2015 年 21 巻 6 号 p. 847-855
2015 年 21 巻 6 号 p. 847-855
Premna ligustroides Hemsl. is a traditional plant food material, but the chemical components, functional ingredients of the leaves and its antioxidant activity of ethanol extracts had never been studied. In this study, the moisture, ash, crude fiber, crude fatty, pectin, and amino acid were 8.95 ± 0.01, 7.76 ± 0.06, 7.86 ± 0.10, 12.93 ± 0.05, 19.21 ± 0.02, and 15.26 ± 0.16 g/100 g dry basis, respectively. Total flavonoids content of leaves was 74.35 ± 0.49 mg/g. Degree of esterification (DE) of the pectin was 66.67 ± 1.02%, and the unsaturated fatty acids occupied 64.71% of the total fatty acids, 17 amino acids which contained seven essential amino acids were detected. Fifty eight volatile compounds were separated and identified. The extracted flavonoids had higher reducing power than ascorbic acid at the same concentration, and had significant scavenging abilities on hydroxyl radicals, superoxide anion radical, and DPPH radical. The results indicated that the leaves of P. ligustroides Hemsl. as a kind of botanical food has a great value of development and utilization.
Plant foods become more and more popular due to the color, smell, taste, as well as the benefits to health. Many food researchers paid more attention to exploiting traditional nature food resources like herbal, trees and shrubs materials and their functional ingredients, which were applied in food process and storage, or herbal medicine (Muhammad and Amusa, 2005; Xie and Leung, 2009). The beneficial effects of plant on health improvement have been partly attributed to their complex mixture of phytochemicals (Farag et al., 2013).
Premna ligustroides Hemsl. (Verbenaceae) is a shrub mainly grown in China, Japan, Malaysia, Philippines. This shrub has been planted for many years, and used to make foods in the local. The seeds, roots and leaves have wide range of medicine values (Zhang and Zhang, 2013). Its leaves are always used to make popular gel food with nature bright green color, unique taste, and health functions. The preliminary experiment showed that there was high flavonoids content in the leaves of Premna ligustroides Hemsl.. An important effect of flavonoids is the scavenging of oxygen-derived free radicals. In vitro experimental systems also showed that flavonoids possess antiinflammatory, antiallergic, antiviral, and anticarcinogenic properties (Nijveldt et al., 2001). What's more, the leaf has been extensively used to make gel (Fig. 1) for its high pectin content, used as nature repellents for its unique smell. It could also be used as an additive in soft drink and noodle making. Additionally, the leaf can also be steamed directly with some meat slices.

The leaves of Premna ligustroides Hemsl. and its food product of grass jelly.
There have not been any reports about the chemical components, functional ingredients for the leaves, as well as the antioxidant activity evaluation for its ethanol extracts. The objective of this study was to systematically analyze the characteristic of the leaves of P. ligustroides Hemsl., including the moisture, ash, pectin, crude fiber, fatty acid, amino acid, volatile components, total flavonoids, and the antioxidant activity of the flavonoids extracts, which will provide theoretical basis for further exploitation and utilization for the botanical food.
Raw material The fresh leaves of P. ligustroides Hemsl. were collected in June, 2012, randomly in different adult trees in Dazhu county, Sichuan province of China (N 29° 23′ 29° 52′, E 105° 28′–106° 2′). They were dried in blast oven at 50°C for 12 h after washed cleanly with deionized water, then grounded to powder and screened by the sieve with 80 meshes. The powder was stored in a brown glass jar at 4°C for further analysis. All chemicals were analytical reagents except noticed.
Determinations of proximate components Proximate composition of the following nutrients was determined using standard procedures of AOAC: moisture, ash, crude fat, and crude fiber (AOAO, 2000).
Total pectin was extracted according to the method described by Wang et al. (2007). Degree of esterification (DE) of the pectin was determined by the titrimetric method of Food Chemical Codex (FCC, 1981).
Fatty acid composition analysis Oil of the leaves was obtained by soxhlet extraction method, and the oil samples were esterified according to the method described by Petrović et al. (2010). Then fatty acid analysis was performed on a GC-MS instrument (Shimadzu QP2010, Japan) equipped with a DB-FFAP column (30.0 m, 250 mm i.d, Agilent). Helium was used as carrier gas with a flow rate of 1.0 mL min−1. Samples were injected at 230°C with a split ratio of 5:1. Oven temperature program initiated at 100°C, increased to 140°C at 12°C/min for 5.0 min, increased to 175°C at 4°C/min for 4 min, and then increased to 205°C at 3°C/min for 2 min, finaly increased to 230°C at 8°C/min for 15 min. Mass spectrometer was operated in electron impact (EI) and full scan monitoring mode (m/z 40 – 400), the electron energy was 70 eV. The ionization source temperature was set at 230°C and the solvent delay time was set at 3 min.
Amino acid composition analysis Amino acid compositions analysis was performed on an Automatic Amino Acid Analyzer (Hitachi L-8800, Japan). About 200 mg of P. ligustroides Hemsl. leaves powder were transferred into a 18 × 180 mm tube, 15 mL of 6 mol/L HCl added, and mixed well. The tube was vacuumed, and then sealed. After that, the tube was heated in a sand bath at 110 ± 10°C for 22 h to hydrolysis protein into amino acids. Then the hydrolysis solution was cooled in room temperature and filtrated. One milliliter of the filtrate was decanted into a beaker and dried at 60°C, 2 mL of HCl (0.02 mol/L) was added to dissolve the samples, and the solution was pipetted into a vial using a filter membrane (25 mm, 0.45 µm), then analyzed using the automatic amino acid analyzer.
Volatile components analysis P. ligustroides Hemsl. leaves (0.25 g) were placed in a solid phase micro extraction (SPME) vial (10 mL) with 2.5 mL deionized water (50°C) and 0.5 g NaCl. Water bath at 50°C for 10 min. The volatile components was extracted by a manual SPME holder at 50°C for 60 min. The SPME fiber (divinylbenzene/carboxen/polydimethylsiloxane, matrix active group 50/30 µm) was purchased from Supelco (Bellefonte, PA, USA). Finaly, the absorbed volatile components in the fiber were desorbed in an injector port of a gas chromatograph at 230°C for 5 min, and then separated by GC-MS (Shimadzu QP2010, Japan). The details of the program used in GC—MS analysis are as follows: helium gas flow-rate of 1.00 mL/min, no split ratio, the initial temperature was 40°C, held for 2 min, and increased to 141°C at a rate of 14°C/min, held for 12 min, increased to 142°C at a rate of 1°C/min, held for 3 min, and finally increased to 230°C at a rate of 12°C with a hold time of 8 min. Other conditions were the same as 2.3.
Antioxidant activity evaluation of crude flavonoids extracts
Concentration determinations of total flavonoids Three gram of dried leaves powder was weighed and put into a conical flask, and 50 mL of 70% (v/v) ethanol was added, and the extraction conducted in an ultrasonic cleaner (KQ3200DB, China) for 30 min at 70°C with the power of 150 W. The upper layer of the solution centrifuged at 3000 × g for 20 min (Eppendorf 5810, Germany), and the solid residue was extracted 3 times. Gathering all the supernatant and transferred into a 250 mL volumetric flask, and adjusted the volume to the line using 70% ethanol.
Total flavonoids content was determined using a colorimetric method described by Zhang et al. (2011): 0.5 mL diluted sample was transferred to 10 mL colorimetric tube, then 0.5 mL of 5% (w/v) sodium nitrite, 0.5 mL of 10% (w/v) aluminum nitrate and 4 mL of 4% (w/v) sodium hydroxide were added according the stated order. The final volume was adjusted to 10 mL with 95% (v/v) ethanol. The mixture was allowed to stand for 15 min, Total flavonoids content (TFC) was determined by spectrophotometry (Shimadzu UV-2450, Japan) at 510 nm. The results were expressed as means (±SD) mg of rutin equivalents per gram dry leaves (mg/g) for the triplicate extracts.
Reducing power assessment The crude flavonoids extracts was diluted into varying concentrations using 70% ethanol. The reducing power of the extract solution was determined by potassium ferricyanide [K3Fe (CN)6] method according to the report of Oyaizu (1986) with some modifications: 2.5 mL of the dilution solution, 2.5 mL of phosphate buffer (0.2 mol/L, pH 6.6) and 2.5 mL of potassium ferricyanide solution (10 mg/mL) were mixed together and incubated at 50°C in water bath for 20 min. After cooling, 2.5 mL of trichloro acetic acid (100 mg/mL) was added then centrifuged at 3000 × g for 10 min. The supernatant (2.5 mL) was mixed with distilled water (2.5 mL) and fresh ferric chloride solution (0.5 mL, 1.0 mg/mL), and then the absorbance was measured at 700 nm. The control was prepared in similar manner excluding samples. Ascorbic acid at various concentrations was used as a comparison group.
Scavenging capacity test for hydroxyl radicals (•OH) Two milliliter of FeSO4 (6 mmol/L), 2 mL of H2O2 (6 mmol/L) and 2 mL the dilution solution (see 2.6.2) were mixed homogeneously. After incubation at room temperature for 20 min, the 2 mL of salicylic acid solution (6 mmol/L) was added, mixed fully and incubated at room temperature for 30 min, and then the absorbance of the mixture was measured at 510 nm. Hydroxyl radical scavenging rate was calculated as (1-absorbance of sample/absorbance of control) ×100% (Sun et al., 2010). Ascorbic acid at various concentrations was used as a comparison group.
Scavenging capacity test for superoxide anion radical (•O2) Appropriate deionized water (4.2 mL) was mixed with 4.5 mL Tris-HCl buffer solution (50 mmol/L, pH 8.2). After water bathed the mixture at 25°C for 20 min, 0.3 mL of pyrogallol solution (3 mmol/L) was added, then the absorbance value was measured with an integral time of 5 s at 325 nm, the accumulated continuously for 300 s, and the changing rate of control group (Fr) was calculated. One milliliter of the dilution solution (see 2.6.2) was added into the above system, and at the same time the volume of the buffer solution was added to keep the volume constant, and the changing rate of test group (Ft) was calculated. The scavenging rate for superoxide anion (•O2) was calculated as: SR % = (Fr-Ft)/Fr ×100% (Sun et al., 2013). Ascorbic acid at various concentrations was used as a comparison group.
Radical scavenging activity test for DPPH After 2.0 mL of the dilution solution (see 2.6.2) was mixed with 2.0 mL of DPPH solution for 30 min, the absorbance of the samples was measured at 517 nm. Radical scavenging activity was expressed as percentage according to the following formula: DPPH radical scavenging activity % = [1-As/A0] × 100. Where As is the absorbance of the samples, and A0 is the absorbance of the DPPH solution. Ascorbic acid solutions of different concentrations were used as positive controls for antioxidant activity (Faller and Fialho, 2010). Ascorbic acid at various concentrations was used as a comparison group.
Statistical analysis All tests were performed in triplicate. Analysis of variance (ANOVA) was performed, and experimental data was expressed as mean values. A Duncan test was conducted to examine significant differences among experimental mean values (P < 0.05).
Determinations of some physicochemical indexes The physicochemical indexes content were shown in Tabel 1. Compared with the pectin content in dry orange peels (5.27%) (Yeoh et al., 2008) and apple pomace (15.75%) (Wang et al., 2007), there was more pectin in the leaves of P. ligustroides Hemsl. (19.21%). The most attractive property of pectin for industrial applications is its gelling activity. The pectin was applied in the food industry as gelling or thickening agent in the beginning, and then, as an excipient for pharmaceutical purposes.
| Parameter | Concentration (g/100 g dry basis) |
|---|---|
| Ash | 7.76 ± 0.06 |
| Moisture | 8.95 ± 0.01 |
| Crude fat | 12.93 ± 0.05 |
| Crude fiber | 7.86 ± 0.10 |
| Pectin | 19.21 ± 0.21 |
The DE of the leaves' pectin was 66.67 ± 1.02%, which is a kind of high DE pectin (DE > 50%). High DE pectin have been developed into hydrogels for drug delivery, for high DE pectin, the formation of hydrophobic areas parallel to the helix axes can expand to such an extent as to dramatically reduce the solubility of pectin. High DE pectin also gel in the presence of large concentrations of sugar (Liu et al., 2003). So with high pectin concentration and high DE value, the leaves of P. ligustroides Hemsl. could be used in food and drug industry.
Fatty acid composition analysis The content of crude fatty acid in the leaves is 12.93 ± 0.02 g/100 g dried samples. Fig. 2 presented a typical total ion current chromatogram of the fatty acids of the leaves of P. ligustroides Hemsl. The analytical results were listed in Table 2. The relative contents were calculated by using the area normalization method. Sixteen kinds of fatty acids in the extracted solution were identified. It could be seen from Table 1 that the leaves of P. ligustroides Hemsl. were remarkably rich in (Z,Z,Z)-9,12,15-octadecatrienoic acid (43.64%) and hexadecanoic acid (15.26%), followed by (Z,Z)-9,12-octadecadienoic acid (14.64%), and the unsaturated fatty acids occupied 64.71% of the total fatty acids.
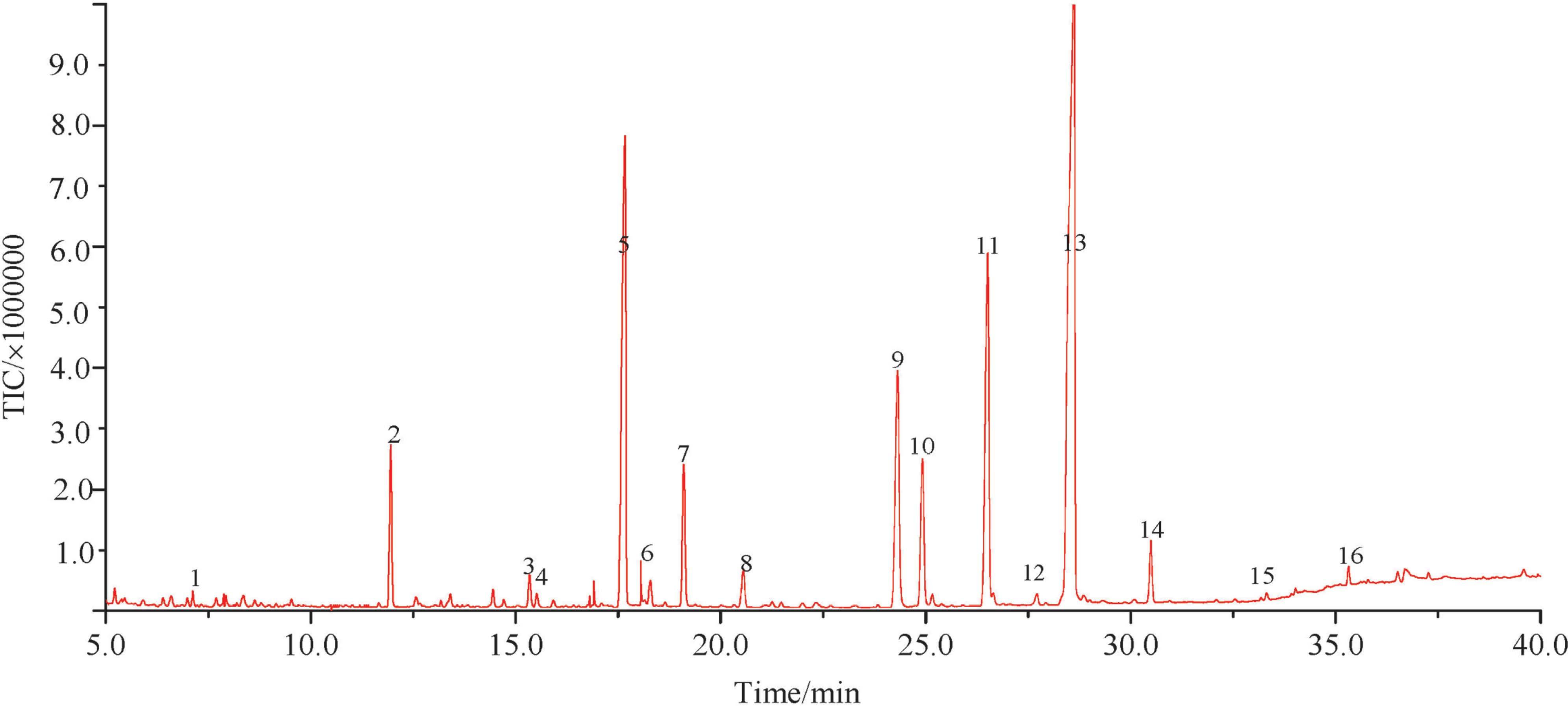
Total ion current chromatograms of fatty acids for leaves of P. ligustroides Hemsl.
| Peak | Retention time/min | Compounds | Molecular Formula | Molecular weight | Relative content/% |
|---|---|---|---|---|---|
| 1 | 7.117 | Dodecanoic acid | C12H24O2 | 200 | 0.2 ± 0.02 |
| 2 | 11.950 | Tetradecanoic acid | C14H28O2 | 228 | 3.66 ± 0.07 |
| 3 | 15.333 | 5-Octadecenoic acid | C18H34O2 | 282 | 0.75 ± 0.02 |
| 4 | 15.508 | Azelaic acid | C10H18O4 | 202 | 0.29 ± 0.01 |
| 5 | 17.658 | Hexadecanoic acid | C16H32O2 | 256 | 15.26 ± 0.71 |
| 6 | 18.283 | 9-Hexadecenoic Acid | C16H30O2 | 254 | 0.85 ± 0.02 |
| 7 | 19.096 | Cyclopropaneoctanoicacid, 2-octyl-, cis- | C19H36O2 | 296 | 3.88 ± 0.22 |
| 8 | 20.550 | Heptadecanoic acid | C17H34O2 | 270 | 1.16 ± 0.01 |
| 9 | 24.308 | Octadecanoic acid | C18H36O2 | 284 | 8.48 ± 0.52 |
| 10 | 24.917 | (Z)-9-Octadecenoic acid | C18H34O2 | 282 | 4.83 ± 0.41 |
| 11 | 26.517 | (Z,Z)-9,12-Octadecadienoic acid | C18H32O2 | 280 | 14.64 ± 0.81 |
| 12 | 27.708 | Nonadecanoic Acid | C19H38O2 | 298 | 0.25 ± 0.01 |
| 13 | 28.617 | (Z,Z,Z)-9,12,15-Octadecatrienoic acid | C18H30O2 | 278 | 43.64 ± 1.63 |
| 14 | 30.483 | Eicosanoic acid | C20H40O2 | 312 | 1.64 ± 0.19 |
| 15 | 33.313 | Heneicosanoic acid | C21H42O2 | 326 | 0.12 ± 0.01 |
| 16 | 35.317 | Docosanoic acid | C22H44O2 | 340 | 0.35 ± 0.02 |
(Z,Z,Z)-9,12,15-octadecatrienoic acid is also named α-linoleic acid (ALA). Humans can obtain α-linoleic acid only through their diets (Goyens et al., 2006). Studies in normal healthy adults show that supplemental ALA raises eicosapentaenoic acid (EPA) and docosahexaenoic acid (DPA) status which can help prevent chronic diseases and important for brain health and normal growth (Brenna et al., 2009). (Z, Z)-9,12-Octadecadienoic acid named linoleic acid is also an essential fatty acid that must be consumed for proper health and can be obtained only through their diets. A diet only deficient in linoleate causes mild skin scaling, hair loss, and poor wound healing in rats (Cunnane and Anderson, 1997; Ruthig and Meckling-Gill, 1999). These two fatty acids occupied more than half of the total fatty acids in the leaves of P. ligustroides Hemsl. It is indicated that the leaves contained many high qualities fatty acids and have high nutritional value.
Amino acids composition analysis The amino acid composition in the leaves of P. ligustroides Hemsl. were shown in Table 3. Totally 17 amino acids were detected in the leaves, and total amino acid content was 15.26 ± 0.16 g/100 g dry basis. It had higher values of glutamic acid (1.88%), leucine (1.64%), aspartic acid (1.46%), alanine (1.18%), tryptophan (1.01%), arginine (1.05%) and lower values of methionine (0.07%), cysteine (0.15%). Except for valine, the other seven essential amino acid were detected, and the content was 39.20% of the total amino acid. What's more, arginine and histidine, which essential for infants and children, were also detected in the leaves. So the leaves can be used as a good resource of vegetable protein.
| Compound | Content (g/100 g dry basis) | Compound | Content (g/100 g dry basis) |
|---|---|---|---|
| Isoleucine‡ | 0.92 ± 0.05 | Aspartic acid | 1.46 ± 0.10 |
| Leucine‡ | 1.64 ± 0.08 | Serine | 0.63 ± 0.04 |
| Lysine‡ | 0.74 ± 0.02 | Glutamic acid | 1.88 ± 0.09 |
| Methionine‡ | 0.07 ± 0.01 | Glycine | 0.98 ± 0.04 |
| Phenylalanine‡ | 0.89 ± 0.02 | Alanine | 1.18 ± 0.08 |
| Threonine‡ | 0.72 ± 0.01 | Cysteine | 0.15 ± 0.05 |
| Tryptophan‡ | 1.01 ± 0.06 | Tyrosine | 0.62 ± 0.05 |
| Arginine† | 1.05 ± 0.05 | Proline | 0.97 ± 0.06 |
| Histidine† | 0.35 ± 0.02 | ||
| Total amino acid content (g/100 g dry basis) | 15.26 ± 0.16 | ||
| Essential amino acids (% of total amino acids) | 39.25 ± 0.21 | ||
Volatile components analysis The unique flavor of P. ligustroides Hemsl. leaf mainly rely on its volatile components. The volatile components analysis results of ion current chromatograms were shown in Fig. 3. The chemical components in the leaves were retrieved by using NIST literature data combined with retention time, and the relative content of each component calculated by area normalization method.

Total ion current chromatograms of volatile components for leaves of P. ligustroides Hemsl.
Twenty-eight volatile compounds were separated and identified by GC-MS. Among which, 19 alkenes compounds account for 39.87%, 10 alcohols compounds account for 34.8%. 8 aldehydes compounds account for 6.09%, 6 ketone compounds and esters compounds account for 9.24% and 2.57%, respectively, 4 alkanes compound account for 3.71%, 2 acids compounds account for 0.45%, 1 phenolic compounds account for 0.26%.
The main volatile compounds were summarized in Table 4, many high content ingredients are common substances applied in food, medicine or cosmetic industry. For example, 1-octen-3-ol (13.87%) is an important flavour component and an effective attractant for mosquitoes (Carvalhoa et al., 2011; Laporta and Sallum, 2011). α-Bisabolo (12.96%) has antitumor effects against cancer and a significant lightening effect in the pigmented skin (Seki et al., 2011; Lee et al., 2010). α-Caryophyllene (5.58%) and caryophyllene (4.52%) show potential antimicrobial activity (Sabulal et al., 2006). β-Ionone (5.3%) is a fragrance ingredient used in many fragrance compounds (Lalko et al., 2007), it is known insect repellants, and can completely inhibited microbial growth in the fruit tissue (Lamikanra et al., 2002).These suggest that the P. ligustroides Hemsl. leaf is a potential material applied in food and medicine industry.
| Peak | Retention time/min | Compounds | Molecular Formula | Molecular weight | Relative contents/% |
|---|---|---|---|---|---|
| 1 | 7.643 | trans-2-Hexenal | C6H10O | 98 | 2.56 ± 0.03 |
| 3 | 9.982 | 1-Octen-3-ol | C8H16O | 128 | 13.87 ± 0.32 |
| 8 | 11.657 | Linalool | C10H18O | 154 | 2.01 ± 0.03 |
| 17 | 16.532 | 2,4- bis (1-methylethenyl)-, (1S,2S,4R)- | C15H24 | 204 | 2.32 ± 0.04 |
| 22 | 16.532 | (-)-Zingiberene | C15H24 | 204 | 2.06 ± 0.15 |
| 25 | 17.510 | Caryophyllene | C15H24 | 204 | 4.52 ± 0.41 |
| 28 | 17.983 | β-Sesquiphellandrene | C15H24 | 204 | 3.67 ± 0.26 |
| 31 | 18.668 | α-Caryophyllene | C15H24 | 204 | 5.58 ± 0.59 |
| 32 | 19.317 | α-Ionone | C13H20O | 192 | 5.3 ± 0.38 |
| 34 | 19.585 | 1,6-Cyclodecadiene,1-methyl-5-methylene-8-(1-methylethyl)-, [1E,6E,8S]- | C15H24 | 204 | 1.79 ± 0.07 |
| 36 | 20.188 | α-Gurjunene | C15H24 | 204 | 2.73 ± 0.22 |
| 37 | 20.610 | α-Cedrene | C15H24 | 204 | 5.5 ± 0.38 |
| 38 | 21.052 | (-)-β-Cadinene | C15H24 | 204 | 1.78 ± 0.13 |
| 40 | 22.294 | Thujopsen | C15H24 | 204 | 6.24 ± 0.34 |
| 47 | 30.735 | α-Bisabolo | C15H26O | 222 | 12.96 ± 0.85 |
Antioxidant activity evaluations of crude flavonoids extracts
Concentration determinations of total flavonoids The total flavonoids concentration in the leaves of P. ligustroides Hemsl. is 74.35 ± 0.49 mg/g. Flavonoids are a class of secondary plant phenolics with significant antioxidant and chelating properties. Most of the beneficial health effects of flavonoids are attributed to their antioxidant and chelating abilities (Kondo et al., 1996). The flavonoids have long been recognized to possess anti-inflammatory, antioxidant, antiallergic, hepatoprotective, antithrombotic, antiviral, and anticarcinogenic activities (Middleton et al., 2000; Tarahovsky et al., 2012). Zhang et al. (2011) reported that the extraction yield of flavonoids, which used rutin as standard, was 3.62% (36.2 mg/g). Siddhuraju et al. (2003) obtained extraction yield of total flavonoids, which used rutin as standard, from freeze-dried Moringa oleifera Lam leaves picked from Nicaragua, India and Niger were 4.43% (44.3 mg/g), 2.10% (21.0 mg/g) and 3.81% (38.1 mg/g), respectively. Sakanaka et al. (2005) got a total of 58.4 mg/g of flavonoids, which used catechin as standard, from the water extract of Japanese persimmon leaf tea (kakinoha-cha). The leaves above, which had been proved to be rich in flavonoids, had lower content of total flavonoids than leaves of P. ligustroides Hemsl. The results indicated that the leaves of P. ligustroides Hemsl. contain high concentration of total flavonoids and could be beneficial to human health.
Reducing power assessment Reducing power of bioactive compounds or food components reflects the electron-donating capacity and is associated with antioxidant activity (Gülçin, 2012). The increase in absorbance of the reaction solution would indicate an increase in the reducing capacity due to an increase in the formation of the complex.
As shown in Fig. 4A, the extracts of leaves had higher reducing power than that of ascorbic acid solutions. The absorbance of ascorbic acid at concentration of 0.03, 0.05, 0.07, 0.09, 0.11, and 0.13 mg/mL was 0.18, 0.28, 0.29, 0.41, 0.51, and 0.61, respectively. But the absorbance of the extracts at the same concentration reached 0.44, 0.72, 1.02, 1.37, 1.43, and 1.54, respectively. As we know, ascorbic acid has a high reducing power. However, the extracts obtained from the leaves of P. ligustroides Hemsl. proved to have a higher reducing power, so we can draw a conclusion that the leaves of P. ligustroides Hemsl. were a better source of antioxidants.

Antioxidant activity of the crude flavonoids extracts compared to ascorbic acid solutions. A is total deoxidization capacity; B is hydroxyl radicals scavenging ability; C is superoxide anion radical scavenging ability; D is DPPH radical scavenging ability.
Scavenging activity assay for hydroxyl radicals (•OH) Hydroxyl radicals can reduce disulfide bonds in proteins, specifically fibrinogen, resulting in their unfolding and scrambled refolding into abnormal spatial configurations (Lipinski, 2011). The presence of too many hydroxyl radicals in the human body will result in their attacking protein, lipid, DNA and other bimolecular, and then result in disease and acceleration of the aging process (Devasagayam et al., 2004).
As illustrated in Fig. 4B, the hydroxy radical scavenging activity initiated by the extracts was stronger than ascorbic acid at the same conditions (from 0.03 to 0.13 mg/mL) in a concentration-dependent manner. This result suggested that the extracts from the leaves of P. ligustroides Hemsl. had significant effect on scavenging hydroxyl radical.
Scavenging ability assay for superoxide anion radical (•O2) Superoxide radical is considered a major biological source of reactive oxygen species. Although superoxide anion is a weak oxidant, it gives rise to generation of powerful and dangerous hydroxyl radicals as well as singlet oxygen, both of which contribute to oxidative stress (Chen and Yen, 2007; Saeed et al., 2012).
As shown in Fig. 4C, the scavenging ability for superoxide anion radical of the extracts from leaves of P. ligustroides Hemsl. were increased along with the increase of the concentration ranged from 0.03∼0.13 mg/mL. The highest scavenging rate of the extracts reached 48.9% at the concentration of 0.13 mg/mL, but the rate of ascorbic acid in this concentration was 29.6%.
Scavenging ability assay for DPPH radical DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule. DPPH has been widely used to evaluate the free radical scavenging effectiveness of various antioxidant substances. The effect of antioxidants on DPPH is due to their hydrogen donating ability.
The results of Fig. 4D indicated that the DPPH radical scavenging ability of the extracts were increased with the concentration increasing of the solutions, and the scavenging rate were ranging from 37.9% to 78.9% with the concentration between 0.03 mg/mL and 0.13 mg/mL. However, compared to the scavenging rate of ascorbic acid, the DPPH radical scavenging abilities of the extracts were less than those of ascorbic acid at the same concentration.
As a traditional plant food material, the leaves of P. ligustroides Hemsl. contain many bioactive components as shown in above results. The pectin content of the dry leaves power was 19.21 ± 0.02 g/100 g dry basis, and the DE of pectin was 66.67 ± 1.02%. The content of crude fatty acid in the leaves was 12.93 ± 0.05 g/100 g dry basis, and sixteen kinds of fatty acids were identified, which include 64.71% unsaturated fatty acids. Total amino acids content was 15.26 ± 0.16 g/100 g dry basis, and seven essential amino acids were detected, which occupied 39.2% of the total amino acid. About 58 volatile compounds were separated and identified by GC-MS. Total flavonoids content in the leaves of P. ligustroides Hemsl. was 74.35 ± 0.49 mg/g, The antioxidant activity of the crude flavonoids extracts suggested that the extracts had higher reducing power than ascorbic acid at the same concentration, and had significant scavenging abilities on hydroxyl radicals, superoxide anion radical, and DPPH radical. All the results indicated that the leaf of P. ligustroides Hemsl. have great potential to be applied in food and pharmaceutical industry.
Acknowledgements This project is financially supported by Chongqing “121” technology demonstration projects (cstc2014zktjccxyyBX0032) and Chongqing science and technology program (cstc2014jcyjA80040).
The authors have declared no conflict of interest.