2015 Volume 21 Issue 4 Pages 623-630
2015 Volume 21 Issue 4 Pages 623-630
The wild yeast Saccharomyces cerevisiae AK46 was isolated from cherry fruits and commercialized as baker's yeast. We isolated a 2-deoxyglucose (2-DOG)-resistant mutant of AK46 (MCD4) and evaluated its leavening ability in bread dough. Our findings show that the maltose utilization of MCD4 was significantly enhanced in the presence of glucose and α-glucosidase activity was increased by approx. 1.6-fold compared to that of AK46, indicating release from catabolite repression. In addition, baking performance results using the sponge dough and no-time dough methods showed that the CO2 production rate of MCD4 was increased by approx. 1.6-fold (sponge and dough method) and the specific volume of bread was increased by 6.3% and 7.8%, respectively. These results demonstrate that the 2-DOG resistance exhibited by MCD4 significantly improved the leavening ability in bread dough.
Bread dough is made from wheat or cereal flour and auxiliary materials such as water, salt, sugar and fat. Yeast is added to expand the dough by fermentation, and the dough is then baked at a high temperature to retain the expanded configuration. In the breadmaking process, yeast provides the characteristic taste and flavor of bread, attributable to the alcohols, organic acids, amino acids, esters and other volatile compounds produced during the degradation and conversion of flour and auxiliary materials. Yeast also contributes to the characteristic texture of bread by promoting the expansion and maturation of dough during fermentation. The most important property of bread yeast is its ability to expand the dough, which is related to its carbon dioxide (CO2) production through the glycolytic pathway.
Wild yeasts are widely distributed in nature; they are present in fruits, nectars, tree saps, soils, active sludge, seawater and insect guts. The yeasts employed in the brewing of beer and sake, and breadmaking are called industrial yeasts (Oda and Ouchi, 1989b; Sujaya et al., 2011). Industrial yeasts suitable for practical use have been developed and selected over a very long period of time, and most of them are classified as Saccharomyces cerevisiae. Yeasts used for commercial bread have higher metabolic activities for maltose and sucrose compared to wild yeasts, and these qualities are used to expand a wide variety of dough types. However, the metabolic activity for maltose of wild yeasts is much lower than for commercial bread yeasts (Bell et al., 2001).
The wild yeast S. cerevisiae AK46 was isolated from cherry fruits in the Tokachi district of Hokkaido, Japan (Oda et al., 2010a). This strain has a high leavening ability in dough with 30% sucrose, indicating that AK46 strain is suitable for breadmaking. However, its baking performance using the sponge and dough method is inferior to that of commercial bread yeast (Oda et al., 2010a). Mutants of S. cerevisiae capable of concurrent metabolization of both glucose and maltose via the release of catabolite repression were obtained among 2-deoxyglucose (2-DOG)-resistant cells (Novak et al., 1990; Randez-Gil and Sanz, 1994). In addition, it was reported that the leavening ability of sweet dough was improved by using 2-DOG-resistant baker's yeast (Rincon et al., 2001). In the present study, we isolated a 2-DOG-resistant mutant of S. cerevisiae AK46, and observed that this mutant (strain MCD4) significantly improved the leavening ability of bread dough by increased utilization of maltose in the presence of glucose, via the release of catabolite repression.
Yeast strains and culture conditions Saccharomyces cerevisiae AK46 strain was isolated from cherry fruits (Ezoyamazakura) in the Tokachi district of Hokkaido, Japan (Oda et al., 2010a), and S. cerevisiae HP216 strain was isolated from commercial baker's yeast as a reference strain (Oda et al., 2010b). Each yeast strain was grown aerobically in 10 mL of YPD medium composed of 1.0% yeast extract, 2.0% polypeptone and 2.0% glucose at 30°C for 24 h in a reciprocal shaker (150 rpm). The whole broth culture was inoculated to 120 mL of YPS medium composed of 2.0% Bacto™ Yeast Extract (Becton, Dickinson and Company, NJ), 4.0% Bacto™ Peptone (Becton, Dickinson and Company, NJ), 2.0% sucrose, 3.0% NaCl, 0.2% KH2PO4 and 0.1% MgSO4·7H2O. After incubation at 30°C for 40 h in a reciprocal shaker, cultured cells were harvested by centrifugation, washed twice with sterilized distilled water, and then suspended in distilled water to make the yeast cell suspension or placed on a porous plate to make a cake yeast containing 33% (w/w) cells as dry matter.
Isolation and selection of strain AK46 mutant 2-DOG mutants were isolated using the method of Oda and Nakamura (Oda and Nakamura, 2009); however, the 2-DOG concentration was decreased to ≤ 0.1% because of the sensitivity of S. cerevisiae to 2-DOG. The parental strain, S. cerevisiae AK46, was grown aerobically in 10 mL of YPD medium at 30°C for 24 h in a rotary shaker (150 rpm). Cultured cells were harvested from the whole broth culture by centrifugation, washed twice with sterilized distilled water, and resuspended in 20 mL of sterilized distilled water. Then, 0.1 mL of the cell suspension was inoculated into minimal maltose agar medium composed of 0.08% 2-deoxyglucose (2-DOG: Sigma-Aldrich, MO), 2.0% maltose, and 0.67% Yeast Nitrogen Base without amino acids (Becton, Dickinson and Company, NJ), and grown at 30°C for 10 d.
Five colonies formed on the agar medium were subcultured twice on minimal maltose agar medium containing 0.09% 2-DOG and then grown on minimal maltose agar medium with 0.10% 2-DOG to obtain five 2-DOG-resistant mutants (strains MCD1, MCD2, MCD3, MCD4, and MCD5). The MCD4 mutant, which exhibited higher cell yield and leavening ability in bread dough, was selected from the resultant five mutants.
Consumption of glucose and maltose One gram of yeast cells as dry matter was inoculated into 50 mL of GM medium composed of 1.0% glucose, 7.0% maltose, 0.5% KH2PO4, and 0.5% (NH4)2HPO4 in a 200-mL Erlenmeyer flask and incubated at 30°C in a rotary shaker (90 rpm). A portion of the culture was withdrawn, and after centrifugation at 15,000 g for 10 min, the amounts of residual glucose and maltose in the supernatant were determined by high-performance liquid chromatography (LaChrom Elite; Hitachi High Technologies, Tokyo) equipped with a packed column (Shodex KS-801; Showa Denko, Tokyo) and refractive index detector.
Fermentative ability Fermentative ability in the liquid medium was measured according to the modified method of the Japan Yeast Industry Association. Briefly, 5 mL of cell suspension containing 200 mg of yeast cells as dry matter was inoculated into 20 mL of liquid medium composed of 8.0% maltose (M8) or 10.0% sucrose (F10) as the carbon source, 0.3% NaH2PO4·2H2O, 0.2% MgSO4·7H2O, 0.08% KCl, 0.002% thiamine, 0.002% pyridoxine and 0.02% nicotine in a 100-mL Erlenmeyer flask and incubated at 30°C for 3 h in a reciprocal shaker (80 rpm). Immediately before and after the incubation, the weight of the flask was measured and the decrease in weight during incubation was calculated as the fermentative ability in liquid medium.
Enzyme assays The invertase activity was determined by the modified method of Oda et al. (Oda et al., 2010a). One milliliter of the yeast cell suspension was added to 1.0 mL of 0.1 M citrate buffer (pH 5.5) containing 5.0% sucrose and incubated at 30°C for 3 min. After the reaction was stopped by the addition of 2.0 mL of 1 N NaOH, the reaction solution was centrifuged and 1 mL of the supernatant was mixed with 1 mL of 3,5-dinitrosalicylic acid (DNS; Nacalai Tesque, Kyoto) coloring reagent, followed by heating at 100°C for 5 min. After cooling on ice, 10 mL of demineralized water was added and the amounts of reducing sugars were spectrophotometrically determined at an absorbance of 540 nm. Specific activity was expressed as nmoles of reducing sugars per min per mg of cells as dry matter.
The α-glucosidase activity was determined by the method of Oda et al. (Oda et al., 2010a) except that ultrasonic breaking was used in place of chloroform and SDS treatments to obtain the cell-free extract (Suzuki and Tamura, 1984). The cell-free extract was prepared by sonication and then filtered (pore size, 0.2 µm). A portion of the extract (0.5 mL) was added to 0.5 mL of 10 mM p-nitrophenyl-α-D-glucopyranoside (Nacalai Tesque, Kyoto) solution and incubated at 25°C for 3 min. The reaction was stopped by the addition of 5 mL of 0.1 M Na2CO3 solution and the released p-nitrophenol was determined at an absorbance of 410 nm. The specific activity of α-glucosidase was expressed as nmoles of p-nitrophenol per min per mg of cells as dry matter.
Baking performance Breads were baked using the sponge dough and no-time dough methods. For the sponge and dough method, based on the baker's percent, 70% flour (Eagle; Nippon Flour Mills Co., Tokyo), 2% cake yeast, 0.1% yeast food (20% NH4Cl, 20% CaSO4, 10% CaCO3, 2% L-cystine, 1% α-amylase, 1% protease, 0.6% L-ascorbic acid, 45.4% mixture of flour, cornstarch, salt, malt powder; Oriental Yeast, Tokyo) and 40% water were combined to make a sponge dough and fermented at 28°C for 4 h. After the remaining ingredients (30% flour, 5% sugar, 2% salt, 5% shortening, 2% powdered skim milk and 25% water) were mixed with the sponge dough, the resulting dough was left to rest at 28°C for 20 min as floor time, divided into 450-g portions, left to rest at 28°C for 20 min as bench-time, molded, proofed at 38°C and 85% humidity for 55 min, and then baked at 210°C for 20 min.
For the no-time dough method, the dough was made by mixing all of the ingredients together. That is, 100% flour as described above, 4% cake yeast, 0.15% yeast food, 0.4% malt extract, 4% sugar, 2.2% salt, 6% shortening, 5% powdered skim milk and 69% water were mixed, fermented at 28°C for 20 min, divided into 450-g portions, left to rest at 28°C for 20 min as bench-time, molded, proofed at 35°C and 85% humidity for 55 min, and then baked at 200°C for 25 min. The specific volume was calculated from volume (mL) per weight (g) of baked bread.
The leavening ability (CO2 [mL] produced from a 40-g piece of dough) of the sponge dough, final dough and no-time dough was monitored at 5 min intervals using a Fermograph II (Atto Corp., Tokyo) according to the instruction manual. For measurement of the leavening ability in unsugared dough, 100 g flour, 2.86 g cake yeast and 57.1 mL water were mixed and the dough was divided into 40-g pieces. The leavening ability was then measured at 28°C for 3 h.
Comparison of DNA and amino acid sequences MCD4 and AK46 were grown in YPD medium at 30°C for 24 h in a rotary shaker, and the cells were then collected by centrifugation at 15,000 g for 10 min at 4°C. Chromosomal DNA in MCD4 and AK46 cells was extracted using a ZR Fungal/Bacterial DNA kit (ZYMO Research, Irvine, CA), and DNA sequences were determined by a next-generation sequencer (Illumina Genome Analyzer IIX system; Illumina, San Diego, CA) in combination with the software program Tablet for next-generation sequence assembly visualization (Milne et al., 2009). To identify the locations of the substituted amino acids of proteins related to maltose metabolism and catabolite repression, we used the multiple sequence alignment editor Bioedit (Ibis Biosciences, Carlsbad, CA) and the BLAST database (http://blast.ncbi.nlm.nih.gov/).
Isolation and selection of mutant AK46 strain It was reported that the 2-DOG mutation led to the utilization of both glucose and maltose via release of catabolite repression, resulting in improved leavening ability of dough (Rincon et al., 2001). In the present study, we isolated 2-DOG-resistant mutants of the wild strain AK46 with improved leavening ability in bread dough (as described in the Materials and Methods) and obtained five spontaneous 2-DOG-resistant mutants (MCD1, MCD2, MCD3, MCD4, and MCD5), among which MCD3 and MCD4 showed almost the same cell yield as the parental strain AK46. These two mutants were investigated for leavening ability in bread dough with or without sucrose (Table 1) and the results showed that both mutants had approximately the same leavening ability values in dough; however, the levels tended to be higher than those of AK46 (Table 1). Since the levels of MCD4 tended to be slightly higher than those of MCD3, we selected MCD4 for use as the mutant strain of AK46 in further tests.
| Strain | Cell yield (g dry wt / L) |
Leavening ability [CO2 (mL) / 2h / 10 g flour] |
|
|---|---|---|---|
| −sucrose | + sucrose (5%) | ||
| AK46 | 10.7 ± 0.1a | 35.2 ± 6.0b | 39.9 ± 9.3a |
| MCD1 | 4.1 | N.D | N.D |
| MCD2 | 3.7 | N.D | N.D |
| MCD3 | 10.3 ± 0.6a | 43.9 ± 6.7ab | 50.8 ± 2.9a |
| MCD4 | 10.2 ± 0.4a | 49.3 ± 4.2a | 51.5 ± 4.5a |
| MCD5 | 8.9 ± 0.4b | N.D | N.D |
The leavening ability in dough was measured as follows (Oda et al., 2010a). Briefly, 10 g flour, 0.2 g yeast cake and 5.5 mL water were mixed with or without sucrose (5%), and CO2 production was measured at 30°C for 2 h.Values are the means ± S .D. of three replicates.
Cell yield of MCD1 and MCD2 was tested once.
In each column, means not followed by the same letter differ significantly at the 5% level according to Student's t-test.
N.D. = not determined.
Maltose utilization by MCD4 in the presence of glucose The parental strain AK46, MCD4 and the commercial baker's yeast HP216 were grown in the presence of both maltose and glucose, and the residual amounts of these sugars in the medium were compared over the 180-min incubation (Fig. 1). The 2-DOG-sensitive strains (AK46 and HP216) showed a similar tendency for sugar consumption during incubation, wherein maltose was immediately consumed after the complete utilization of glucose within 60 min. The residual amounts of maltose in the medium decreased gradually after the consumption of glucose; however, the residual concentration was still approx. 5% at 180-min incubation, indicating that catabolite repression had a functional role in the metabolism of sugars (Fig. 1).
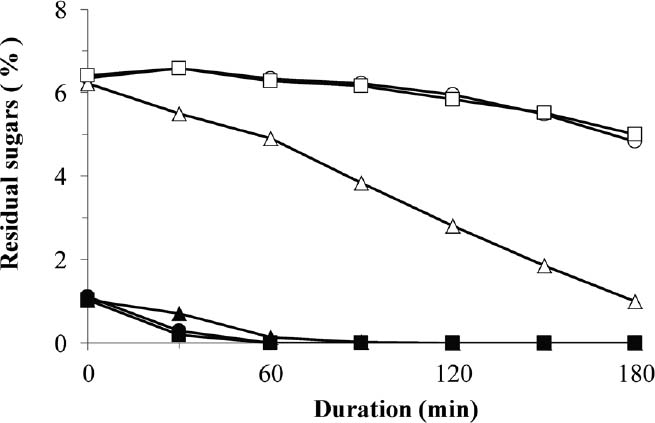
Residual maltose and glucose concentrations in the medium of AK46, MCD4 and HP216.
AK46, MCD4 and HP216 strains were grown in GM medium at 30°C, 90 rpm for 180 min and a portion of the medium was withdrawn every 30 min to measure the residual maltose (open symbols) and glucose (closed symbols) concentrations (%) as described in the Materials and Methods. Symbols: ○, ●: AK46; ▵, ▴: MCD4; ■ , □: HP216.
However, the 2-DOG-resistant strain (MCD4) consumed both glucose and maltose simultaneously during incubation, i.e., even in the presence of glucose, the residual amounts of maltose started to decrease immediately upon incubation and the residual concentration decreased considerably to only approx. 1% at 180-min incubation, indicating that catabolite repression was released in MCD4 (Fig. 1). These results demonstrated that MCD4 acquired a much higher capacity to utilize maltose, even in the presence of glucose, compared to AK46 and HP216.
Properties required for baker's yeast Table 2 shows the cell yield, discharge of CO2 from the liquid medium, and the α-glucosidase and invertase activities of MCD4, AK46 and HP216. Results showed that the cell yield of MCD4 was comparable to that of AK46 and HP216, and the level was approx. 10 g dry weight per L, indicating that MCD4 exhibited sufficient growth as baker's yeast.
| Strain | Cell yield (g dry wt / L) |
Fermentative ability (decrease in weight (g)) |
Enzyme activity (U / mg dry wt of cell) |
||
|---|---|---|---|---|---|
| F10 | M8 | α-Glucosidase | Invertase | ||
| AK46 | 10.1 ± 0.4a | 279.2 ± 13.4a | 29.3 ± 3.8a | 28.7 ± 0.7a | 74.1 ± 23.2a |
| MCD4 | 10.4 ± 0.4a | 312.5 ± 13.7b | 111.1 ± 8.2c | 45.1 ± 4.8b | 70.5 ± 33.9a |
| HP216 | 10.7 ± 0.1a | 437.1 ± 13.4c | 48.7 ± 4.9b | 24.3 ± 4.2a | 1248.4 ± 179.0b |
Fermentative ability was calculated from the decrease in weight (g) after 3 h of incubation with 10% sucrose (F10) or 8% maltose (M8).
Values are the means ± S .D. of three replicates.
In each column, means not followed by the same letter differ significantly at the 5% level according to Student's t-test.
We evaluated the fermentative ability of MCD4 in M8 medium by the decrease in the weight of the culture as discharged CO2. Results showed that the amounts of CO2 discharged from MCD4 were significantly (approx. 3.8- and 2.3-fold) higher compared to the amounts discharged from AK46 and HP216, respectively. In addition, the α-glucosidase activity of MCD4 was approx. 1.6- and 1.9-fold higher than for AK46 and HP216, respectively. These results indicated that the ability of MCD4 to ferment maltose was markedly improved by the enhanced α-glucosidase activity (Table 2). The fermentative ability of MCD4 in F10 medium was observed to be slightly higher than that of AK46, but there was no significant difference in invertase activities between the strains, indicating that there was no correlation between invertase activity and the ability to ferment sucrose (Table 2). It is well known that both α-glucosidase and invertase are important for baking performance (i.e., fermentative ability of dough, specific volume of bread). However, the baking performance of yeast is not only determined by these enzyme activities. Particularly, it was reported that there was an inverse correlation between invertase activity and carbohydrate resistance of yeast in high-sugar doughs. Moreover, the fermentative ability of yeast having high invertase activity was repressed by the increase of osmotic pressure in dough caused by the breakdown of sucrose (Sato, 1966).
We obtained haploid cells from MCD4 and tested their susceptibility to 2-DOG by tetrad analysis, according to the method of Sherman and Hicks (Sherman and Hicks, 1991). The results showed that haploid MCD4 was composed of 2-DOG-resistant and -sensitive haploids at a ratio of approx. 1:1, wherein the 2-DOG-resistant haploid showed significantly higher ability to ferment maltose compared to the 2-DOG-sensitive haploid, indicating that the acquisition of 2-DOG resistance led to improved maltose fermentative ability via increased α-glucosidase activity (data not shown). Oda and Ouchi (1989a) reported the correlation between α-glucosidase activity in yeast and the leavening ability of maltose derived from starch by wheat amylase in dough without the addition of sucrose. However, we observed that the ability of AK46 to ferment maltose was lower than HP216 even though these strains exhibited similar α-glucosidase activities, suggesting that the ability to ferment maltose is influenced by maltose permease and maltose metabolism in the glycolytic pathway.
Baking performance Figure 2 shows the leavening ability (CO2 production rate) of unsugared dough and that obtained by the various bread production processes (sponge dough, final dough and no-time dough methods) using MCD4, AK46 and HP216. The results for the unsugared dough showed that the leavening ability of MCD4 was considerably higher than that of AK46 during all leavening periods, and the rate was significantly higher compared to that from HP216 at 30 to 150 min after the onset of leavening (Fig. 2A). Results of the sponge and dough method showed similar leavening ability; MCD4 exhibited higher leavening than AK46 during the entire leavening period and reached approx. 1.6-fold of AK46 within 1 h of leavening (Fig. 2B). In particular, the level was significantly higher than that for HP216 from 30 to 150 min of leavening (Fig. 2). These results demonstrated that MCD4 showed improved leavening ability in sponge dough as well as in unsugared dough.
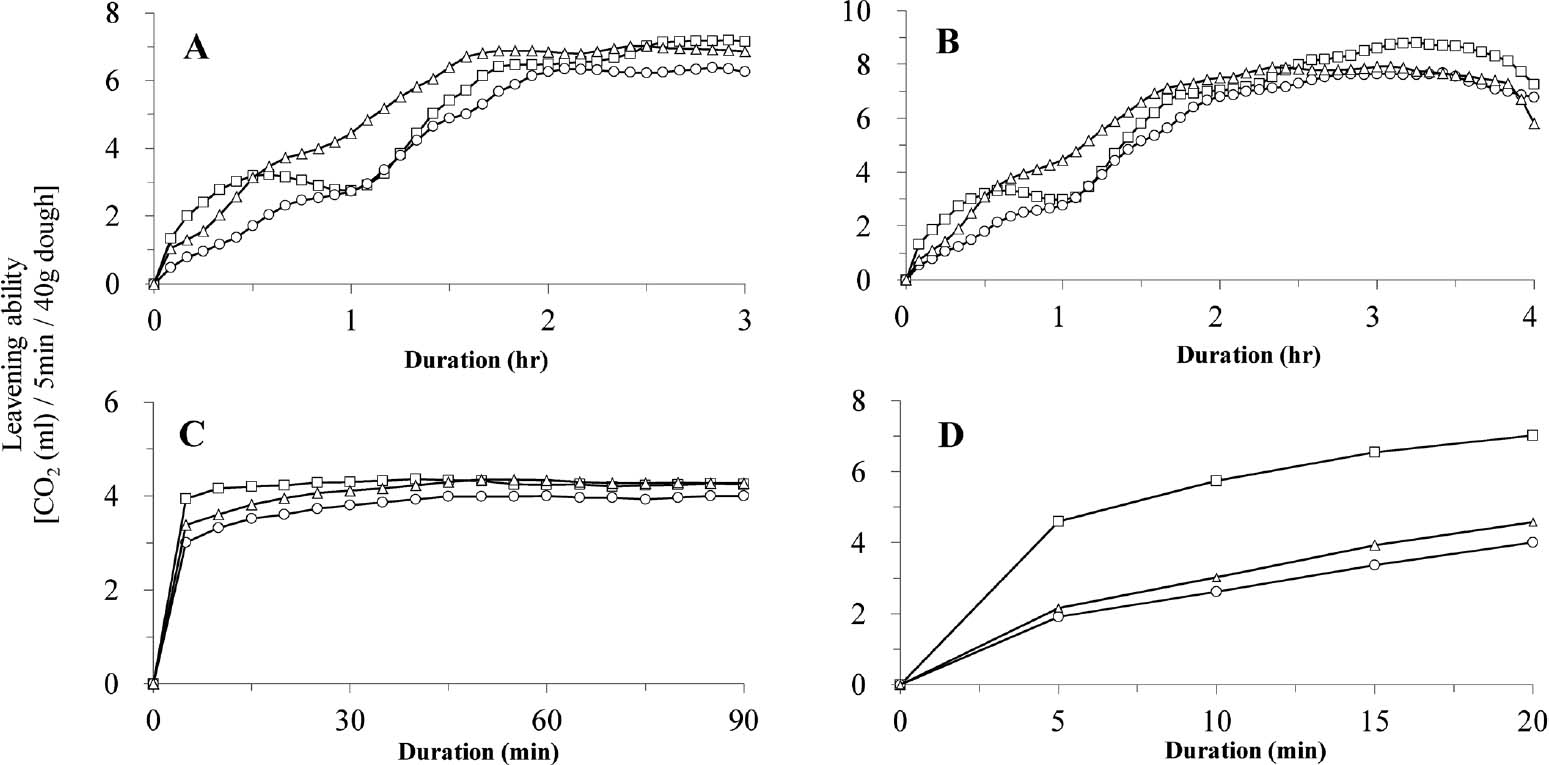
Leavening ability of AK46, MCD4 and HP216 in unsugared dough (A), sponge dough (B), final dough (C) and no-time dough (D). Leavening ability (CO2 production rate from each dough: mL/5 min/40 g dough) was monitored by Fermograph II as described in the Materials and Methods. Values are the means of three replicates. Symbols: ○, AK46; △, MCD4; □, HP216.
Profiles of leavening ability in the unsugared dough and the sponge dough with HP216 showed two conspicuous peaks; the first at 0–1 h and the second at 1–2 h of leavening (Fig. 2A,B). The first peak was only shown by HP216 and is thought to represent the fermentation of slight amounts of sugars, such as monosaccharide and fructosides, present in the flour before the maltose fermentation. It was reported that this first peak was detected by the addition of invertase or the use of yeast strains with high invertase activity (Tanaka and Sato, 1969). Thus, the first peak might not have been exhibited by both MCD4 and AK46 because of their considerably low invertase activities compared to HP216 (Fig. 2A,B).
The second peak, on the other hand, was possibly caused by maltose fermentation, and was shown by both MCD4 and AK46, although their peaks were not sharp. In addition, the results obtained using the sponge dough (i.e., final dough) and no-time dough methods also showed that the CO2 production rate of MCD4 tended to be higher than that of AK46 during the entire leavening period, although these rates were below those of HP216 (Fig. 2C,D). These results prompted our evaluation of the baking performance of MCD4 by the sponge dough and no-time dough methods.
For the sponge and dough method, the sponge dough, composed of flour, water and yeast, is fermented first and then mixed with the remaining ingredients. Advantages of this method include improved bread mechanical tolerance by increased dough extensibility and flexibility with delayed bread staling; this method is used in industrial mass production. On the other hand, for the no-time dough method, all ingredients are mixed simultaneously, shortening the required time for breadmaking via a drastic reduction in fermentation time.
Results of bread production and images of cross-sections are shown in Table 3 and Figure 3, respectively. For the sponge and dough method, the specific volume of the bread using MCD4 (5.4 ± 0.09 mL/g) was significantly higher than that of AK46 (5.08 ± 0.06 mL/g) and the value tended to increase compared to HP216 (5.32 ± 0.10 mL/g) (Table 3, Fig. 3). In a previous study, bread was produced by the sponge and dough method using AK46, in which the amount of cake yeast was 1.2-fold higher than that used for HP216; however, the specific volume provided by AK46 was still lower than that for HP216 (Oda et al., 2010a). These results demonstrated that the 2-DOG-resistant mutant MCD4 could significantly improve the baking performance of AK46 and produced similar or better results compared to the commercial strain HP216. For the no-time dough method, the specific volume using MCD4 (5.10 ± 0.07 mL/g) tended to increase compared to AK46 (4.73 ± 0.25 mL/g); however, the value was lower than that of HP216 (5.96 ± 0.10 mL/g) (Table 3, Fig. 3). Since this method requires a yeast strain that can exert strong leavening activity in a short period of time, it seems that the lower CO2 production rates of MCD4 and AK46 from the dough in the initial stage of fermentation are responsible for the decrease in specific volume of the bread compared to HP216 (Fig. 2D).
| Baking Method | Strain | Volume (mL) (A) |
Weight (g) (B) |
Specific Volume (A / B) |
|---|---|---|---|---|
| Sponge Dough | AK46 | 2035.4 ± 66.9 | 400.8 ± 8.3 | 5.08 ± 0.06a |
| MCD4 | 2143.2 ± 44.8 | 397.1 ± 7.3 | 5.40 ± 0.09b | |
| HP216 | 2124.9 ± 40.5 | 399.2 ± 9.0 | 5.32 ± 0.10b | |
| No-Time Dough | AK46 | 1892.2 ± 84.1 | 399.9 ± 3.8 | 4.73 ± 0.25c |
| MCD4 | 2025.3 ± 18.7 | 397.3 ± 4.1 | 5.10 ± 0.07c | |
| HP216 | 2330.1 ± 20.2 | 391.2 ± 4.7 | 5.96 ± 0.10d |
Values are the means ± S .D. of three replicates.
Means not followed by the same letter differ significantly at the 5% level according to Student's t-test.

Images of cross-sections of breads produced using sponge dough (A) and no-time dough (B) methods.
Breads were baked using sponge dough and no-time dough methods with AK46, MCD4 and HP216 as described in the Materials and Methods.
Comparison of DNA and amino acid sequences between MCD4 and AK46 The DNA and amino acid sequences related to maltose metabolism and catabolite repression were compared between MCD4 and AK46. Five MAL loci (MAL1, 2, 3, 4 and 6) were identified in S. cerevisiae strains, and the presence of at least one of these was required for maltose fermentation (Charron et al., 1989; Higgins et al., 1999; Novak et al., 2004). Each MAL locus was composed of three genes (gene 1, maltose permease; gene 2, α-glucosidase; gene 3, maltose activator) and functions of these genes were required for maltose fermentation (Charron et al., 1986; Naumov et al., 1994).
The results of our DNA sequence analyses showed that the AK46 strain had two MAL loci (MAL1 and 3) with three well-known regulator genes (MIG1, SNF1, and TUP1) (Hu et al., 1995; Treitel and Carlson, 1995). The MAL1 locus was located on chromosome VII and was composed of three MAL genes (MAL11, MAL12, and MAL13), while the MAL3 locus was located on chromosome II and was composed of three MAL genes (MAL31, MAL32, and MAL33). Compared with the DNA sequence of AK46, MCD4 had some mutations only for MAL11, MAL13, MAL31 and MAL33 genes. Amino acid sequence analysis indicated that a certain level of amino acid substitutions and deletions also occurred in these MAL gene products (Table 4). Since none of the mutated sequences were involved in the active domain, the structural modification could have occurred in these Mal proteins. We suspect that MCD4 contains a deletion mutation causing a translation stop at the 594th position in Mal11, the 319th position in Mal13, and the 379th position in Mal 31 (Table 4). Since Mal13 acts as a maltose activator protein for both Mal11 (maltose permease) and Mal12 (α-glucosidase), these results indicate the possibility that maltose permease and/or α-glucosidase activity were enhanced in a manner that caused the structural modification of this activator protein. Further studies at the molecular level are thus needed to clarify the mechanism underlying the increase of maltose utilization by MCD4.
| Proteins | Description | Number of total amino acids | Amino acid substitutions* |
|---|---|---|---|
| Maltose metabolism | |||
| Mal11 | sugar transporter | 616 | H591L /D592I /S593R /I594X** |
| Mal12 | alpha-glucosidase | 584 | none |
| Mal13 | activator | 473 | T299I /T318A /S320X /N327Y /T330I,V / S333A /R336W /R337H /I341V /N361R / G362A /Q363H /I364V /R370S /E381D,K,N /D385E,G /V391I /V393A,I,M,T /T395A / L396I /I398V /T400N |
| Mal31 | sugar transporter | 614 | H49R /A122S /S146P /Q166H /M175L / Q261T /A265P /E268N /E339K /T349S / V354L /G357S /I358V /C374I,S /S375T / A378T /S379P,Q,X /S394G /V508A,I,T / T509R,S /K526L /F534L /L536F /A540V / V544I |
| Mal32 | alpha-glucosidase | 584 | none |
| Mal33 | activator | 468 | S240A /V243I /H244D,K,Q /Q257L /F260V /D269E /F272L /M274V /F286Y /E292V / K305R /K308N /A313T /L315H /E316D / I327F /F329C /S330F,L /H332P /A336T / F343L /Q344H /N346K /K365R /D369E,G / I371M,T /S390A /V393I /K403Q /Y404H / H406K,N,Q |
| Catabolite repression (regulator) |
|||
| Mig1 | transcription factor | 504 | none |
| Snf1 | carbon catabolite derepressing protein kinase | 633 | none |
| Tup1 | glucose repression regulatory protein | 713 | none |
Strain MCD4, a 2-DOG-resistant mutant of the wild yeast S. cerevisiae AK46, showed enhanced maltose utilization in the presence of glucose, indicating release from catabolite repression. MCD4 significantly improved baking performance using the sponge and dough method, as shown by the increase in leavening ability.
Acknowledgements We are very grateful to Dr. Yuji Oda of Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Hokkaido, Japan for critical reading of the manuscript, thoughtful discussions and advice.