2015 Volume 21 Issue 5 Pages 695-704
2015 Volume 21 Issue 5 Pages 695-704
Free radical scavenging activities of two series of tripeptide libraries were investigated using ABTS, DPPH, and ORAC assays. Two Tyr-containing tripeptides showed higher scavenging activities against the hydrophilic ABTS and AAPH radicals than two His-containing tripeptides, showing that Tyr residues play important roles in free radical scavenging activity. Higher concentrations of tripeptides were required to reveal apparent DPPH radical scavenging activity. The antioxidant activities of the tripeptide libraries obtained from this study were compared with previous results obtained using several assays, including antioxidant activity against the peroxidation of linoleic acid, FRAP assay, and peroxynitrite (PN) scavenging activity. The antioxidant activity of the tripeptides against the peroxidation of linoleic acid showed high correlations with the ABTS and ORAC assays (correlation coefficients (R) = 0.725 and 0.731, respectively), and low correlations with FRAP, PN scavenging, and DPPH assays. The highest correlation was found between the ABTS and ORAC assays (R = 0.905).
After the enzymatic digestion of various protein sources, such as plant, animal and food by-products, a number of antioxidant peptides have been isolated and identified (Elias et al., 2008; Hartmann and Meisel, 2007; Sarmadi and Ismail, 2010; Bernardini et al., 2011; Cavazos and Gonzales de Mejia, 2013; Udenigwe and Aluko, 2012; Samaranayaka and Li-Chan, 2011). Although proteins themselves can interact with reactive oxygen species and free radicals to show antioxidant activity, the peptides typically exhibit higher activities than intact proteins due to enhanced accessibility of amino acid residues capable of scavenging free radicals and chelating prooxidative metals (Elias et al., 2008). It is generally accepted that the nucleophilic sulfur-containing amino acids, cysteine (Cys, C) and methionine (Met, M), the aromatic amino acids, tryptophan (Trp, W), tyrosine (Tyr, Y), and phenylalanine (Phe, F), and the imidazole-containing amino acid, histidine (His, H), play important roles in the antioxidant activity (Davies, 2005; Stadtman and Levine, 2003). However, free amino acids are not effective as antioxidant peptides in many cases (Zhou and Decker, 1999). The higher antioxidant activity of peptides compared to free amino acids is attributed to the unique chemical and physical properties conferred by their amino acid sequences, especially the stability of the resultant peptide radicals, which do not initiate or propagate further oxidative reactions (Elias et al., 2008).
A number of different assays based on various principles have been used in the screening for antioxidant peptides and protein hydrolysates (Elias et al., 2008; Hartmann and Meisel, 2007; Sarmadi and Ismail, 2010; Bernardini et al., 2011; Cavazos and Gonzales de Mejia, 2013; Udenigwe and Aluko, 2012; Samaranayaka and Li-Chan, 2011). Lipid peroxidation inhibition assays have been frequently used by using of the ferric thiocyanate method (Mitsuda et al., 1966). Although this method is reliable and practical, measurements are time consuming. In recent years, radical scavenging assays based on free radicals or free radical generating compounds, such as 2,2-azo-bis(2-methylpropionamidine) dihydrochloride (AAPH) (Cao et al., 1993; Ou et al., 2001), 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Miller et al., 1993; Re et al., 1999) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) (Bondet et al., 1997; Cheng et al., 2006) have gained popularity because of their ease of use and convenience. The oxygen radical absorbance capacity (ORAC) assay measures the ability of a free radical scavenger to quench peroxyl radicals generated by AAPH and protect the fluorescent marker, fluorescein (Cao et al., 1993; Ou et al., 2001).
Six antioxidant peptides were isolated from a protease digest of soybean protein (Chen et al., 1995). The peptides were composed of 5 – 16 amino acid residues, including hydrophobic amino acids, valine (Val, V) or leucine (Leu, L), at the N-terminal positions, and proline (Pro, P), His, or Tyr in the sequences. On the basis of the smallest peptide, Leu-Leu-Pro-His-His (LLPHH), which was derived from a soy protein hydrolysate, structurally related peptides were synthesized (Chen et al., 1996). Their antioxidant activities against the peroxidation of linoleic acid were compared in an aqueous system, and Pro-His-His (PHH) was found to be the most active among the peptides tested (Chen et al., 1998). For further exploration of antioxidant properties of peptides, two series of combinatorial tripeptide libraries were constructed, based on an antioxidant peptide isolated from a soybean protein hydrolysate (Saito et al., 2003). One was a library of tripeptides containing either two His or two Tyr residues. The other was a library of tripeptides related to PHH, which had been identified as an active core of the antioxidant peptide. The antioxidant properties of these libraries were examined by several assays, such as the antioxidant activity against linokeic acid peroxidation, ferric reducing antioxidant power (FRAP) assay, ABTS assay, and peroxynitrite (PN) scavenging assay. Two Tyr-containing tripeptides showed higher activities than those of the two His-containing tripeptides in the peroxidation of linoleic acid. Tyr-His-Tyr (YHY) showed strong synergistic effects with phenolic antioxidants such as butylated hydroxyanisole and d-tocopherol. However, the tripeptide had only marginal reducing activity and moderate PN scavenging activity. Cys-containing tripeptides showed high PN scavenging activity. Changing either the N-terminus or C-terminus of PHH to other amino acid residues affected their antioxidant activities.
These results demonstrated the importance of amino acid composition, sequence, and size in the antioxidant activities of peptides. Furthermore, it is evident that different amino acid residues and peptide sequences are responsible for the inhibition of oxidative reactions initiated by different types of free radicals or pro-oxidants such as metal ions, as well as in different molecular environments, e.g., aqueous, lipid, or emulsion systems; different pH conditions; and the presence of other compounds in food matrices or biological systems, etc.
Based on the chemical reactions involved, the antioxidant assays could be classified into several groups according to the ability of an antioxidant to donate hydrogen atoms, transfer electrons (i.e. reducing capacity), or chelate metal ions (Huang et al., 2005). Because the antioxidant activity assays are based on different chemical reactions, a tested sample could exhibit different antioxidant activity depending on the assay used (Huang et al., 2005; Prior et al., 2005; Frankel and Finley, 2008). Due to these reasons, it is recommended that the antioxidant activity of the peptide of interest be evaluated using more than one assay.
The purpose of this study is to compare the free radical scavenging activities of tripeptide libraries using ABTS, ORAC, and DPPH assays. This comparison will provide further evidence to understand the structure-activity relationship of antioxidant peptides. The results are collated with previous data (Saito et al., 2003) obtained with the same peptide libraries, and further insights into the antioxidant properties of these peptides are presented.
Chemicals and reagents 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), sodium fluorescein (FL), 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) were purchased from Wako Chemicals (Osaka, Japan). 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was purchased from Sigma Chemical (St. Louis, MO, USA). Amino acids were from Nacalai Tesque (Kyoto, Japan). Amino acid derivatives, coupling reagents, and resins for peptide-assembly were from SynProPep reagents (Shimadzu Scientific Research Inc., Kyoto, Japan).
Tripeptide library Peptides were prepared by solid-phase synthesis with the fluorenylmethoxycarbonyl (Fmoc)-strategy, using an automated simultaneous multiple peptide synthesizer (model PSSM-8; Shimadzu, Kyoto, Japan) as described previously (Saito et al., 2003), manual multiple synthesis using multiple LibraTube (HiPep Laboratories, Kyoto, Japan) or Module SRM96 (HiPep Laboratories) (Yasuhara and Nokihara, 1999). The two His-containing (XHH, HXH, HHX) and two Tyr-containing (XYY, YXY, YYX) tripeptide library were constructed by incorporating various amino acids, which were classified into eight categories depending on side chain groups, to the position X; acidic (Asp: D, Glu: E), basic (His: H, Lys: K, Arg: R), aliphatic (Ala: A, Ile: I, Leu: L, Val: V), aromatic (Phe: F, Trp: W, Tyr: Y) and neutral (Gly: G, Asn: N, Gln: Q) amino acids, and amino acids possessing thioether (Met: M), hydroxyl (Ser: S, Thr: T), and sulfhydryl (Cys: C) groups.
Another tripeptide library consisting of 108 peptides was also constructed. Tripeptides were classified into two groups; a His- containing group; Leu-His-X (LHX), Pro-His-X (PHX) and Arg-His-X (RHX), a Trp-containing group; Leu-Trp-X (LWX), Pro-Trp-X (PWX) and Arg-Trp-X (RWX). At position X, 18 of the 20 common amino acids, except Cys and Pro, were incorporated.
ABTS assay The ABTS assay is based on the ability of antioxidants to scavenge the ABTS radical cation (Miller et al., 1993; Re et al., 1999). Samples (final concentration: 8.2 µM) were dissolved in 0.1 M sodium phosphate buffer (pH 7.0). Fifteen microliters of 20 µM metmyoglobin, 20 µL of 0.9 mM ABTS and 50 µL of 0.1 M sodium phosphate buffer (pH 7.0) were mixed and pre-incubated at 40°C. The radical cation was generated by incubating the mixture for 18 min after the addition of 15 µL of 0.6 mM hydrogen peroxide. One hundred microliters of the reaction mixture was added to 20 µL of peptide sample in a 96-well titer plate. The plate was shaken for 5 sec and absorbance at 630 nm was measured by a plate reader (Model 680 Microplate Reader; Bio-Rad, Hercules, CA, USA). The radical scavenging activity was measured by spectrophotometric changes of the ABTS radical cation using Trolox as a standard. The relative activity was calculated by using a Trolox calibration curve and converted to a Trolox equivalent antioxidant capacity (TEAC) value.
ORAC assay The ORAC assay was carried out as described previously (Ou et al., 2001; Ou et al., 2002). One hundred eighty microliters of 75 mM sodium phosphate buffer (pH 7.4) and 10 µL of sample (final concentration: 0.5 – 5 µM) were mixed in a black titer plate, and incubated at 37°C for 15 min. After incubation, 5 µL of 2 µM fluorescein was added, and the initial fluorescence intensity was measured at Ex: 493 nm/Em: 515 nm by a fluorescent plate reader (Gemini XPS; Molecular Devices, Sunnyvale, CA, USA). Keeping the plate at 37°C, the radical reaction was initiated by the addition of 5 µL of 400 mM AAPH, and the fluorescence intensity was measured every minute. All measurements were expressed relative to the initial reading. The oxygen radical absorbance capacity was calculated using the differences of areas under the fluorescence decay curves between the blank and samples. The relative activity was calculated by using a Trolox calibration curve and converted to a TEAC value.
DPPH assay To peptides (final concentration: 100 or 500 µM) or Trolox (40 – 320 µM) dissolved in 50 µL of 200 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 6.0) and 100 µL of methanol in 96-well titer plates, 50 µL of 800 µM DPPH in methanol was added (Bondet et al., 1997; Cheng et al., 2006). The mixture was then shaken vigorously and kept for 20 min in the dark. The absorbance was measured at 540 nm by a plate reader. The DPPH radical scavenging activity of the sample was calculated by using a Trolox calibration curve and converted to a TEAC value.
Statistical analysis Results were expressed as means ± standard deviation (SD) of three measurements. Statistical analysis was performed using the Student's t-test and p < 0.05 was considered to be significant. Correlations among the data obtained were calculated using the MS Excel software correlation coefficient statistical option.
Two series of tripeptide libraries, which were constructed based on antioxidant peptides from soybean protein hydrolysates, were subjected to free radical scavenging activity assays using ABTS, ORAC, and DPPH assays in this study. Figure 1 shows the ABTS radical scavenging activities of tripeptides (final total concentration for each category: 8.2 µM) containing either two His or Tyr residues in the peptides. The free radical scavenging activity was converted to a TEAC value. In the two His-containing tripeptide group, the tripeptides, which contained aromatic amino acids the position X showed high free radical scavenging activities. Two Tyr-containing peptides showed higher activity than two His-containing peptides. There was little difference in the activity among different categories of two Tyr-containing peptides. In terms of position X, His-X-His and X-Tyr-Tyr tended to show higher activities than other positions in their groups.

ABTS radical scavenging activity of tripeptide libraries. (A) Two His-containing tripeptide libraries. (B) Two Tyr-containing tripeptide libraries. The peptide library was constructed by incorporating various amino acids, which were classified into eight categories depending on side chain groups, to the position X. X: (a) D, E; (b) H, K, R; (c) A, I, L, V; (d) F, W, Y; (e) G, N, Q; (f) M; (g) S, T; (h) C. The total concentration of tripeptide was 8.2 µM for each category. The activity is expressed as the mean of TEAC ± SD of three independent experiments.
Figure 2 shows the free radical scavenging activity of the tripeptide libraries measured by the ORAC assay. The results are similar to those obtained by the ABTS assay. In all the groups the two Tyr-containing peptides showed higher activity than the two His-containing peptides. Two His-containing peptides containing aromatic amino acids showed higher activity than the other two His-containing peptides, but the activity was lower than those of the two Tyr-containing peptides.

The radical scavenging activity of tripeptide library measured by the ORAC assay. (A) Two His-containing tripeptide libraries. (B) Two Tyr-containing tripeptide libraries. The same peptide library was used as in Fig. 1. The total concentration of tripeptide was 0.5 µM for each category. The activity is expressed as the mean of TEAC ± SD of three independent experiments.
Figure 3 shows the radical scavenging activity of 108 tripeptides, which are classified into two groups: the His-containing group (LHX, PHX, and RHX) and Trp-containing group (LWX, PWX, and RWX). It is apparent that the Trp and Tyr groups had higher activity than the His group. The peptides containing Trp or Tyr residues at the C-terminus had the highest activities in both groups. These results indicate that aromatic amino acids are closely linked to ABTS radical scavenging activity.

ABTS radical scavenging activity of PHH and its structurally related peptides. (A) His group having the structures: Leu-His-X, Pro-His-X and Arg-His-X. (B) Trp group: Leu-Trp-X, Pro-Trp-X and Arg-Trp-X. At the position X, 18 amino acids were incorporated. The concentration of tripeptide was 8.2 µM. The activity is expressed as the mean of TEAC ± SD of three independent experiments.
The ORAC assay gave similar results of radical scavenging activity (Fig. 4) as the ABTS assay. However, differences in the activities among the Trp group were much smaller than those measured by the ABTS assay. This result indicates that the Trp residue scavenges the AAPH radical as efficiently as the Tyr residue, though the Trp residue is less effective in the scavenging of the ABTS cation radical than the Tyr residue.

The radical scavenging activity of tripeptide libraries measured by the ORAC assay. (A) His group having the structures: Leu-His-X, Pro-His-X and Arg-His-X. (B) Trp group: Leu-Trp-X, Pro-Trp-X and Arg-Trp-X. At the position X, 18 amino acids were incorporated. The concentration of tripeptide was 5.0 µM. The activity is expressed as the mean of TEAC ± SD of three independent experiments.
Figure 5 shows the radical scavenging activities of tripeptides containing Tyr and/or Trp residues, and their constituent amino acid mixtures measured by ABTS and ORAC assay, respectively. The tripeptides containing a Tyr residue had markedly higher activities than the constituted amino acid mixtures, whereas some tripeptides containing

The radical scavenging activity of tripeptides and their constituent amino acid mixtures measured by ABTS (A) and ORAC (B) assays. The concentrations of tripeptides and amino acids were (A) 8.2 µM and (B) 5.0 µM. The activity is expressed as the mean of TEAC ± SD of three independent experiments.
Trp residue, such as Leu-His-Trp (LHW) and Leu-Trp-Trp (LWW), showed lower activities than their constituent amino acid mixtures in both assays. Although both free forms of Tyr and Trp have radical scavenging activities (Figs. 6 and 7), the activities of the constituent amino acids were only enhanced after incorporation of Tyr into the peptides.

The radical scavenging activity of 10 µM amino acids measured by the ABTS assay. The activity is expressed as the mean of TEAC ± SD of three independent experiments.

The radical scavenging activity of 10 µM amino acids measured by the ORAC assay. The activity is expressed as the mean of TEAC ± SD of three independent experiments.
The DPPH assay required higher concentrations of tripeptides to exhibit positive results of radical scavenging activity. At a concentration of 100 µM, only a few tripeptides, such as CHH, HCH, HHC, YY(F/W/Y), CYY, and YYC, showed significant activities (Fig. 8). By increasing the peptide concentration to 500 µM, more tripeptides exhibited activity, though the peptides containing a Cys residue revealed much higher activities than other peptides (Fig. 9). However, YCY showed only weak radical scavenging activity.
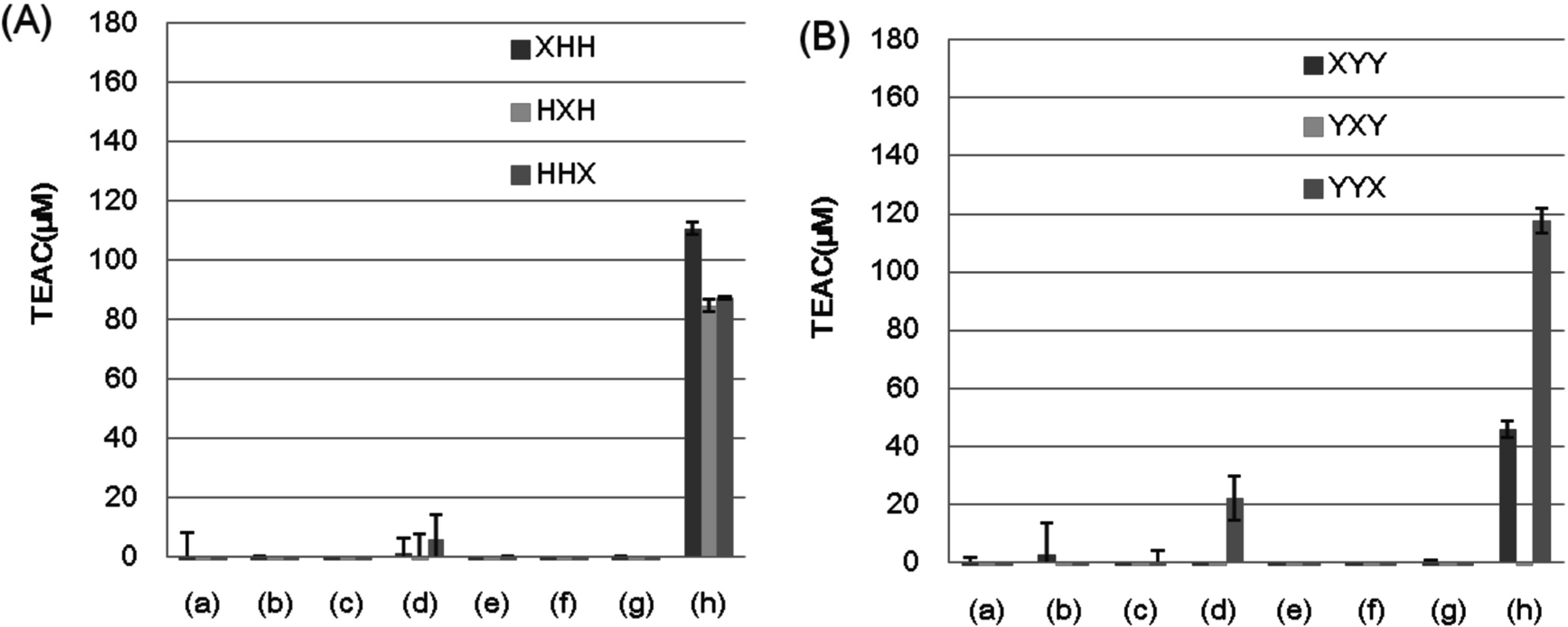
The radical scavenging activity of tripeptide library measured by the DPPH assay. (A) Two His-containing tripeptide libraries. (B) Two Tyr-containing tripeptide libraries. The same peptide libraries were used as shown in Fig. 1. The total concentration of tripeptide was 100 µM for each category. The activity is expressed as the mean of TEAC ± SD of three independent experiments.

The radical scavenging activity of tripeptide library measured by the DPPH assay. (A) Two His-containing tripeptide libraries. (B) Two Tyr-containing tripeptide libraries. The same peptide libraries were used as shown in Fig. 1. The total concentration of tripeptides was 500 µM for each category. The activity is expressed as the mean of TEAC ± SD of three independent experiments.
A number of antioxidant peptides with distinct amino acid sequences have been isolated from various protein hydrolysates and screened using a variety of assays for antioxidant activity (Elias et al., 2008; Hartmann and Meisel, 2007; Sarmadi and Ismail, 2010; Bernardini et al., 2011; Cavazos and Gonzales de Mejia, 2013; Udenigwe and Aluko, 2012; Samaranayaka and Li-Chan, 2011). Since the mechanism for antioxidant activity differs from one assay to another, peptides show different results depending on the assay used. Thus, there is still little information available about the structural requirements for antioxidant activity. In our previous communication we analyzed tripeptide libraries to investigate antioxidant properties using several assays, such as the antioxidant activity against linoleic acid peroxidation, the FRAP assay, and the peroxynitrite (PN) scavenging assay (Saito et al., 2003). In this study, the same tripeptide libraries were subjected to ABTS, ORAC, and DPPH assays, and the relative activities of the tripeptides measured by all six assays were compared to reveal the antioxidant properties of the peptides.
Except for two His-containing peptides containing Cys, the two Tyr-containing peptides showed higher activities than the two His-containing peptides in most of the assays employed (Table 1). The order of the antioxidant activity against the peroxidation of linoleic acid showed correlation coefficients (R) of 0.297, 0.095, 0.725, 0.731, and 0.187 for FRAP, PN scavenging, ABTS, ORAC, and DPPH assays, respectively (Table 2). FRAP, PN scavenging, and DPPH assays showed low R values as compared to other methods. ABTS and ORAC assays showed the highest R value of 0.905. On the basis of the chemical reactions, these assays are classified into two groups: methods based on hydrogen atom transfer (HAT) and electron transfer (ET) (Huang et al., 2005). The ORAC assay is an example of an HAT-based assay. ABTS, FRAP, and DPPH assays are examples of ET-based assays. The classical method, which measures antioxidant activity against the peroxidation of linoleic acid by the ferric thiocyanate method, correlated well with the ORAC and ABTS assays.
| A | B | C | D | E | F | A | B | C | D | E | F | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ① | XHH | 31 | 16 | 39 | 34 | 33 | 31 | ① | X Y Y | 8 | 4 | 22 | 12 | 21 | 29 |
| HXH | 28 | 9 | 18 | 31 | 35 | 25 | Y X Y | 17 | 23 | 12 | 14 | 13 | 16 | ||
| HHX | 42 | 48 | 28 | 45 | 47 | 39 | Y Y X | 4 | 28 | 9 | 21 | 16 | 35 | ||
| ② | XHH | 48 | 11 | 40 | 38 | 29 | 38 | ② | X Y Y | 18 | 3 | 40 | 3 | 7 | 22 |
| HXH | 47 | 8 | 40 | 30 | 30 | 30 | Y X Y | 1 | 26 | 6 | 8 | 6 | 14 | ||
| HHX | 43 | 40 | 40 | 45 | 44 | 39 | Y Y X | 2 | 33 | 22 | 17 | 12 | 39 | ||
| ③ | XHH | 20 | 13 | 20 | 42 | 40 | 31 | ③ | X Y Y | 13 | 1 | 12 | 2 | 4 | 15 |
| HXH | 31 | 6 | 11 | 35 | 45 | 7 | Y X Y | 8 | 18 | 8 | 7 | 1 | 12 | ||
| HHX | 46 | 47 | 25 | 41 | 45 | 39 | Y Y X | 11 | 20 | 28 | 15 | 3 | 11 | ||
| ④ | XHH | 26 | 12 | 40 | 25 | 24 | 28 | ④ | X Y Y | 25 | 2 | 12 | 1 | 8 | 10 |
| HXH | 20 | 32 | 40 | 26 | 19 | 8 | Y X Y | 19 | 18 | 15 | 6 | 5 | 9 | ||
| HHX | 7 | 43 | 40 | 27 | 27 | 19 | Y Y X | 5 | 10 | 33 | 10 | 2 | 6 | ||
| ⑤ | XHH | 30 | 15 | 28 | 40 | 38 | 36 | ⑤ | X Y Y | 3 | 5 | 33 | 4 | 14 | 24 |
| HXH | 37 | 36 | 17 | 36 | 40 | 18 | Y X Y | 10 | 22 | 40 | 11 | 10 | 13 | ||
| HHX | 40 | 44 | 25 | 45 | 40 | 39 | Y Y X | 13 | 30 | 24 | 5 | 11 | 33 | ||
| ⑥ | XHH | 20 | 16 | 37 | 39 | 35 | 39 | ⑥ | X Y Y | 24 | 7 | 19 | 22 | 26 | 36 |
| HXH | 36 | 39 | 28 | 36 | 40 | 27 | Y X Y | 27 | 29 | 36 | 16 | 15 | 26 | ||
| HHX | 43 | 47 | 27 | 44 | 28 | 39 | Y Y X | 23 | 37 | 7 | 24 | 22 | 39 | ||
| ⑦ | XHH | 29 | 46 | 38 | 43 | 37 | 39 | ⑦ | X Y Y | 13 | 24 | 15 | 8 | 17 | 16 |
| HXH | 38 | 38 | 40 | 31 | 47 | 20 | Y X Y | 11 | 25 | 21 | 13 | 9 | 21 | ||
| HHX | 43 | 44 | 32 | 45 | 39 | 39 | Y Y X | 5 | 33 | 33 | 18 | 18 | 33 | ||
| ⑧ | XHH | 35 | 40 | 3 | 29 | 33 | 4 | ⑧ | X Y Y | 41 | 14 | 1 | 23 | 25 | 2 |
| HXH | 31 | 31 | 3 | 28 | 31 | 3 | Y X Y | 13 | 27 | 10 | 19 | 23 | 22 | ||
| HHX | 31 | 35 | 5 | 33 | 32 | 5 | Y Y X | 39 | 20 | 2 | 20 | 20 | 1 |
The order of the activity was numbered from the highest to the lowest. The peptide library used was the same as shown in Figure 1. A: Inhibitory activity against the peroxidation of linoleic acid measured by the ferric thiocyanate method, B: FRAP assay, C: PN scavenging activity, D: ABTS assay, E: ORAC assay, F: DPPH assay.
| B | C | D | E | F | |
|---|---|---|---|---|---|
| A | 0.297 | 0.095 | 0.725 | 0.731 | 0.187 |
| B | 0.066 | 0.522 | 0.429 | 0.286 | |
| C | 0.244 | 0.172 | 0.474 | ||
| D | 0.905 | 0.478 | |||
| E | 0.390 |
PN produced by the reaction between nitric oxide and superoxide can produce some strong oxidants, such as hydroxyl radical, nitronium ion, and nitrogen dioxide (Beckman et al., 1994). It appeared that Cys residues in tripeptides were the prime target for PN, though Trp and Tyr residues were also known to be modified with the oxidant. The latter two amino acids alone showed higher scavenging activities than other amino acids against ABTS and AAPH radicals (Figs. 6 and 7). Among all the amino acids, only Cys showed high scavenging activity against the DPPH radical (Fig. 10). The DPPH assay showed a relatively low R value, 0.474, compared to the PN scavenging assay.

DPPH radical scavenging activity of 100 µM amino acids. The activity is expressed as the mean of TEAC ± SD of three independent experiments.
The present study revealed several significant antioxidant properties of the tripeptide libraries. Firstly, Trp, which was highly active by itself did not increase the activity by forming peptides with other amino acids, whereas Tyr increased the activity mostly by forming peptides. The antioxidant activities of Trp and Tyr may be explained by the special capability of phenolic and indolic groups to serve as hydrogen donors. The phenoxyl and indoyl radicals are much more stable and have longer periods of activity than simple peroxy radicals; thus, any reverse reaction or the propagation of the radical-mediated peroxidizing chain reaction are inhibited (Hawkins and Davies, 2001). It should be noted that there continues to be ongoing discussion concerning the influence of the peptide bond and the conformation of the peptide on the antioxidant activity of constitutive amino acids. β-Lactoglobulin-derived peptides, Trp-Tyr, Trp-Tyr-Ser, and Trp-Tyr-Ser-Leu-Ala-Met, showed higher radical-scavenging activity than the corresponding equimolar amino acid mixtures analyzed using the ORAC assay, indicating that the peptidic bond and/or structural peptide conformation improved the hydrogen donor capacity of the amino acid residues, enhancing their antioxidant activity (Hernandez-Ledesma et al., 2005). However, the antioxidant activity of Trp-Tyr-Ser-Leu-Ala-Met-Ala-Ala-Ser-Asp-Ile was lower than that of Trp alone and an equimolar mixture of the constitutive amino acids. In addition, the antioxidant activity of Tyr-Gly-Ser, which was isolated from a defatted peanut meal hydrolysate, was lower than that of the mixture of constituent amino acids, though the peptide showed a 3-fold higher antioxidant activity than glutathione analyzed using the ORAC assay (Zheng et al., 2012).
Secondly, the amino acid sequence of peptides plays an important role in their antioxidant activity as shown by the high DPPH radical scavenging activity of Cys-containing peptides except for Tyr-Cys-Tyr (Figs. 8 and 9). The positional selectivity and rates of radical attack on peptides and proteins were accounted for by the presence of the deactivating protonated amino group and the radical stabilizing groups on side chains. Site-specific oxidation can also arise from the binding of a metal ion or other initiating species at particular sites on peptides (Stadtman, 1993). Selective modifications of His, Pro, Met, Cys, Arg, Lys, and Trp residues have been observed with various peptides and proteins. These distinct physicochemical properties of peptides are related to their antioxidant activities.
Thirdly, the DPPH assay requires more peptide samples than other radical scavenging assays, though the assay is sensitive toward Cys-containing peptides. It was also shown that Tyr-Gly-Ser displayed negligible DPPH radical scavenging activity and reducing power (Zheng et al., 2012). Although the DPPH assay is widely used for detecting phenolic compounds and other phytochemicals, and has also been applied to analyze peptides, one should keep in mind the specificity of the assay as mentioned above when antioxidant peptides are screened.
Fourthly, six different assays gave distinct results regardless of the classification on the basis of the chemical reactions. It is known that the use of different antioxidant assays makes it difficult to compare antioxidant potential and mechanisms of reported peptide sequences. The correlation among six different assays demonstrated in this study may be useful to discussion of the structure-activity relationship of antioxidant peptides. The appropriate combination of the assays may be effective to isolate bioactive peptides or cryptides from protein sources upon hydrolysis (Mora and Hayes, 2015), because a wide range of peptides can be screened by these assays.
The Quantitative Structure-Activity Relationships (QSAR) concept has been extended to antioxidant peptides by two research groups (Chanput et al., 2010; Li et al., 2011a; Li et al., 2011b). They used the same experimental results reported by Saito et al. (2003); i.e. the antioxidant activities of the tripeptide libraries against the peroxidation of linoleic acid. The one research group indicated that hydrophobic amino acids such as Ile, Leu, Phe, Trp, Tyr, and Val were required for antioxidant peptide segments (Chanput et al., 2010). The other research group showed that the N-terminal amino acid should be a high hydrophobic and low electronic amino acid (such as Ala, Gly, Val, and Leu); the center amino acid would be an amino acid that possesses a high hydrogen bond property (such as basic amino acids Arg, Lys, and His) (Li et al., 2011a; Li et al., 2011b). The methodology of the QSAR modeling relies on the idea that biological activity is a function of chemical structures, which can be described by molecular or physicochemical variables, e.g., electronic attributes, hydrophobicity, and steric properties. The data obtained in this study would be useful for QSAR modeling of unique antioxidant peptides as well as for finding and screening cryptides, which are food-derived peptides and often multifunctional (Cavazos and Gonzalez de Mejia, 2013; Mora and Hayes, 2015).
In conclusion, six different antioxidant assays of combinatorial tripeptide libraries showed several aspects concerning structure-activity correlations. Two Tyr-containing tripeptides showed higher scavenging activities against hydrophilic ABTS and AAPH radical than two His-containing tripeptides, indicating that Tyr and Trp residues play an important role in expressing the radical scavenging activities. Tripeptides except Cys-containing peptides, showed only weak scavenging activity against the hydrophobic DPPH radical. The antioxidant activity of the tripeptides against the peroxidation of linoleic acid showed high correlations with the ABTS and ORAC assays, and low correlations with FRAP, PN scavenging and DPPH assays. The highest correlation was found between the ABTS and ORAC assays. The combined use of these assays holds potential as an effective approach to screen the antioxidant activity in the course of antioxidant peptide isolation.
Acknowledgement This work was supported by JSPS KAKENHI grant no. 26292111.