2016 Volume 22 Issue 1 Pages 101-109
2016 Volume 22 Issue 1 Pages 101-109
The objective of this study was to investigate the effects of superfine grinding on the physicochemical and antioxidant properties of oat bran polysaccharides (OBP). The solubility of OBP increased from 8.1 to 11.3 mg/mL. The average molecular weights of raw OBP (rOBP) and superfine ground OBP (sOBP) were 655.6 and 489.6 kDa, respectively. The intrinsic viscosity of sOBP (4.58 mL/mg) was extremely lower than that of rOBP (94.75 mL/mg). The endothermic enthaphy change (ΔH) values of OBP with different water contents (0%, 50%, and 75%) changed from −328.79 J/g, −942.41 J/g, and −1.94 kJ/g to −197.01 J/g, −703.29 J/g, and −1.50 kJ/g, respectively. After being superfine grinding treated, sOBP exhibited higher antioxidant activities than rOBP based on FRAP, DPPH radical scavenging, and ABTS radical scavenging activities. The results suggested that superfine grinding treatment enhanced OBP's antioxidant activities. Physicochemical properties, including molecular weight, monosaccharide composition and solubility were key factors in OBP's antioxidant activities.
Natural polysaccharides and their conjugates have been widely used in food and medicine for a long time. Numerous favorable biological and pharmacological effects of natural polysaccharides have been extensively studied in vitro and in animal models in vivo, and more and more kinds of natural polysaccharides have been tested and even applied in therapies (Wang and Fang, 2004). It was demonstrated that some natural polysaccharides were effective at preventing oxidative damage in living organism, thus could be a potential resource of novel antioxidants (Ge et al., 2009; Tsiapali et al., 2001; Xu et al., 2009).
Oat belonging to the genus Avena in the family Gramieae has been widely cultivated and was used as a traditional Chinese herb medicine (Duan et al., 2014; Hu et al., 2014; Zhang et al., 2012). In addition, oat bran is an important source of natural functional polysaccharides. The high content of functional polysaccharides in oat bran has showed significant positive health effects in lowering cholesterol, modulating glucose absorption, improving gastrointestinal function, and preventing heart diseases (Alfredo et al., 2009; Chen, He, et al., 2006; Tapola et al., 2005). Oat bran polysaccharides (OBP) have been considered as potential ingredients in health-promoting functional foods because of their health benefits, including antioxidant properties (Lazaridou et al., 2006).
Superfine grinding is a new technology, which is a useful tool for making superfine powder normally with a particle size of less than 10 – 25 µm and good surface properties (Tkacova and Stevulova, 1998; Hu et al., 2012). The surface of superfine powder can undergo some changes, which bring out a series of important characteristics that coarse particles do not possess. Nowadays, superfine grinding technology has shown a great potential in producing nutraceuticals and functional foods (Chen, Weiss, et al., 2006). Some studies have shown that the superfine powder possessed higher dispersibility, solubility, and water holding capacity, and thus the quality of food products produced by superfine grinding technology was improved (Wu et al., 2012; Zhao et al., 2009). Furthermore, it considerably enhances the efficiency of the extraction and is friendly to environment, and thus has been widely employed to extract natural polysaccharides from different bioresources with great extraction efficiency and antioxidant properties (Chun-yu et al., 2004; Hu et al., 2012; Zhang et al., 2014).
Physicochemical properties of OBP play fundamental roles in their functionality in food systems. Various OBP sources have developed by different processing methods, such as acid or enzymatic hydrolysis, extrusion, and steam heating, for improving their functionality (Gamel et al., 2014; Zhang et al., 2009). These studies revealed the alterations in the molecular weight and structure of OBP during processing, as well as their effects on the physicochemical, rheological and functional properties of OBP (de Moura et al., 2011). Nevertheless, so far, the effects of superfine grinding treatment on the physicochemical and antioxidant activities of OBP were unknown yet. Further in-depth studies on the effect of food processing methods on the physicochemical and functional properties of OBP will widen their applications in food industry and open new possibilities for designing natural polysaccharide-enriched products (Rosell et al., 2009).
In this study, the superfine grinding technology was applied to influence the physicochemical and antioxidant properties of OBP. The raw OBP (rOBP) and superfine ground OBP (sOBP) extracts were obtained by ethanol precipitation. The physicochemical properties, thermodynamic properties, and antioxidant activities of OBP were investigated. The structure-activity relationships of OBP were also discussed to explore new biological function principle for use in food industry.
(1) Materials Naked oat (Avena nuda L.) bran was obtained from Yanbei Health-Care Food Co. (Wanquan County, Hebei Province, China). Trifluoroacetic acid (TFA) and the standard monosaccharides (fucose, rhamnose, ribose, arabinse, xylose, mannose, galactose, glucose, sorbose), T-series dextran standards, 1,1-diphenyl-2-picrilhydrazyl (DPPH˙), and 2,2-Azino-bis(3-ethyl-benzthiazoline-6-sulphonic acid) (ABTS˙+) were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO). All other chemicals and reagents were purchased locally and were of analytical grade.
(2) Superfine grinding treatment of oat bran and particle size measurement Dried oat bran was milled in a common pulverizer, and was sieved through a 40-mesh screen. Then the normal grinding oat bran (raw oat bran) was ball-milled in a CJM-SY-B type high-energy nano-mill (Taiji Ring Nano Products Co., Ltd., Qinhuangdao, China) with fast swing motion (100 – 400 times/min, motor power: 4 kW) for 8 h, and the superfine ground oat bran powder was obtained. The high-energy nano-mill utilized in the present study included ball-mill system and cooling circulation system as illustrated in Fig. 1.

The ball mill system.
Small angle X-ray scattering (SAXS) (Rigaku-3014, Rigaku, Japan) was employed to determine the particle size of superfine ground oat bran by following China National Standard GB/T 13321-2004 (Korhonen et al., 1998). Briefly, sample powders were dispersed in a celloidin-acetone solution and were dried at 20 – 50°C to remove acetone completely. Samples were analyzed at 35 kV, 20 mA with Co kα radiation. The scattering angle 2θ was set as 0 – 3°. A background scattering intensity of multilayer filter was subtracted from the sample intensities for data correction. The mean size (D), median size (d) and distribution spread (B) were calculated using the dividing distribution function (DDF) method according to GB/T 13221-2004.
(3) Extraction of polysaccharides Oat bran polysaccharides were extracted from raw and superfine grinding-treated oat bran. Briefly, 50 g of raw or superfine grinding-treated oat bran powder was extracted refluxed with 400 mL of 75% (v/v) ethanol at 80°C for twice to remove most of the polyphenols, flavonoids, lipids and monosaccharides. The residues of oat bran extracts were dried in air and were extracted with distilled water (1000 mL, 3 times) at 60°C for 30 min. All supernatants were combined and hydrolyzed with α-amylase (480 units/mL, Beijing Aoboxing Biotechnology Co., Beijing, China) at 60°C for 30 min and β-amylase (4700 units/mL, Beijing Aoboxing Biotechnology Co., Beijing, China) at 40°C for 30 min to remove starch. The supernatants were concentrated to 10% of the original volume under vacuum pressure and precipitated by adding 4 times of volume of 95% (v/v) ethanol at 4°C overnight. The mixture was filtered and vacuum freeze-dried. The protein in oat samples was then removed by the Sevag method. OBP samples from raw and ultrafine grinding-treated oat bran were obtained and named as rOBP and sOBP, respectively.
(4) Physicochemical properties of oat bran polysaccharides
a) Dietary fiber composition The dietary fiber composition was determined according to China National Standard GB/T 5009.88-2008. Briefly, OBP samples were hydrolyzed by α-amylase, protease, and glucosidase at 60°C for 30 min, respectively, to remove protein and starch completely. The hydrolysates were then precipitated by adding 95% (v/v) ethanol at 60°C for 1 h. The mixtures were filtered and were washed with ethanol and acetone for twice. The residues were dried at 105°C for 4 h. The total dietary fiber (TDF) content was obtained by weighing the dried residues. The insoluble dietary fiber (IDF) content was obtained by filtering the hydrolysates directly, then washing with hot water, and drying to constant weight. The filtrate was precipitated by adding 4 times of volume of 95% (v/v) ethanol, and the soluble dietary fiber (SDF) content was obtained by filtering the sediment the and drying to constant weight.
b) Monosaccharide composition The composition of neutral monosaccharide in OBP was determined by gas chromatography (Korhonen et al., 1998). Generally, 10 mg of polysaccharide sample was hydrolyzed with 1 mL of 2 M TFA at 120°C for 4 h. TFA was evaporated to dryness at 60°C under vacuum pressure. The hydrolyzed polysaccharide sample with fucose (Fuc), rhamnose (Rha), ribose (Rib), arabinose (Ara), xylose (Xyl), mannose (Man), galactose (Gal), glucose (Glu), sorbose (Sorb) as monosaccharide standard, were dissolved in 2 mL distilled water, reduced by 30 mg NaBH4 for 1.5 h at room temperature, treated with acetic acid, and dried at 60°C under vacuum pressure. The HCl-methanol solution was added to help removing NaBH4 at 105°C for 15 min. Then 0.5 mL acetic anhydride and 0.5 mL pyridine were added and reacted in a boiling water bath for 1 h. Gas chromatography was performed on a 7890 A GC instrument (Agilent Technologies), 3 µL acetate derivatized samples were injected into an OV-1701 capillary column (30 m × 0.32 mm, 0.50 µm thickness). The operation was performed using the following conditions: H2 40 mL/min; air 450 mL/min, N2 34 mL/min; initial temperature 150°C, held 1 min; 10°C/min from 150°C to 200°C, 200°C for 10 min; then increase temperature by 5°C/min for 4 min to 220°C, held 5 min; 1.5°C/min from 220°C to final temperature 240°C, 240°C hold for 20 min.
c) Molecular weight determination Molecular weight (Mw) distributions of OBP samples were determined using a Shimadzu LC-20AT HPLC system (Shimadzu, Japan) equipped with a RID-10 A refractive index detector (Shimadzu Scientific Instruments Inc., Kyoto, Japan) and a Shodex Ohpak SB-804HQ column (8.0 × 300 nm, 10 µm) placed in a 30°C column oven. OBP samples (1% w/v) were dissolved in ultrapure water and were filtered through 0.45 µm filter. The flow rate of the mobile phase (ultrapure water) was set at 0.8 mL/min and sample injection volume was 20 µL. Calibration of the column was performed according to five different Mw standards (Pharmacia International Ltd.) in the range of 40 – 359 kDa. The Mw of OBP samples were calculated from the calibration curve.
d) Particle size determination The mean diameter (D) of OBP samples were conducted using a BI-200SM dynamic light scattering (DLS) system (Brookhaven Instruments Corp., New York, USA) equipped with a MGL-III model 100 mV He-Ne laser (γ=532 nm), a computer-controlled BI-200SM goniometer, and a BI-9000AT digital correlator. Light scattering was monitored at a 90° angle and the temperature of the sample holder was controlled at 25°C via a recirculating water bath. Sample solutions (1 mg/mL) were carefully filtered through a 0.45 µm membrane directly into a borosilicate glass tube and were measured within 5 minutes. Particle sizes of OBP species were obtained by CONTIN model.
e) Solubility OBP supersaturated solutions were prepared in flasks and were incubated at 90°C for 1 h with magnetic stirring. All flasks were then left to cool to room temperature and were centrifuged at 1,611 xg for 30 min. The supernatant (V1 mL) was decanted into a breaker and dried to a constant weight (m1 g) in an oven at 110°C The solubility (g OBP/100 mL) was calculated using the following equation:
 |
f) Water holding capacity (WHC) The WHC of OBP samples were determined using the procedure as follows. A 2.5% w/w dispersion was prepared through dissolving 0.1 g dried sample by distilled water in a 50 mL pre-weighted centrifuged tube and agitated thoroughly by a vortex mixer. Samples were placed at 4°C for 24 h, and then were centrifuged at 1,611 xg for 30 min. The supernatant was decanted and the wet sediment was weighed. WHC was defined as follows:
 |
(5) Flow properties
a) Intrinsic viscosity Viscosity of OBP solutions were measured at 25°C using a 4 − 0.55 Ukrainian-type capillary viscometer, which is suspended in a thermostatic water bath under precise temperature control. Using exactly 7 mL of solution sample, the system was manually diluted after generating at least three efflux time readings at each concentration. The OBP sample viscosity (η) was converted to specific viscosity (ηsp), which was calculated as follows:
 |
where ηs is the viscosity of the solvent (deionized water).
The intrinsic viscosity [η] was usually obtained from extrapolation of ηsp/c to infinite dilution according to the Huggins empirical expression (Huggins, 1942) as follows:
 |
where the Huggins coefficient k1 is a measure of polymer/polymer interaction in dilute conditions and also depends upon the extent of coil expansion of the polymer coil; c is the concentration of OBP sample.
b) Flow behavior Rheological properties of OBP samples (0.5% and 1.0%) were taken using a rotational rheometer (Brookfield Engineering Laboratories model DV-III+ULTRA, USA) with concentric cylinders spindle ULA. The temperature was controlled at 25°C. A dependence of apparent viscosity on shear rate was observed in controlled rate mode. The shear rate was linearly increased from 10 to 250 s−1. The experimental data were fitted by a power law constitutive equation:
 |
where τ is the shear stress (Pa); K is the consistency coefficient (Pa sn); γ is shear rate (s−1); n is the flow behavior index (dimensionless) describing the divergence from the Newtonian model, n < 1 for a shear-thinning fluid and n = 1 for a Newtonian fluid.
(6) Thermal stability Differential scanning calorimetry (DSC) measurements on rOBP and sOBP were carried out using a DSC-60 A calorimeter (Shimadzu Scientific Instruments Inc., Maryland, USA). Samples were prepared by adding water with a microsyringe to OBP (2.0 mg) in an aluminium capsule and sealed hermetically. Then samples were scanned from 30 to 300°C under a dry nitrogen atmosphere at a heating rate of 10°C/min, against an empty reference capsule. The peak onset temperature (To), peak maximum temperature (Tp), endset temperature (Te) and the endothermic enthalphy change (ΔH) were determined.
(7) Antioxidant activity
a) DPPH radical scavenging assay DPPH radical scavenging activity (RSA) was measured according to the method with some modification (Sun et al., 2009). Here, 2.5 mL of 0.2 mM DPPH ethanol solution was mixed with 0.5 mL of OBP sample. The mixture was vigorously shaken and left to stand at room temperature for 10 min in the dark. The absorbance value (Ai) of this solution was measured at 517 nm using an UVmini-1240 spectrophotometer. DPPH solution that did not contain OBP sample was used as a control (A0). Aj is the absorbance value of the mixture of 2.5 mL anhydrous ethyl alcohol and 0.5 mL OBP sample. RSA was calculated as follows:
 |
b) ABTS RSA ABTS RSA was measured according to the method with some modifications (Choi et al., 2009). ABTS radical cation was produced by reacting 7 mM ABTS solution with 2.45 mM potassium persulfate and allowing the mixture to stand in the dark at room temperature for 16 h. In the moment of use, the ABTS solution was dilute with PBS (pH 7.0) to an absorbance of 0.70 ± 0.02 at 734 nm. 0.15 mL OBP sample was added to 2.85 mL of ABTS solution and mixed vigorously. The reaction mixture was allowed to stand at room temperature for 6 min and the absorbance at 734 nm was immediately recorded. The ABTS scavenging effect was calculated as follows:
 |
where A0 is the absorbance value of ABTS without sample, A is the absorbance value of OBP sample and ABTS, and Ab is the absorbance value of OBP sample without ABTS.
c) FRAP assay The ferric reducing/antioxidant power (FRAP) assay was measured according to the method of Benzie et al. (2004). FRAP solution was preheated to 37°C and 2.7 mL of the solution was mixed with 0.3 mL of OBP. The mixture was allowed to keep at room temperature for 10 min in the dark condition. The absorbance value (A) of the mixture was recorded at 593 nm. The standard curve was linear between 0.1 and 1.0 mmol/L FeSO4 and the FRAP value was calculated as follows:
 |
where C is the FeSO4 concentration (mmol/L), and A is the absorbance value at 593 nm.
(8) Statistical analysis Results were analyzed by one-way ANOVA and Student's t-test. Differences were considered statistically significant at p < 0.05 for all tests.
(1) Particle size of superfine ground oat bran powder The intensity profile of SAXS scanning is closely related to the particle size distribution of the sample, and SAXS results always provides the primary particle size rather than internal crystallite or external agglomerate size (Troy et al., 1999). Therefore, SAXS is an effective technique to determine the particle size distribution of the superfine powder. As seen in Table 1, the particle size of the superfine ground oat bran powder was distributed in the range from 10 nm to 620 nm and mostly concentrated in the range of 200 – 620 nm with an average particle size of 397.6 nm, which is much less than the average diameter of plant cell (10 – 200 µm).
| Size interval (nm) | Distribution frequency (%/nm) | Volume fraction (%) | Cumulative volume fraction (%) |
|---|---|---|---|
| 10–18 | 0.22 | 1.8 | 1.8 |
| 18–36 | 0.06 | 1.1 | 2.8 |
| 36–60 | 0.01 | 0.1 | 3.0 |
| 60–96 | 0.01 | 0.3 | 3.3 |
| 96–140 | 0.01 | 0.6 | 3.9 |
| 140–200 | 0.04 | 2.1 | 6.0 |
| 200–300 | 0.20 | 20.0 | 26.1 |
| 300–430 | 0.22 | 28.6 | 54.6 |
| 430–620 | 0.24 | 45.4 | 100.0 |
Volume mean diameter (Dv) = 397.6 (nm), Volume median diameter (dv) = 408.9 (nm), Distribution spread (Bv) = 134.8 (nm).
(2) Physicochemical properties of oat bran polysaccharides
a) Dietary fiber composition The dietary fiber compositions of rOBP and sOBP samples are shown in Table 2. After the superfine grinding process, the IDF and TDF content in OBP sample decreased from 29.09 and 38.07 to 20.06 and 32.51 g/100 g, respectively; whereas the SDF content significantly increased from 8.49 to 11.12 g/100 g. Generally, the polysaccharide chain of IDF is longer than that of SDF, it is likely that the IDF polysaccharide chains were apt to break into SDF during the superfine grinding process.
| Physicochemical properties | rOBP | sOBP |
|---|---|---|
| Insoluble dietary fiber (g/100 g) | 29.09 ± 0.74a | 20.06 ± 0.3b |
| Soluble dietary fiber (g/100 g) | 8.49 ± 0.18a | 11.12 ± 0.14b |
| Total dietary fiber (g/100 g) | 38.07 ± 0.17a | 32.51 ± 0.26b |
| Monosaccharide composition (mol.%) | ||
| Arabinose | 2.30 | 4.61 |
| Xylose | 1.55 | 4.03 |
| Glucose | 95.55 | 83.56 |
| Mannose | 1.54 | 1.00 |
| Galactose | 1.00 | 1.43 |
| Average molecular weights (× 103 Da) | 655.6 | 489.6 |
| Particle size (nm) | 139.87 ± 1.40a | 111.90 ± 1.65b |
| Solubility (mg/mL) | 8.1 ± 0.53a | 11.3 ± 0.71b |
| WHC (g/g) | 13.94 ± 0.40a | 10.44 ± 0.35b |
Values are expressed as mean ± SD (n = 3) of three replicated determinations. Means with the different letter in the same index are significantly different (p < 0.05).
b) Monosaccharide composition The monosaccharide analysis of OBP sample was conducted by GC method. As shown in Table 2, both rOBP and sOBP were composed of arabainose, xylose, glucose, mannose, and galactose, and glucose had a predominate ratio in OBP samples. After the superfine grinding treatment, the contents of arabinose, xylose, galactose slightly increased, whereas the glucose content decreased by 11.99%. Oat is an excellent source of different dietary fiber types, such as mixed-linked (1→3), (1→4)-β-D-glucan, arabinoxylans, cellulose, and so on (Drzikova et al., 2005). The major component of the endosperm cell walls of the oat bran is a (1→3), (1→4)-β-D-glucan, and mainly composed of glucose. In this study, the monosaccharide composition of OBP was changed after superfine grinding treatment as shown in Table 2. This result was different from Virkki and others' study (2005), in which OBP was composed of arabinose, xylose, as well as glucose, and the amount ratio of them differed according to the different extracted fraction and processing method used. This might be due to the change of oat bran cell walls after the processing and different constituents were extracted.
c) Molecular weight distribution and particle size HPLC chromatograms of OBP samples are shown in Fig. 2. It showed that the superfine grinding process induced a decrease in the amounts of high-molecular-weight OBP. As shown in Table 2, the average molecular weights of rOBP and sOBP were 655.6 and 489.6 kDa, respectively, which suggested that superfine grinding treatment might cause the pulverization of the high molecular weight fiber in the ultrafine grinding process. The average diameters of OBP samples in dilute water solution by DLS. The average diameters of rOBP and sOBP were 139.87 nm and 111.90 nm, corresponding with the average molecular weights of 655.6 and 489.6 kDa.
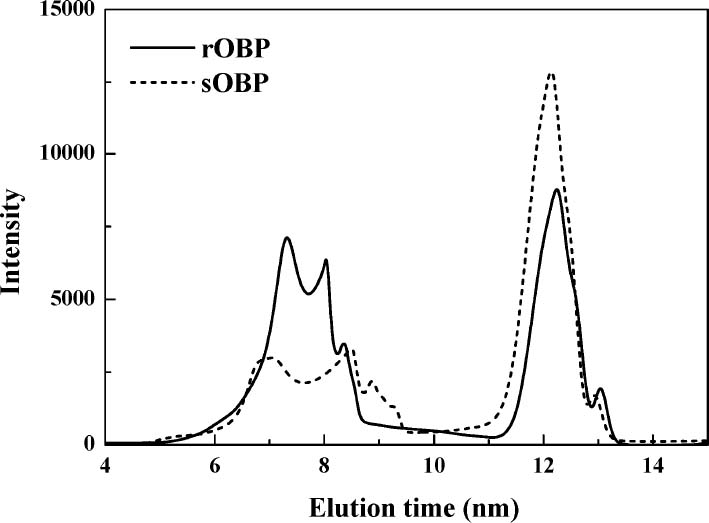
HPLC chromatograms of rOBP and sOBP.
d) Solubility and water holding capacity Solubility and water holding capacity of OBP separated from superfine grinding-treated oat bran differentiated each other (Table 2). Solubility of OBP samples varied from 8.1 to 11.3 mg/mL, and WHC values were changed from 13.94 to 10.44 g/g. Solubility was observed to increased significantly by superfine grinding treatment. The change trend of WHC value was opposed to that of solubility. The WHC value of rOBP was higher than that of sOBP. WHC value has been reported to be influenced by strongly bonded micellar networks and amylopectin molecular structure (Kratz et al., 2013). The higher solubility and lower WHC value of sOBP may be attributed to the degradation of long polysaccharide chains by superfine grinding treatment.
(3) Flow properties of oat bran polysaccharides
a) Intrinsic viscosity The typical Huggins plots for calculation of OBP's intrinsic viscosity are represented in Fig. 3A. The instrinsic viscosities, [η], were determined using the Huggins empirical expression. The fitting equations for rOBP and sOBP were y=6.347x+94.677 (R2=0.998) and y=5.977x+4.573 (R2=0.998), respectively, where y is ηsp/c and x is c. It can be seen that the intrinsic viscosities of rOBP and sOBP were 94.677 and 4.573 mL/mg, respectively, indicating that sOBP had much smaller intrinsic viscosity than that of rOBP. The relatively high viscosity of OBP will limit their application in food processing. The intrinsic viscosity of OBP significantly decreased after superfine grinding treatment, which render OBP might be of potential use in the food industry.

Intrinsic viscosity (A) and shear rate dependence of viscosity for rOBP (B) and sOBP (C) solutions with different concentrations: ◯ 0.5%; ◆ 1.0%.
b) Flow behavior To better understand the processing behavior, the shear rate dependence of apparent viscosity for rOBP and sOBP dispersions was investigated. The apparent viscosity of rOBP was much higher than that of sOBP over the entire measured shear rates at both 0.5% and 1.0% OBP concentration (Fig. 3B and C). 1.0% of rOBP and sOBP showed shear-thinning behavior typical of polysaccharides, since longer polymers tend to participate in more entanglements which are responsible for the shear-thinning phenomenon (Brummer et al., 2014). 0.5% of rOBP and sOBP maintained the viscosity with increasing shear rate. It can be seen that the apparent viscosities of dispersions were dependent on the concentration and molecular weight of OBP. The larger OBP concentration and molecular weight, the higher were the apparent viscosities of rOBP and sOBP dispersions (Xu et al., 2013). In addition, the flow behavior can be characterized by a power law constitutive equation. The dispersions of 1.0% rOBP and sOBP, which had relatively high polysaccharide contents, possessed smaller flow behavior index of 0.918 and 0.987, respectively; while the dispersion of 0.5% rOBG and sOBG, which had relatively small polysaccharide contents, possessed higher flow behavior index of 0.977 and 1.031, respectively. This result showed that rOBP at different concentrations (0.5% and 1.0%) and sOBP at a high concentration of 1.0% exhibited a shear-thinning character, whereas sOBP at lower concentration of 0.5% showed a flow behavior similar to Newtonian fluid, suggesting that the molecular weight and concentration of OBP samples would affect their flow behavior.
(4) Thermal properties of oat bran polysaccharides Gelation of oat bran polysaccharides involves the formation of network structures through inter-chain segment association and aggregated junction zones (Rodarte et al., 2004; Troy et al., 1999). Endothermic peaks during the gel→sol transition from DSC measurements can provide the information on the melting process of polysaccharides. DSC was performed to further evaluate the effect of superfine grinding on the thermostability of OBP. As shown in Fig. 4, melting peak temperatures (Tp) of rOBP with the water content of 0%, 50%, and 75% were 49.88, 157.81, and 147.89°C, respectively; whereas those of sOBP were 49.93, 167.29, and 126.19°C. And the heat enthalpy change (ΔH) of OBP decreased from −328.79 J/g, 942.41 J/g, and −1.94 kJ/g to −197.01 J/g, −703.29 J/g, and −1.50 kJ/g, respectively, indicating that sOBP required less energy for melting. This result suggested that Tp and ΔH have closely relationship with the molecular weight of OBP.

DSC curves of rOBP and sOBP with different water contents.
(5) Antioxidant activity of oat bran polysaccharides
a) DPPH RSA Antioxidant activities of OBP extracts were investigated using the DPPH radical scavenging, ABTS radical scavenging and FRAP assay. As shown in Fig. 5A, the DPPH RSA of OBP was much smaller than that of ascorbic acid (Vc), but significantly increased from 13.46% to 19.10% (p < 0.05) by superfine grinding treatment with the OBP concentration of 5 mg/mL. The result suggested that some active residues might be exposure from intramolecular polysaccharide chains, and thus the DPPH RSA of OBP was enhanced by superfine grinding treatment.

Antioxidant activities of rOBP and sOBP. (A) DPPH RSA assay, (B) FRAP assay, and (C) ABTS RSA assay.
b) FRAP activity The RSA assay was generally concomitant with reducing power. The FRAP activity was defined as the corresponding FeSO4 concentrations of the UV value, mmol/L. As shown in Fig. 5B, the FRAP value of absorbance at 593 nm in the calibration, namely FRAP sOBP samples was higher than that of rOBP samples in the concentration range of 1 – 5 mg/mL. Similar with the results of DPPH assay, the FRAP value of OBP was much smaller than that of Vc, and significantly increased from 0.33 to 0.74 at the OBP concentration of 5 mg/mL. This result indicated that the total antioxidant activity of OBP sample was obviously enhanced during superfine grinding processing, as the FRAP value of sOBP was approximately twice higher than that of rOBP.
c) ABTS RSA The ABTS RSA of OBP samples at different concentrations are shown in Fig. 5C. Consistent with the results of DPPH and FRAP assays, the ABTS RSA of sOBP sample was also higher than that of rOBP sample at different OBP concentration. Specially, the ABTS RSA of OBP increased from 38.87% to 62.29% at the OBP concentration of 5 mg/mL. In addition, the sOBP sample showed a relatively high ABTS radical scavenging activity (62.29%) as compared with that of DPPH radicals (19.10%) at the same OBP concentration (5 mg/mL), indicating that rOBP can be a good scavenger of ABTS radicals.
The DPPH RSA, FRAP value, and ABTS RSA of OBP increased with decreasing of the molecular weight by the superfine grinding treatment, suggesting that molecular weight played an important role in OBP's antioxidant activities. According to Sun et al. (Sun et al., 2009), high-molecular weight polysaccharides from P. cruentum had no obvious antioxidant activity, but low-molecular weight fractions after degradation exerted an inhibitory effect on oxidative damage (Choi et al., 2009). This may due to that high-molecular weight polysaccharides has a compact structure, and thus the effect of intramolecular hydrogen bond is stronger. The strong effects of intramolecular hydrogen bonds could weaken the activities of hydroxyl groups and their exposure to the surface of molecular chains, which would account for less radical scavenging and total antioxidant activity. In addition, the changes in the physicochemical properties of OBP samples after superfine grinding treatment, for instance, molecular weight and monosaccharide composition, might lead to the increase in the antioxidant activities of sOBP samples. Especially, the increase of the solubility of the sample seems also to be a main factor for the higher antioxidant activities of rOBP samples.
OBP from superfine grinding treated and untreated oat bran were extracted. The physicochemical properties, flow properties of aqueous dispersion, thermal properties, and antioxidant activities of rOBP and sOBP samples were investigated. The results showed that superfine grinding treatment could decrease OBP's molecular weight and apparent viscosity, enhance OBP's solubility and antioxidant activities, suggesting that superfine grinding technology had a great potential to be applied in functional polysaccharide food. The physicochemical properties, viscosity, thermal stability, and antioxidant activities have closely relationship with the molecular weight of polysaccharides.
Acknowledgments The project was supported by the National Natural Science Foundation of China (No. 31171781) and the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20121208110003) and the fund of Tianjin University of Science and Technology (No. 2014CXLG01).