2015 Volume 21 Issue 4 Pages 557-562
2015 Volume 21 Issue 4 Pages 557-562
Several polyphenolic compounds which were derived from the fermented soybean had been identified and investigated for their potential effects on some diseases. However, such a biotransformation phenomenon is seldom discussed and has not been studied by using submerged culture with isoflavone, such as pure daidzein, as a substrate. In this study, the conditions for hydroxylation of pure daidzein into 8-hydroxydaidzein by Aspergillus oryzae were systemically investigated. Our results indicated that the optimal culture conditions for the hydroxylation were 200 rpm and pH 6. Moreover, the production was significantly increased 57.4% by an optimized concentration of 3 g/L MgSO4. On the other hand, 77.8% high biotransformation efficiency of 8-hydroxydaidzein could be obtained by providing pure daidzein; that can avoid many derived polyphenolic compounds were generated by isoflavone biotransformation. Our results suggest that the biotransformation of 8-hydroxydaidzein can be performed through a highly selected catalysis under specified conditions using certain microorganisms.
Soybeans, produced by the most cultivated plant in the world, are rich in proteins (40 – 50%), lipids (20 – 30%) and carbohydrates (26 – 30%). They are consequently the subject of extensive scientific research (Bernard et al., 2004). In Asian countries, there are many traditional fermented products that use soybeans as the fermented substrate, including miso (Japanese fermented soybean), sufu (Chinese fermented soybean), and tempeh (Indonesian fermented soybean). These nutritious foods have been dietary staples for a long time (Villares et al., 2011). Recently, these foods were found to have greater antioxidant effects than unfermented steamed soybeans and to possess several naturally occurring polyphenolic compounds (Esaki et al., 1997; Esaki et al., 1998; Esaki et al., 1999; Romero et al., 2004; Lin et al., 2006). Isoflavones are one of a kind of polyphenolic compound. Beyond their nutritional value, isoflavones are of increasing research interest due to their potential benefits relating to various aspects of human health and chronic diseases such as symptoms related to menopause, various types of hormone-dependent cancers, cardiovascular disease, osteoporosis and the efficacy of dipeptidyl peptidase IV inhibitor (Messina, 1999; Delmonte et al., 2006; Imamura et al., 2011). The main polyphenols in soybeans are isoflavone analogues classifiable under three main types: daidzein, genistein and glycitein. These isoflavones are present as aglycone, glucoside, acetyl-glucoside or malonyl-glucoside (Wang and Murphy, 1994). Since soybean foods fermented using microorganisms show increased antioxidative activity, several compounds have been identified and investigated for their potential effects on some diseases (Chen et al., 2005).
One of these polyphenolic compounds, 8-hydroxydaidzein (7,8,4′-trihydroxyisoflavone), is discussed in this research. 8-Hydroxydaidzein from soybean fermented with Aspergillus saitoi has been identified as a potent antioxidant (Esaki et al., 1998), and the same compound derived from Aspergillus sp. has been identified as a human aldose reductase inhibitor (Fujita et al., 2004). 8-Hydroxydaidzein from soybean miso has high DPPH radical-scavenging activity, antimutagenic activity and antiproliferative activity toward HL-60, A549 and B16 melanoma 4A5 cell lines (Hirota et al., 2000; Chen et al., 2003), while 8-hydroxydaidzein from soygerm koji has potent suicide substrates of mushroom tyrosinase (Chang et al., 2007b; Chang, 2007). Moreover, 8-hydroxydaidzein not only inhibits both the monophenolase and diphenolase activities of tyrosinase, it is also an irreversible inhibitor of mushroom tyrosinase (Chang, 2007). This capacity is very different from that of the four common isoflavones, daidzein, glycitein, daidzin and genistin, which are competitive inhibitors only for the monophenolase activity of mushroom tyrosinase. Therefore, 8-hydroxydaidzein is a relatively unique isoflavone and worthy of further investigation.
After 8-hydroxydaidzein was purified and proven to be a potent antioxidant (Esaki et al., 1998), the same researchers further confirmed the formation mechanism in solid and liquid cultures using soybean as a substrate with Aspergillus saitoi (Esaki et al., 1999). Daidzin in steamed soybeans was transformed into 8-hydroxydaidzein by a two-step reaction in fungi fermentation. First, daidzin was gradually converted into daidzein by β-glucosidase, and then hydroxylated enzymatically to produce the 8-hydroxydaidzein. The transformation of daidzein into ortho-hydroxydaidzein derivatives was dominated by cytochrome P450 monooxygenases, a super-family of heme-containing enzymes (Bernhardt, 2006). This protein could have wide-ranging applications in the production of drugs and drug metabolites or as catalysts (Jung et al., 2011). Therefore, the recombinant strains have been obtained and studied in the recent years (Roh et al., 2009; Pandey et al., 2011). The maximal conversion 36.3% of daidzen to 6-hydroxydaidzein was achieved in a recombinant strain of Pichia pastoris (Chang et al., 2013). For the hydroxylations of dainzein and genistein in whole cell, 15.9% conversion was obtained in screening some microorganisms (Roh et al., 2009).
In this study, that transformation had been verified by directly providing the key substrate daidzein to investigate the hydroxylation behavior. The microorganism used is a key point for this study since not all of the fungal strains from dou-chi, miso, sake, soy sauce or sufu have the ability to process the biotransformation (Chang et al., 2007a). Even among the Aspergillus species, only some can metabolize daidzin; for example, Aspergillus sojae, Aspergillus tamari and Aspergillus oryzae. In this study, we used Aspergillus oryzae BCRC 32288 since its transformability was the best in the previous report (Chang et al., 2007a).
Materials The daidzein was purchased from Sigma. Glucose was purchased from Riedel-de Haën. Malt extract was purchased from BD. Soya peptone was purchased from HIMEDIA. Casein from bovine milk was purchased from Sigma and was technical grade. CaCl2 and MgSO4 · 7H2O were purchased from Merck. CaCO3 was purchased from J. T. Baker. 8-hydroxydaidzein was kindly donated by Dr. Te-Sheng Chang at the National Tainan University, Tainan, Taiwan (ROC). HPLC-grade acetonitrile and acetic acid were purchased from Sigma. Other reagents and solvents were analytical grade.
Strain and medium Lyophilized culture of Aspergillus oryzae BCRC 32288 was obtained from the Bioresources Collection and Research Center (BCRC, Food Industry Research and Development Institute, Hsinchu, Taiwan, ROC). The stock culture was grown on potato dextrose agar (PDA) and maintained at 25°C.
Culture conditions The culture medium contained 2 g/L glucose, 20 g/L malt extract and 1 g/L soya peptone. Prior to sterilization at 121°C, the pH value of the medium was adjusted to 7.0. For the culture condition test, the organisms were grown in a 250 mL flask with working volume 100 mL of medium containing 10% inoculum. The strain was inoculated with final concentration 1x107 spores/mL and incubated at 30°C at 200 rpm. The culture medium used for the transformation of daidzein was the same as the preculture medium except for the addition of daidzein dissolved in DMSO at 0.2 g/L. The cultivation time was five days. For different initial pH values, the pH of medium was adjusted using PBS to obtain different initial pH values, and was not controlled during the progress of biotransformation.
Analytical methods Sample from cultivation broth 1.5 mL was centrifuged 12000 rpm for 10 min. The 0.5 mL supernatant was collected and then mixed with equal volume of 95% ethanol and extracted for 1 hr at 200 rpm. The pellet was extracted with 0.5 mL of 95% ethanol for 1 hr at 200 rpm. These samples were filtered through a 0.2 mm nylon filter, and 20 µL of each sample was used for HPLC analysis. HPLC analysis was performed on a Hitachi D-7000 HPLC system (Hitachi, Ltd., Tokyo, Japan) equipped with an L-7400 UV detector and a 250x4.6 mm i.d., Mightysil C18 reversed-phase column (Kanto Chemical Ltd.). The operating conditions were as follows: solvent, 25% acetonitrile/water containing 1% acetic acid; flow rate, 1.0 mL/min; detection, 260 nm. The standard form of 8-hydroxydaidzein was purified from soy germ koji fermented with A. oryzae BCRC 32288, and the procedure for that was described in previous report (Chang et al., 2007a).
Effect of rotating speed There are many traditional fermented products that use soybeans as the fermented substrate. The production process for those products involves a solid-state culture. In this study, we investigated the biotransformation in a submerged culture. The oxygen requirement for achieving maximum biotransformation is governed by the characteristics of the culture. The rotating speed effect on oxygen transfer was analyzed using 50, 100 and 200 rpm for maximum 8-hydroxydaidzein production. The results were shown in Figure 1. The optimal rotating speed for maximum extracellular 8-hydroxydaidzein production was 200 rpm with the 0.024 g/L product. The results also showed that the oxygen requirement for 8-hydroxydaidzein production was significant since the productivities for 100 and 50 rpm were very low. The production phenomenon was also discussed in the study by Esaki et al. (1999) for liquid culture of a soybean extract fermented by A. saitoi. In their results, daidzin, which existed in the soybean extract at day 0, decreased with increasing incubation time, while daidzein increased to its highest level after 1.6 days. Meanwhile, 8-Hydroxydaidzein was first present at 1.6 days, which was the start of sporulation. The amount of 8-hydroxydaidzein after 3.6 days was about 0.015 g/L of culture filtrate which were lower than our data 61.7% in terms of maximum product concentration. It may be due to different microorganism used since BCRC 32288 was the best one in 8-Hydroxydaidzein production which has been screened in the previous report (Chang et al., 2007a).

Amounts of 8-hydroxydaidzein produced at different rotating speed.
Effect of initial pH One of the important factors affecting the microbial growth is the pH in the medium. Since the common pH value for cultivating A. oryzae is around 7.0, we set initial pH values of the substrates between 4.0 and 8.0. The results indicated that the optimal initial pH for biotransformation was 6.0 (Figure 2). The production of 8-hydroxydaidzein was increased about 43% than the production at conventional pH 7.0. The final concentration of 8-hydroxydaidzein was significant decreased when the variation of pH above 1 and seriously decreased at pH below 5 and higher than 7.0. The low concentration of 8-hydroxydaidzein produced in higher pH may be due to the instability property of this compound (Chang, 2009). Therefore, the pH level is a significant factor in the biotransformation of 8-hydroxydaidzein.

Effects of different pH levels on the biotransformation of daidzein into 8-hydroxydaidzein.
Addition of other compounds By employing the insights gleaned from the culture environment investigation above, the maximum product concentration was 0.033 g/L. In other words, the biotransformation ratio of daidzein into 8-hydroxydaidzein was only 17%. To increase the transform efficiency still further, some commonly used compounds were used as substrates, with the added concentrations being 5 g/L in the total working volume. The results are shown in Figure 3. Four compounds were used and only MgSO4 significantly increased 8-hydroxydaidzein production, while the other compounds had differing degrees of negative effect on 8-hydroxydaidzein production. Consequently, we tested various amounts of MgSO4 to find the optimal concentration. The results of the tests (Figure 4) indicate that the optimal concentration of MgSO4 for production of 8-hydroxydaidzein was between 3 and 7 g/L. Therefore, 3 g/L MgSO4 was the best choice for the economic reason and the 8-hydroxydaidzein concentration was 0.052 g/L which is 57.4% increased from 0.033 g/L.

Amounts of 8-hydroxydaidzein produced via biotransformation of daidzein using various additional compounds as substrates.
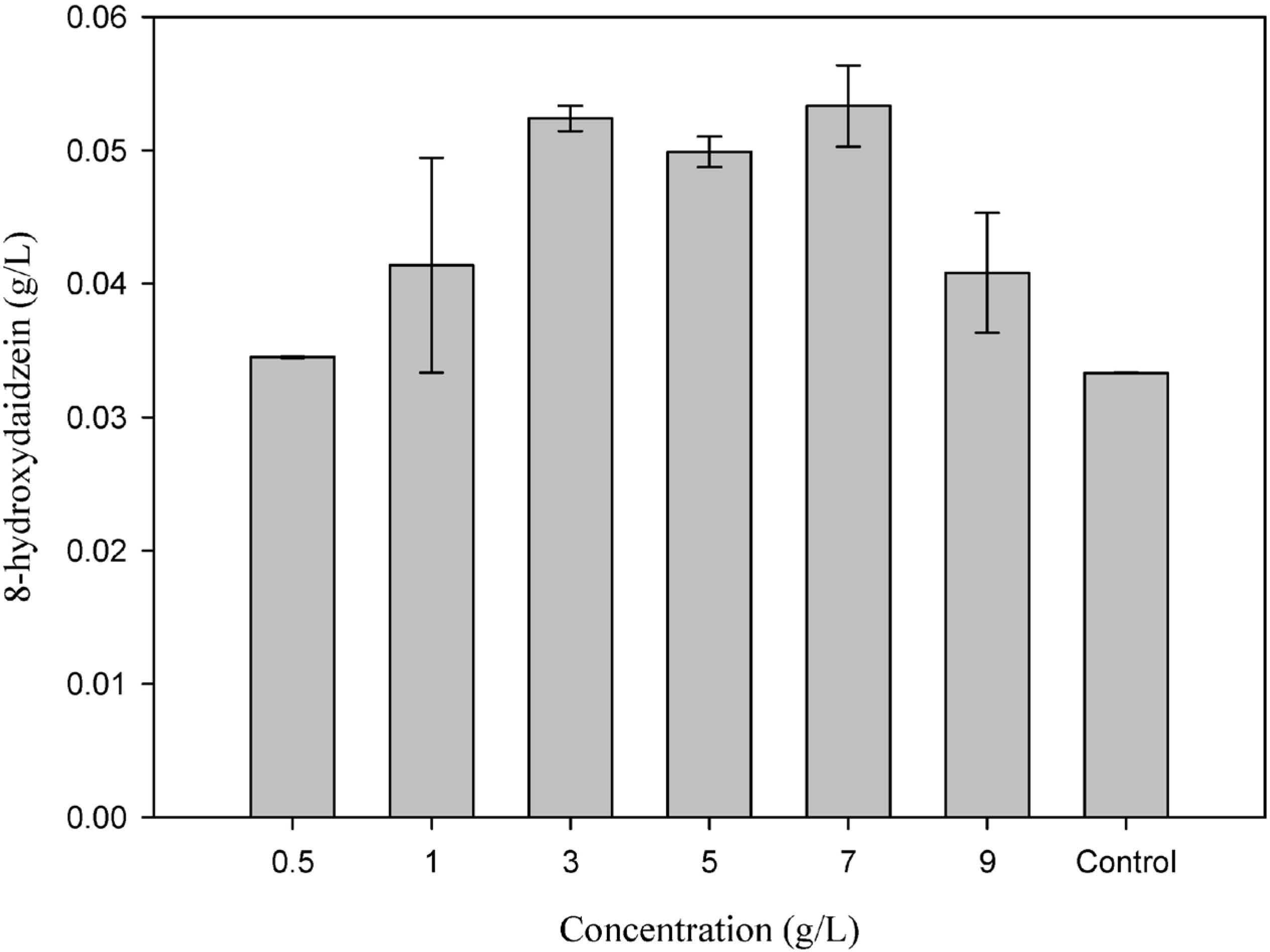
Effects of different concentrations of MgSO4 on the biotransformation of daidzein into 8-hydroxydaidzein.
Effect of daidzein To test the biotransformation ability, different daidzein concentrations were added as the substrate, and the optimal concentration was found (Figure 5). The highest amount of 8-hydroxydaidzein was produced with a 0.2 g/L daidzein concentration, while the product concentration was reduced when too much daidzein was added. It was speculated that these differing production rates may have been due to the substrate inhibition effect on cell growth or biotransformation efficiency. To test these hypotheses, the cell dry weights were measured on the final day of culture, and the results showed that the dry weights of the A. oryzae at different daidzein concentrations 0.1 to 0.3 g/L were the same 6.0 (g/L) (Figure 5). Compared to the dry weight production in 0.2 g/L, the results were 35.0% higher for lower daidzein concentration 0.05 g/L and 13.3% lower for highest daidzein concentration 0.4 g/L, indicating daidzein may change the growth characteristics of A. oryzae. Moreover, daidzein may also influence the metabolism of daidzein into 8-hydroxydaidzein since the dry cell weight was the same at daidzein concentration 0.1 to 0.3 g/L. On the other hand, the biotransformation efficiencies obtained for the differing daidzein concentrations were also shown in Figure 5. The efficiencies were significantly decreased with the increasing daidzein concentrations. The highest catalytic efficiency, then, for converting daidzein to 8-hydroxydaidzein (77.8%) was obtained when the substrate concentration was 0.05 g/L.

Amounts of 8-hydroxydaidzein produced (bar), conversion rate (closed circles) and dry cell weight (closed triangles) via biotransformation of different amounts of the daidzein substrate.
The production of 8-hydroxydaidzein from soybean extract was investigated by Seo et al. (2013) recently using selected A. oryzae KACC 40247 from 60 fungal strains. Under optimal culture conditions, 0.062 g/L of 8-hydroxydaidzein from 0.106 g/L of daidzin plus daidzein at a conversion yield of 58% (w/w) can be obtained in the fermenter. In this study, the conversion is much higher than the highest value 36.3% obtained by Pandey et al. (2011) and 58% obtained by Seo et al. (2013). Therefore, we can obtain high biotransformation efficiency of daidzein to 8-hydroxydaidzein under certain conditions using an A. oryzae culturing system.
Hydroxylation by biotransformation was systematically investigated through the hydroxylation of daidzein into 8-hydroxydaidzein. The target compound 8-hydroxydaidzein is usually produced through the biotransformation of soybean using certain specific microorganisms, but the amount produced is low. Since the applications of 8-hydroxydaidzein are wide-ranging, however, the details of its production are well worth systematic investigation. The results of the present study indicate that the optimal culture conditions for maximum production of 8-hydroxydaidzein was at 200 rpm of rotating speed and culture medium at pH 6. Furthermore, the addition of MgSO4 to the medium in the optimal concentration enhanced 8-hydroxydaidzein production with 57.4% increased. Overall, the 8-hydroxydaidzein production was improved from 0.024 g/L to 0.052 g/L that about 117% production was increased. Moreover, a high biotransformation efficiency of 77.8% and maximum 8-Hydroxydaidzein production 0.052 g/L were achieved under different special culture conditions. Therefore, the biotransformation of 8-hydroxydaidzein can be performed through a highly selected catalysis under specified conditions using certain microorganisms.
Acknowledgment We are very grateful for the financial support of grant from the Fooyin University, Taiwan (FYU1300-102-18).