2015 年 21 巻 3 号 p. 479-487
2015 年 21 巻 3 号 p. 479-487
The applicability of Einstein's and Krieger-Dougherty's theories of viscosity was examined using soymilk. Under dilute conditions, the relative viscosity of the emulsion with suspended oil bodies was proportional to the volume fraction of oil bodies, and the slope increased to greater than 2.5. Under concentrated conditions, the oil body suspension showed a Krieger-Dougherty-like dependency on volume fraction. The oil bodies in soymilk behaved as suspended substances and it was possible for us to predict the relative viscosity from the volume fraction of oil bodies. We also focused on the coagulation of soymilk by magnesium chloride and examined the validity of the novel viscous model, combining the extended Einstein equation and the Krieger-Dougherty equation, on the effect of cross-linkers. At 10°C and 25°C, the equation could be applied to various soymilk samples. In addition, the viscosity during coagulation could be predicted when the parameter hc of the dispersion system was utilized.
Soymilk production has increased because of the heightened consumer interest in the functionality of this protein-laden beverage. There has been a significant amount of research published about soybean proteins (Mori et al., 1986; Thanh and Shibasaki, 1977; Yuan et al., 2009) and soybean protein isolates as new food materials (Campbell et al., 2009; Fukushima, 2001). However, very little information about the physicochemical properties of soymilk is available (Nik et al., 2008; Nsofor and Osuji, 1997; Ringgenberg et al., 2012). Understanding the physicochemical properties of soymilk, including viscosity, is required in the development of soymilk products. Viscosity equations were developed in order to predict viscosity in colloidal food systems. Viscosity is a physical property that can be effectively utilized in characterizing the effect of quality control of a manufacturing process or mechanical and thermal processing. Soymilk is a colloidal system produced as an extract from swelled and ground soybeans; therefore, almost all of the components (protein, lipid, and saccharides) of the soybean seeds are present in the soymilk (Shurtleff and Aoyagi, 2000). It was reported that about 70% of the lipids in soybeans were extracted to the soymilk (Poysa and Woodrow, 2002). Dispersed substances consist of oil bodies and protein particles and the dispersion medium is the liquid containing the dissolved proteins, sugars, and minerals such as potassium. Oil bodies consist of a triacylglycerol (TAG) matrix core surrounded by a layer of phospholipids and intrinsic oleosin (Li et al., 2001; Tzen and Huang, 1992). The average particle diameter of an oil body in soymilk is approx. 250 nm (Iwanaga et al., 2007). Oil bodies containing soymilk lipids were stabilized by the presence of oleosin and phospholipids on their surface (Chen and Ono, 2010).
Einstein developed a theory for the viscosity of a dilute suspension of small hard spheres in a continuous fluid (Einstein, 1905 and 1911). Viscosity causes dissipation of energy in a suspension because of the hydrodynamic effect. Therefore, the following equation based on hydrodynamics was described:
 |
 |
 |
This study aimed to systematically understand the viscosity of a multicomponent system that exhibits intermolecular interactions, namely soymilk, and to propose a viscosity theory applicable to a wide range of volume fractions. In particular, we experimentally investigated how the coefficient of viscosity changed with the volume fraction. The dependence of the coefficient of viscosity as a function of lipid content was evaluated and the relationship between viscosity and lipid content was examined in order to predict soymilk viscosity during the production process. We also focused on the coagulation of soymilk by magnesium chloride, where the dispersion state changed notably in the presence of the coagulant. Furthermore, we also examined the applicability of the novel model of the extended Einstein equation and the Krieger-Dougherty equation to explain the effect of cross-links. In addition, the relationship of each parameter was investigated and the physical meaning of the parameter was discussed.
Analysis of viscosity with the extended Einstein equation and the Krieger-Dougherty equation As long as rigid bodies are suspended and diluted with the dispersion medium, equation (3) will guarantee that there is a domain where the viscosity is proportional to the volume fraction φ. However, it becomes impossible to ignore the thickness of the boundary layer that forms near the particle surface since there is a hydrodynamic effect in actual particles. Then, if the effective volume fraction of a dispersed particle represents φe and the parameter of boundary layer thickness represents h,
 |
 |
Krieger and Dougherty (Krieger and Dougherty, 1959) proposed an equation that related ηr to φ for concentrated suspensions
 |
A novel viscous model combining the extended Einstein equation and the Krieger-Dougherty equation with the effect of cross-linkage Although there are many foods possessing cross-linkers or coagulants, there are only a few models of viscosity that are applicable to such systems. To determine the viscosity of a colloidal dispersion system aggregated with a coagulant under a wide range of conditions, it is necessary to evaluate and analyze the influence of coagulant addition on the formation of clusters.
The apparent viscosity of a colloidal dispersion system before coagulant addition is ηc0. Addition of a coagulant leads to cross-link formation between colloidal particles and generation of bulky clusters. The apparent viscosity increase is in accordance with the hydrodynamic effect of the amount and shape of these bulky clusters. The supposed increment in the effective volume fraction of a cluster (φc) is proportional to the coagulant concentration C,
 |
 |
The Krieger-Dougherty equation can be applied to a concentrated colloidal dispersion system with coagulant addition:
 |
 |
First, when the coagulant concentration is low, the parameter hc is calculated using equation (8) from the dimensionless viscosity ηd of each soymilk. Dimensionless viscosity ηd is obtained by dividing the viscosity ηc by coagulant-free viscosity ηc0. Second, Kc is calculated using hc and equation (10).
Materials In this study, various soybeans were used in order to examine versatile formulations. Tanrei (Japan, 2009), Enterprise (Canada, 2009), and OAC-KENT (Canada, 2011) were selected as soybean samples. Soymilk was provided by Taishi Food Inc. (Aomori, Japan).
Adjustment of the solid content of soymilk To prepare diluted soymilk, soymilk made from Tanrei soybeans (Japan, 2009) was utilized. The solid content of soymilk (12.4%) was adjusted to 11.2% and 9.9% with deionized water, representing concentrations within the range usually used in the manufacture of tofu. To prepare a formulation used to investigate the stability of soymilk, soymilk made from Enterprise soybeans (Canada, 2009) was utilized. Soymilk used for the isolation of oil bodies was made from the OAC-KENT soybeans (Canada, 2011). The moisture content was determined by placing the samples in aluminum cups and oven drying at 105°C for 24 h.
Determination of lipid and protein The lipid content was determined by the Southgate method (Southgate, 1971). Celite (5 g) was added to the sample (10 g), which was then extracted with 60 mL chloroform / methanol 2:1 (v/v) at 65°C for 1 h and the extract was then filtered through glass microfiber filters 40 µm in diameter. The filtrate was washed with 35 mL chloroform / methanol 2:1 (v/v). The extract was rotary evaporated at 40°C. After cooling, 25 mL petroleum ether was added and the sample was shaken after the addition of anhydrous sodium sulfate. The petroleum ether layer was centrifuged at 3,000 rpm for 5 min, and the 15 mL supernatant fraction was rotary evaporated and the weight measured. The protein content was measured by the improved Dumas method using a nitrogen analyzer (SUMIGRAPH NC-220F; Sumika Analysis Chemical Service, Ltd., Tokyo, Japan).
Preparation of soymilk with different lipid contents To separate the lipid content of soymilk, the soymilk (3.2% lipid) was centrifuged at 10,000 rpm for 10, 20, 30, and 60 min at 10°C. The floating layer was then removed. The lipid content of each soymilk sample was determined to be 2.6%, 2.1%, 1.8%, and 1.0%, respectively. The 1.0% lipid soymilk was used as the dispersion medium of oil bodies because it contained few oil bodies. These samples were used within 24 h of preparation.
Isolation of oil bodies and sample preparation Oil bodies were physically isolated from soymilk (Fujiki et al., 1982; Loer and Herman, 1993). Soymilk (100 mL), Tris(hydroxymethyl) aminomethane (12.1 g), and NaCl (29.2 g) were added to a 200-mL beaker and mixed. The pH of each sample was adjusted to 8.6 by 1 N HCl and then the samples were centrifuged at 10,000 rpm for 20 min at 10°C. The resulting floating layer was collected and suspended in a chilled 0.1 M buffer solution of sodium hydrogen carbonate. The sample was incubated for 30 min at 4°C and centrifuged again. The floating layer was suspended in a 50 mM phosphate buffer (pH 6.5) and the sample was centrifuged twice at 10,000 rpm for 20 min at 10°C. The collected floating layer was stored at 4°C and was subsequently diluted in the phosphate buffer. Additionally, the floating layer was diluted in the 1.0% lipid soymilk. The lipid contents of the soymilk samples were 1.6%, 3.3%, 5.5%, 9.8%, and 13.0%. The lipid contents were measured by the Southgate method (Southgate, 1971) described above. The ratio of the weight fraction of the oil body was converted into volume fraction by using the density of the oil body. The density of the oil body was measured using a pycnometer.
Measurement of viscosity Viscosity measurements were carried out with a rotating cylinder viscometer (model DV-E; Brookfield, MA, USA), using spindle LV2 at 10°C or 25°C. The viscometer motor rotated the spindle at 60 rpm and the viscometer measured the rotational resistance and reported a viscosity value. The sample (307 g) was put into a 300-mL beaker and the viscosity was measured after 1 min in order to stabilize the reading on the digital viscometer. The viscosity of the isolated oil bodies diluted in the phosphate buffer and soymilk with 1.0% lipids was measured after centrifugation. The relative viscosity ηr was obtained by dividing the viscosity of the solution by the viscosity of the phosphate buffer. The viscosity before coagulant addition was defined as coagulant-free viscosity ηc0. The coagulant was mixed into the solution with a stir bar at 400 rpm for 20 s. The viscosity ηc was measured after 10 min.
Analysis of the viscosity of the oil body dispersion system under dilute conditions with the extended Einstein equation Recent research suggested that oil bodies act like hard spheres in tofu (Ito and Idogawa, 2013). Therefore, Einstein's equation was used for the analysis because it could be considered that the oil bodies in soymilk were spheres covered with oleosin proteins.
The applicability of suspension viscosity in soymilk to the dependence on particle concentration was evaluated. We first attempted to apply the extended Einstein equation to understand the viscosity of the soymilk colloidal system. Figure 1 shows the dependence of the relative viscosity of soymilk on the volume fraction of lipids in the oil bodies. The volume fraction of lipids was calculated from the lipid content of soymilk. The oil body density was 0.9806 g/cm3. Three samples were used: the sample where the oil bodies were separated in the phosphate buffer, the sample in which the oil bodies were removed by centrifugal separation from soymilk, and the sample in which the separated oil bodies were mixed by centrifugal separation with the soymilk containing 1.0% lipids (low-fat soymilk). The apparent viscosity depended on the volume fraction of oil bodies for each soymilk sample. Moreover, the relative viscosities of soymilk samples subjected to centrifugal separation and the samples containing a mix of the separated oil bodies and low-fat soymilk suspension were in agreement with the slope predicted from the formulation of oil body suspensions. The parameter h is related to boundary layer thickness, and it could be considered as the hydrodynamic molecular volume of the solvent molecules attached to the solute surfaces (Toda and Furuse, 2006). In the three samples, h was almost the same and was approximately estimated to be 2.5. This suggested that no molecule other than the water in the solvents affected the hydrodynamic volume of the oil bodies in the dispersions. That is, in dilute soymilk, the oil bodies existed as independent spheres whose surface was hydrated. The relative viscosity of the oil body suspension in phosphate buffer could be explained by equation (6) for φ values up to approximately 0.25. However, we noted a deviation from the straight line at 10% of the oil bodies' volume fraction in the low-fat soymilk suspension. Protein particles and dissolved proteins exist in low-fat soymilk. It appeared that the protein interacted with over 10% of the oil bodies' volume fraction.

Relative viscosities plotted against volume fraction. The circles represent the oil bodies in a phosphate buffer; triangles represent the oil bodies in low-fat soymilk; crosses represent centrifuged soymilk. The dotted line represents the extended Einstein equation where h = 1 and the solid line represents the extended Einstein equation where h = 2.5.
Analysis of the viscosity of the oil body dispersion system under concentrated conditions using the Krieger-Dougherty equation The viscosity of the oil body dispersion system is shown in Fig. 2. The coefficient of viscosity increased as the oil bodies increased, rapidly increasing at high volume fractions. The applicability of the Krieger-Dougherty equation (6) to explain the particle concentration dependence of relative viscosity in the highly concentrated oil body dispersion system was evaluated. For oil bodies suspended in phosphate buffer, the viscosity parameters [η] and K were determined to be 3.11 and 1.62, respectively (Fig. 2a). From this, the maximum volume fraction was estimated to be 0.617 and the value was quite close to the expected value for the random close packing of hard spheres (0.64). When the equation is applied to a suspension of dispersed particles with shapes other than uniform hard spheres, the value of the parameter [η] = 2.5 or the maximum volume fraction = 0.6 shifts greatly. This result indicated that the oil body was globular because the value of the maximum volume fraction of the sample was around 0.6. The equation, when applied to oil bodies suspended in low-fat soymilk, gave viscosity parameters [η] = 3.68 and K = 5.82 (Fig. 2b). When compared with the system suspended in buffer, there was no significant difference in parameter [η]. However, K differed greatly. It was hypothesized that the existence of proteins in the low-fat soymilk made it impossible to have tightly packed oil bodies. Strikingly, the maximum volume fraction of oil bodies, which was as high as 0.6 in phosphate buffer, was only 0.17 in low-fat soymilk. It seems that the cause of increased viscosity with increasing volume fraction of oil bodies was due to various colloidal interactions (Van der Waals force, electrostatic repulsion, and hydrophobic interaction etc.). It was reported that a suspension of chocolate and sugar showed a similar Krieger-Dougherty dependency on volume fraction and the melt rheology of the chocolate was dominated by hydrodynamic interactions (Taylor et al., 2009). Hydrodynamic interactions among particles in the phosphate buffer suspension caused an increase in viscosity. On the other hand, oil bodies, protein particles, and dissolved proteins exist in the low-fat soymilk suspension. In order to accommodate protein-oil body interactions, the low-fat soymilk suspension had a higher viscosity as compared to the viscosity of the phosphate buffer suspension.

Relative viscosity plotted against volume fraction. (a) Circles represent the relative viscosity of oil bodies in a phosphate buffer. The solid line shows the Krieger-Dougherty equation with [η] = 3.11 and K = 1.62. (b) Triangles indicate the relative viscosity of oil bodies in low-fat soymilk. The solid line shows the Krieger-Dougherty equation with [η] = 3.68 and K = 5.82.
Application of the novel viscous model, combining the extended Einstein equation and the Krieger-Dougherty equation, to soymilk coagulation with the effect of cross-linkage In the soymilk industry, tofu is traditionally produced by the reaction of soymilk with magnesium chloride as a coagulant. Here, we were able to evaluate the aggregation process of soymilk using the parameters hc and Kc at 10°C and 25°C. Mixing of the coagulant in tofu production has been performed at various temperatures (Onodera et al., 2009; Shih et al., 1997). Figure 3 shows the dimensionless viscosities of the diluted soymilk samples and the values predicted by the equation at 10°C. Predictions of the parameters were possible in the soymilk with various solid contents, 12.4%, 11.2% and 9.9%. hc became small as the solid content became low. This result showed that the size of the bulky aggregate bridged by the same coagulant concentration became small when the solid content was low. Moreover, Kc shows the index of filling of the bulky aggregates in a concentrated system and they became a dense structure as the solid content increased. In Fig. 4, the difference in the stability of soymilk during the coagulation reaction at 10°C is shown. Figure 4b shows the dimensionless viscosity of the soymilk from Fig. 4a after 24 h. Since hc decreased after 24 h, the amount of bulky aggregates formed by the coagulant was small. There was a possibility that the reactivity of proteins and coagulants decreased after 24 h. In this study, it was possible to apply the combined model of the extended Einstein equation and the Krieger-Dougherty equation with the effect of cross-linkage, even if the conditions of the concentration of soymilk, materials etc. differed.

Dimensionless viscosities of soymilk at 10°C plotted against a molar concentration of MgCl2·6H2O. (a) cross, 12.4%; (b) circle, 11.2%; (c) triangle, 9.9% solid content of soymilk. The solid line shows the viscous model combining the extended Einstein equation and Krieger-Dougherty equation.

Dimensionless viscosities of soymilk at 10°C plotted against a molar concentration of MgCl2·6H2O. (b) shows the dimensionless viscosity of the soymilk used in (a) at 10°C for 24 hours. The solid line shows the viscous model combining the extended Einstein equation and Krieger-Dougherty equation.
In Fig. 5, the differences in soymilk with different lipid volume fractions in the coagulation reaction at 25°C are shown. The effect of the coagulation reaction on lipid content was examined. Each soymilk sample contained 5.0% protein. The viscosity model used in this study was applicable for the investigated soymilk samples. Here, although the example of soymilk with different lipid volume fractions was shown, hc and Kc differed in each of the soymilk samples. hc is a parameter that depends on the effective size of the bulky cluster formed by cross-linkage. When the oil bodies were removed, hc became small. hc is a parameter that shows the index in which the protein of the continuous phase aggregated by cross-linkage in the case of low lipid concentration. Since hc became large as the number of oil bodies increased, the existence of oil bodies might have promoted the formation of the aggregates and a synergistic effect might have appeared between the proteins and oil bodies. Therefore, it was possible that oil bodies were bound to the aggregates formed by a coagulant. Additionally, Kc is a parameter that shows the degree of filling of bulky aggregates when the coagulant concentration is high. When the oil bodies were removed, Kc became large. The soymilk samples investigated here were the same except for the amount of oil bodies, and hc and Kc expressed the colloidal characteristics of each soymilk. As mentioned above, the combined model of the extended Einstein equation and the Krieger-Dougherty equation with the effect of cross-linkers described the cross-link concentration dependence quite well.
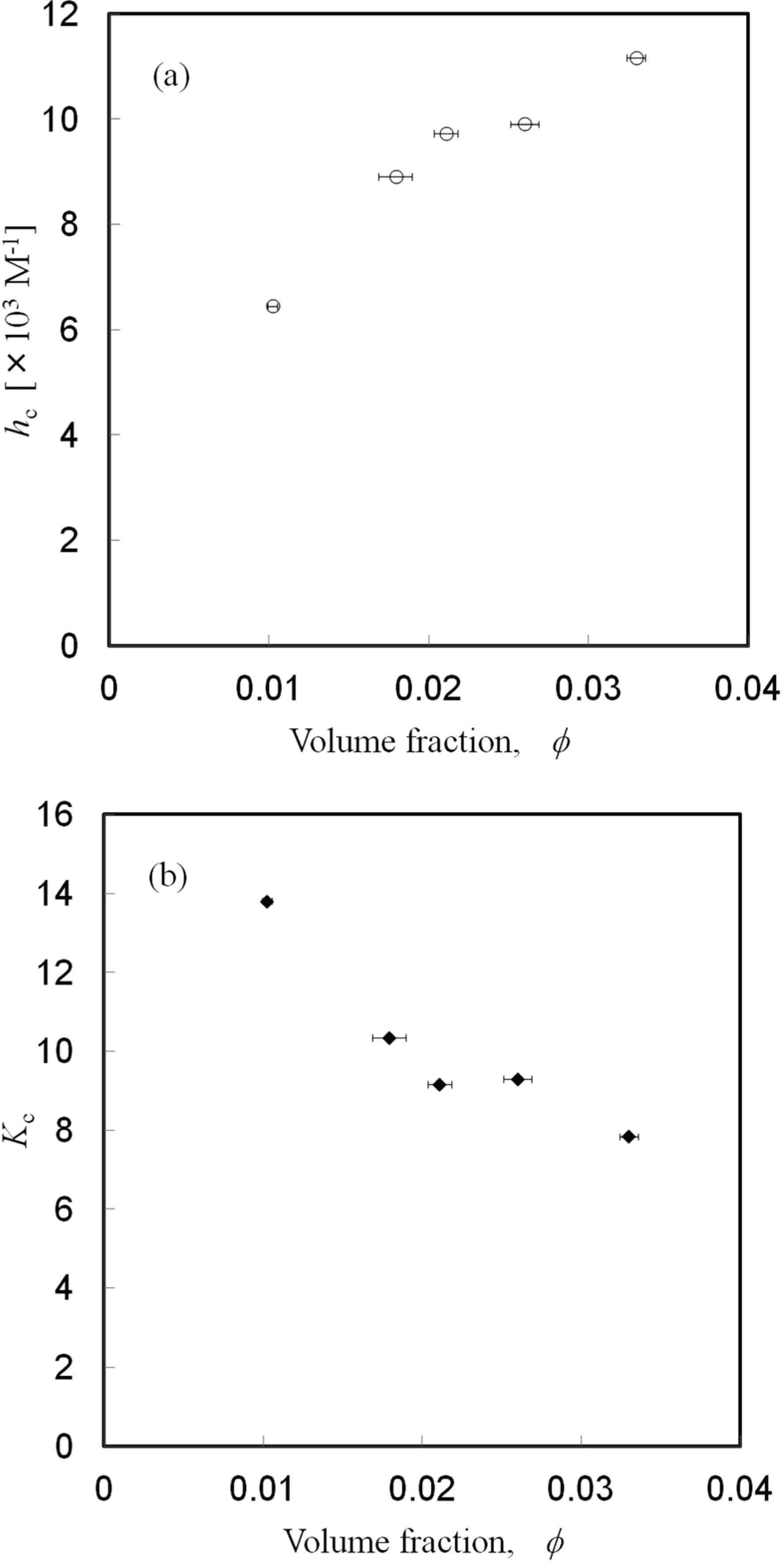
Dependence of each parameter on lipid volume fraction. Each soymilk sample contained 5.0% protein.
The relationship between hc and Kc in the magnesium chloride-containing soymilk samples is shown in Fig. 6. This result indicated a correlation between hc and Kc. Therefore, the viscosity during aggregation with coagulates could be predicted when the parameter hc of the dispersion system was given. Moreover, the difference was shown by the relationship of the parameter at 10°C and 25°C. The radius of the soymilk protein particles was smaller at low temperatures due to the formation of a more compact particle, resulting from the increase in hydrogen bonding (Ringgenberg et al., 2013). The graph showing the effect at 10°C was likely shifted to the lower left. The relationship of each parameter could be described by a power law and could predict the aggregation process quantitatively. The aggregation process in terms of hc and Kc is shown in Fig. 7. One reason for the smaller hc was the formation of clusters containing fewer oil bodies (Fig. 5). Moreover, hc became small so Kc was large (Fig. 6). The structure of a bulky aggregate denoted by Kc was related to the attractive force of particles (Dickinson, 1992). When the attractive force of particles is small, adhesion of particles does not always take place. Therefore, a bulky cluster and oil body encounter a bulky aggregate and the bulky aggregate becomes larger. Consequently, a bulky aggregate takes on the comparatively close-packed structure. On the other hand, when the attractive force of the particles is large, particles take on a structure that adheres strongly at several points and does not take on a closely-packed structure with many adhesions. In soymilk, Kc was interpreted as the filling factor of the bulky aggregates with bulky clusters and oil bodies aggregated by the addition of a coagulant (Guo et al., 1999; Ren et al., 2009). In this study, the lipid concentration affected the reaction with added coagulants and the importance of this was demonstrated.

Relationship between hc and Kc at 10°C and 25°C.

A diagram of the different forms of aggregation.
Although an understanding of the aggregation process is very important, the effect of cross-links remains to be clarified (Chiou et al., 2006; Senff and Richtering, 2000). Because the physical meaning of the parameter of the Krieger-Dougherty equation has been clarified and evaluated in terms of the applicability to coagulant-free systems, this equation was used in this study. The novel viscous model combined the extended Einstein equation and the Krieger-Dougherty equation to consider the effect of cross-links on the aggregation process of soymilk. In addition, at high coagulant concentrations, Kc could be calculated using hc at low coagulant concentrations, and the viscosity of the aggregation process was predictable from the size of bulky clusters at low coagulant concentrations. In other words, the change of the isothermal coefficient of viscosity could be predicted. Tofu is formed by adding a coagulant and heating. As such, this work might be applicable not only to soymilk but also to numerous other colloidal products. Hence, we aim to evaluate the rheological characteristics and show applications in other systems with cross-linkers.
Acknowledgements This work was partly funded by a Scheme to Revitalize Agriculture and Fisheries in Disaster Area through Deploying Highly Advanced Technology, Ministry of Agriculture, Forestry and Fisheries of Japan.