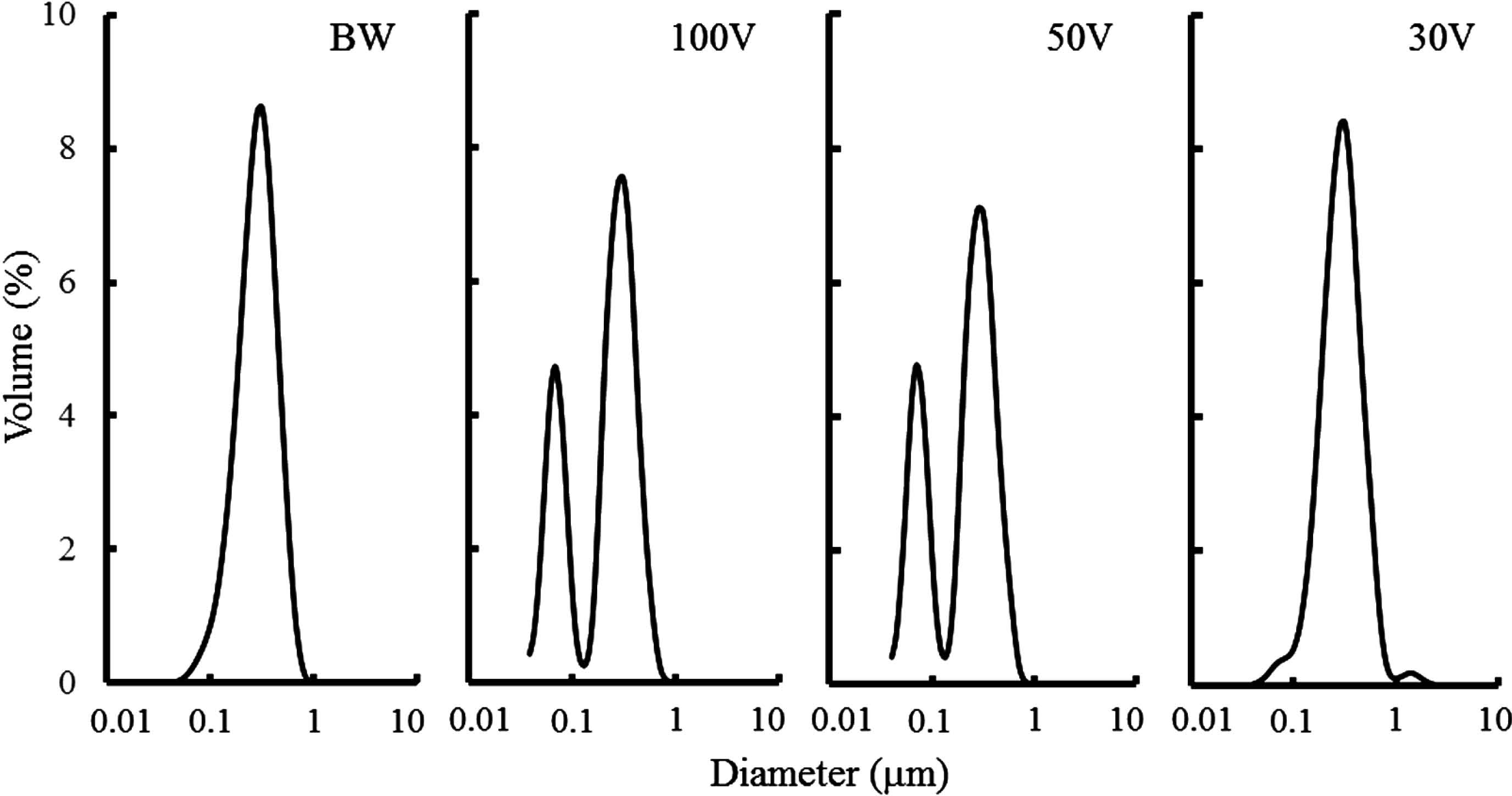
Particle size distributions of soymilk prepared by ohmic heating.
BW, boiling water heating; 100 V, 50 V, 30 V, applied voltages in ohmic heating.
The size distribution of soymilk ohmically heated is also shown in Fig. 5. Ohmic heating at voltages of 50 and 100 V, which respectively resulted in a similar or more rapid heating rate than observed with boiling water heating, showed bimodal distributions of 40 to 120 nm as protein particles (Ono et al., 1991; Guo et al., 1997) and 120 to 800 nm as oil body-like particles. Thus, ohmic heating may differ somewhat from boiling water heating, although the volume of soymilk heated in boiling water differed from that ohmically heated. A more rapid and/or uniform heating compared to thermal conduction from the exterior is possibly related to this result. However, ohmic heating at 30 V, which resulted in a much slower heating rate than other heating conditions, produced a much smaller distribution of protein particles, with a primary distribution of protein particles and a secondary distribution ranging from 1 to 2 µm. The secondary distribution was assigned to flocculation or coalescence of oil droplets in reference to previous papers (Toda et al., 2007; Shimoyamada et al., 2010). The above data suggest that the size distribution of particles in the soymilk could be attributed to the heating rate. The disappearance of the protein particles in soymilk ohmically heated at 30 V suggests that the size of the particles either decreased to <40 nm or increased to within the range of the oil body-like particles (ca. 100 – 800 nm).
Ono et al. (1991) measured the content of protein particles using ultracentrifugation. The ohmically heated soymilk was therefore also subjected to ultracentrifugation to estimate the effect of voltage on the ratio of protein particles (Fig. 6). As the result, soymilk samples ohmically heated at 100, 50 and 30 V showed almost equivalent protein particle contents (50 ∼ 55%); thus, the protein particle content was considered to be maintained. Since the protein particle content did not change, the disappearance of particles 40 to 100 nm in size at 30 V was attributed to the enlargement of protein particles, with particles possibly located in the peak due to emulsion droplets. That is, the slower heating rate at 30 V allowed the protein particles to further agglomerate.

Content of protein particles in soymilk prepared by ohmic heating.Closed bar, protein particles (soluble aggregate): open bar, soluble protein (monomer and low aggregate).Raw, raw soymilk (unheated); BW, boiling water heating; 100 V, 50 V, 30 V, applied voltages in ohmic heating.
Tofu curd formation with soymilk prepared by ohmic heating The soymilk samples prepared by ohmic heating at various voltages were subsequently subjected to tofu curd formation by GDL. Stress-strain curves of the tofu curd (Fig. 7) indicated that curd made from soymilk prepared by ohmic heating at 50 and 100 V showed a slope comparable to that of soymilk heated in boiling water. However, the curves showed higher breaking stress and strain. Figures 5 and 6 show that ohmically heated soymilk contained a larger protein particle fraction than did the soymilk heated in boiling water. Ohmic heating is therefore thought to result in firmer tofu curd through the increased number of protein particles. This is further supported by a previous study in which the ratio of protein particles was shown to affect tofu curd formation (Guo and Ono, 2005). On the other hand, the sample prepared by ohmic heating at 30 V formed a much less coagulated tofu curd. Thus, very slow heating results in insufficient coagulation. Figures 4 and 5 also imply that heating at 30 V further increased the agglomerate protein particle fraction, possibly giving precipitable or less dispersible aggregates. This suggests that a very large aggregate potentially inhibits the uniform coagulation of tofu curd.

Stress-strain curves of tofu made from soymilk produced by ohmic heating. BW, boiling water heating (solid line); 30 V, ohmic heating at 30 V (dotted line); 50 V, ohmic heating at 50 V (dashed line); 100 V, ohmic heating at 100 V (dotted and dashed line).
Soymilk was prepared by ohmic heating. Precipitation, viscosity and surface hydrophobicity of proteins were increased with the decrease in voltage applied, namely a slower heating rate. Furthermore, ohmically heated soymilk at 50 and 100 V was shown to generate firmer tofu curd than that produced by 30 V and boiling water heating. This difference was considered to be due to the influence on protein particle formation. The possibility exists that the heating rate also affects the thermal denaturation of proteins, resulting in soymilk of modified quality.