2016 Volume 22 Issue 2 Pages 173-183
2016 Volume 22 Issue 2 Pages 173-183
The optimal initial gas composition (CO2, O2 and N2) and its effects on quality and shelf-life of Pacific white shrimp (Litopenaeus vannamei) under controlled freezing-point storage at −0.8°C was determined through microbial flora, pH, total volatile basic nitrogen (TVB-N), exudates and sensory analyses. Pacific white shrimp under gas to product ratio of 3:1 and gas composition spanning the whole area from 0 to 100% based on a simplex centroid mixture design were analyzed after 2, 4 and 6 days of storage. Mesophilic and psychrotrophic bacteria counts, pH and TVB-N contents decreased and formation of exudates increased with increasing of CO2 levels, but odor and appearance scores decreased and mesophilic and psychrotrophic bacteria counts, pH and TVB-N contents increased in the shrimp with decreasing CO2 and increasing N2 concentration. Besides, low O2 levels could favor the odor of raw shrimp. Finally, The optimum gas composition for modified atmosphere packaged Pacific white shrimp under controlled freezing-point storage at −0.8°C was determined to be 75%CO2, 10%O2 and 15%N2, and it could give the most suitable formation of exudates and lowest TVB-N and inhibit the growth of microbial flora; and at the same time maintain high odor and appearance scores in packaged Pacific white shrimp, and the shelf-life was extended to 11 – 12 days, which would be obviously beneficial for the exploitation of quality control, shelf-life extension and development of active packaging on shrimps.
Pacific white shrimp (Litopenaeus vannamei) is one of the most representative fishery products for its positive culinary quality and high nutritional values (Oosterveer, 2006; Qian et al., 2013). Since fresh shrimps have a short shelf life, which causes substantial practical problems for its transportation and distribution, and they are very sensitive to microbial spoilage and can be contaminated by bacteria naturally present in the marine environment (Jeyasekaran et al., 2006; Wang et al., 2010, 2014), and moreover parts of shrimp tissues after death are still active and biochemically alive, the organic decomposition or the change of shrimp composition may be triggered by various factors, i.e. enzymes and microbiological activities, the need to develop methods for maintaining good postmortem quality of shrimps on their way to the market increases (Cui et al., 2013; Wang et al., 2014).
Among the technologies that maintain the quality with minimum losses, modified atmosphere packaging (MAP), along with preservatives or low-temperature storage, have become increasingly popular preservation techniques, which have brought major changes in storage, distribution, and marketing of raw shrimps to meet consumer demands (Chinivasagam et al., 1996; Lakshmanan et al., 2002; Lalitha and Surendran, 2006; Thepnuan et al., 2008; Lu, 2009; Wang et al., 2014). However, MAP solutions often hampered by high gas volume to product volume ratio (g/p) and gas concentration (i.e. CO2, O2 and N2), especially the optimum level of each gas for each food product must be determined and used in order to maximize the positive and minimize the negative effects of each gas and thereby obtain the appropriate shelf-life extension (Floros and Masos, 2005; Al-Nehlawi et al., 2013). Up to now, only little information is available on the optimum gas mixture for MAP of aquatic products (Fletcher et al., 2004; Simpson and Carevic, 2004; Sivertsvik, 2007), and moreover to our knowledge, most of MAP samples were kept in cold storage or in freezers. However, more recently, an additional storage technique based on the Japanese concept of ‘Hyo-on’ has attracted considerable interest. ‘Hyo-on’, also called controlled freezing-point storage, employs a temperature range, in which foods remain in a non-frozen temperature-zone between the freezing point of water and that of an individual material (Yamane, 1982; Fukuma et al., 1998). Storing food at controlled freezing-point temperatures can be advantageous in terms of maintaining food freshness and suppressing harmful microorganisms as compared to other storage temperature (Yamane, 1996; Wang et al., 2010, 2014). In particular, controlled freezing-point storage, combined with the optimized gas mixtures, will be a considerably better method to maintain the freshness and extend the shelf-life of Pacific white shrimp (Litopenaeus vannamei).
Therefore, the purpose of this study was to find the optimal modified atmosphere for packaging of Pacific white shrimp (Litopenaeus vannamei) under controlled freezing-point storage at −0.8°C by using a simplex centroid mixture design with 3 components (CO2, O2 and N2) spanning the whole area from 0 to 100%, in which microbial flora, pH, total volatile bases nitrogen (TVB-N), exudates and sensory attributes were measured. In this context optimum gas composition obtained was used to pack fresh Pacific white shrimp and assess its effects on quality and shelf-life of shrimp samples. MAP with 40%CO2/30%O2/30%N2 was used as control for comparison.
Samples preparation Fresh Pacific white shrimps (Litopenaeus vannamei), of the size of 55 – 65 shrimps/kg, were procured from local aquatic products market. The shrimps with signs of visual defect or breakage were removed, and guaranteed the average length and weight of shrimp were ca. 14 cm and 17 g, respectively. After procurement, the live shrimp were placed in a large polyethylene bag with sufficient oxygen and transported to laboratory within 1 h, and then washed in filtered clean flowing water and stored in ice until used (not more than 2 h).
Study design The study was designed to find the optimized MA for packaging of Pacific white shrimp by using a simplex centroid mixture design spanning the whole area from 0 to 100% among CO2, O2 and N2 (Fig. 1), and then on this basis evaluate the quality and shelf-life characteristics of Pacific white shrimp under controlled freezing-point storage at −0.8°C. Prior to all analyses aliquots of 12 specimens were individually weighed and placed separately in a thermoplastic starch tray (TPS tray, 238 × 173 × 25, length × width × depth, in mm) (THP-34, Tianhe Environmental Technology Co., Ltd., Zhejiang). Then all trays were packed inside polyamide and polyethylene bags (33.5 cm long × 27 cm wide; 160 µm in thickness; water vapor transmission rate of 1.30 – 1.52 g per m2 at 37°C, 90% relative humidity, 24 h; oxygen transmission rate of 16.8 – 20.2 cm3 per m2 at 23°C, 75% relative humidity, 24 h) and the gas mixtures applied using a Model DQ360 machine (Qingpa Co., Ltd., Shanghai, China). Firstly, the air was evacuated from packages and immediately selected gas mixtures was flushed with gas to product ratio of 3:1 and the bag sealed.

Experimental design for optimizing the modified atmosphere for packaging of Pacific white shrimp.
Twelve batches were then obtained (12 bags per batch) as displayed in Table 1. Immediately after packing, all the packs were kept in a precise refrigerator (Model SHP-2500, P&S Co., Ltd., Shanghai, China) (Storage temperature as mentioned in the results of initial freezing point) for a total of six days. Sampling took place after 2, 4, 6 days of storage, and for day 0, fresh Pacific white shrimp was directly used.
| Experimental Variables/Levels | Axial Point (3) | Center Point (3) | Vertex (3) | Double Blend (3) |
|---|---|---|---|---|
| − 1 | 0 | + 1 | + 2 | |
| CO2:O2:N2 | 67:17:17 (G1) | 33:33:33 (G0) | 100:0:0 (G4) | 50:50:0 (G7) |
| 17:67:17 (G2) | 33:33:33 (G0) | 0:100:0 (G5) | 50:0:50 (G8) | |
| 17:17:67 (G3) | 33:33:33 (G0) | 0:0:100 (G6) | 0:50:50 (G9) |
Note: G0 and G (1⋯9) represented center mix (n = 3) and all 9 gas mixes, respectively.
Differential Scanning Calorimeter measurement Tests were conducted on a differential scanning calorimeter (DSC, 200PC, Netzsch Co., Ltd., Germany) with automatic data analysis software. Fresh shrimp samples were beheaded, peeled and ground to obtain uniformity in clean condition, and small samples (8–10 mg each) were hermetically closed in aluminum DSC pan and very precisely weighed. Prior to measurements, the DSC was calibrated for temperature and energy sensitivities using indium, Hg and C6H12. Samples were frozen in situ in the calorimeter with liquid nitrogen cooling and stabilized at −40°C and then heated from −40°C to 20°C at a rate of 2°C/min. An empty pan was used as a reference and the baseline was optimized using two empty pans (one placed at the reference oven compartment and the other at the sample oven compartment) and sapphire was used as a standard with a known specific heat value. Six replications were performed and specific heat capacity of these runs was calculated using the following equation (Eq. 1) and results were displayed as average curve with standard deviation in the figure.
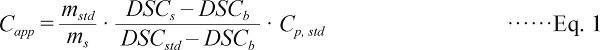 |
Where Capp and Cp, std are the specific heats of sample and sapphire, respectively (kJ/kg °C), ms and mstd are the weights of sample and sapphire, respectively (kg), and DSCs, DSCstd and DSCb are the heat flow rates of sample, sapphire and baseline, respectively (mW) (Wang et al., 2015).
Microbiological analysis A sample of approximately 25 g was taken aseptically from each designed gas mixtures within the same replication, transferred to a Stomacher bag and 225 mL of 0.1% sterilized peptone (Bacto peptone, BD211667, NY, USA) water with salt (NaCl, 0.85%, wt/vol) were added, and the mixture was homogenized for 120 s in a Stomacher F-200 Laboratory Blender (Specimen and model factory, Shanghai, China). Then serial decimal dilutions of each homogenate were carried out with the same diluents. For the mesophilic bacteria and psychrotrophic bacteria, samples (1.0 mL) of serial dilutions of shrimp homogenates were inoculated into 10 mL of molten (45°C) Plate Count Agar (PCA, BD247940, NY, USA). After setting, a 10-mL overlay of molten medium was added, and the plates were incubated at 37°C for 2 days and 7°C for 10 days, respectively. Three replicates of at least three appropriate dilutions were enumerated. Microbiological data was transformed into logarithms of the number of colony-forming units (log CFU/g).
Physicochemical analysis For pH determination, 5 g of shrimp meat was homogenized with 45 mL deionised water for 90 s and the homogenate was kept at room temperature for 5 min. The pH was measured using a pH meter (PHS-3C, Shanghai, China) (Wang et al., 2010, 2014).
TVB-N contents in shrimp meat were determined using the Conway micro-diffusion method (Conway and Byrne, 1936). Briefly, shrimp meat (2 g) was mixed with 8 mL of 4% TCA and homogenized at 6500 rpm using the Stomacher F-200 Laboratory Blender for 1 min. The homogenates were filtered through a filter paper (Whatman No. 41, Buckinghamshire, UK) and filtrates were used for analyses. Sample extract (1 mL) was placed in the outer ring, and then 1% boric acid containing the Conway indicator was pipetted into the inner ring. To initiate the reaction, saturated K2CO3 solution (1 mL) was mixed with sample extract. The Conway unit was closed and incubated at 37°C for 60 min. The inner ring solution was then titrated with 0.02 N HCl until the green color turned to pink. The contents of TVB-N were calculated and expressed as mg N/100 g shrimp meat.
The exudates in the packages during storage were measured gravimetrically. The entire package (sample and film) was weighed. Then, the samples and any purge were removed from the package, and the shrimp and the entire package surface were wiped clean with a paper towel. Finally, the shrimp samples were placed back into its package and re-weighed. The mass of the exudates (g) was divided by the initial mass of the product (g) and reported as a g/100 g initial weight (Fernández et al., 2009).
Sensory evaluation Sensory evaluation panel (12 persons) was recruited from a pool of panelists trained in descriptive analysis. Training consisted of preliminary trials and training of sensory evaluation standard. Eventually Standard references for raw shrimp odor and appearance were established based on Erickson and Wang's descriptive methods (Erickson et al., 2007; Wang et al., 2010, 2014) (Table 1). Briefly, raw shrimp was presented to a panel of trained panelists for sensory evaluation. Panelists were asked to rate samples as acceptable or unacceptable on the basis of odor and appearance using a 10-point scale, where 10 corresponded to a product of highest quality and 0 corresponded to a poor quality of product. Scores of 6.0 and above were considered acceptable. Above of all, raw shrimps were placed into a TPS box (167 mm length × 134 mm width × 50 mm depth, THH-09, Tianhe Environmental Technology Co., Ltd., Zhejiang) and held in a refrigerator (4°C) for less than 1 h before evaluations were conducted.
Statistical analysis and parameters verification In order to obtain the optimized CO2, O2 and N2 levels, Minitab's response/optimizer in Minitab's DOE package (Minitab Release 16, Minitab Ltd., Coventry, UK) was used to explored the experimental data to find a numerical solution, and in the meantime the optimizer does the same as a graphical solution using contour plots with target values and upper or lower boundaries. However, considering the effects of different gas mixtures on responses at sampling, the graphical solution could be more difficult to interpret in this case because of the number of responses. Therefore, global solutions were determined for both weighted and un-weighted responses but microbiological, physiochemical and sensory results were in turn given different weight and importance. The data from each replication was averaged, and means and standard deviations were calculated and the statistical significance of experimental data was also analyzed including multivariate contrasts and multiple comparisons by Tukey's test using the Origin 8.0 for Windows software (OriginLab Inc., Hampton, MA, USA). A confidence interval at the 95% level (P < 0.05) was considered in all cases.
| Category | Attribute | Definition | Reference/source |
|---|---|---|---|
| Odor | Old shrimp | Associated with old fish, from slight to strong | Shrimp powder |
| Ocean/seawater | Associated with the ocean or seawater, from slight to strong | Clam juice (QianChuan Rubber Co., Ltd., Qingdao, China) | |
| Appearance | Darkness | The intensity of the shell color, from light to dark | White bond paper (L = 91.32, a= 0.03, b = 0.01) |
| Black bond paper | |||
| Stripe darkness | The darkness of the stripes on the shell, from light to dark | White bond paper | |
| Black bond paper | |||
| Brown color | The brownness of the shell (from one section to six), from white to brown | White bond paper | |
| Light brown paper (L = 65 . 62, a = 8 . 12, b = 10 . 18) | |||
| Chocolate syrup (Deli Foods Co., Ltd., Wuhu, China) | |||
| Blotchiness | The amount of coverage of dark spots on the surface of the meat, from not blotchy to blotchy | None | |
| Fully covered | |||
| Glossiness | The amount of light reflected from the shell, from dull to glossy | White bond paper | |
| Laminated card |
Thereafter verification of the optimum MA for packaging of Pacific white shrimp (Litopenaeus vannamei) under controlled freezing-point storage at −0.8°C was performed. Briefly, the fresh shrimp samples packaged were experimentally analyzed on day 0, 2, 4, 6, 8, 10, 12, 14 of storage, in which MAP with 40%CO2/30%O2/30%N2, reported by many researchers (López-Caballero et al., 2002; Lu, 2009; Wang et al., 2010, 2014), was used as the control.
Initial freezing point Experimental data of specific heat capacity versus temperature for fresh Pacific white shrimp samples is shown in Fig. 2. In all replicates, the mean value of specific heat and standard deviation gave a coefficient of variation lower than 10%, indicating that the variability in the measurements was not excessive.

Experimental data of specific heat capacity versus temperature for fresh Pacific white shrimp samples
Specific heat depends strongly on temperature (Karunakar et al., 1998; Ngadi et al., 2003). Just as illustrated in Fig. 2, specific heat capacity of the samples increased with temperature until the values peaked (154.38 ± 7.48 kJ/kg °C), and immediately dropped from peak values to 4.56 ± 0.23 kJ/kg °C due to phase change. Subsequently the value became flat no matter how the temperature rose in the non-frozen state. This trend indicated that specific heat capacity of samples in frozen status was lower than that in non-frozen ones. Obviously it was due to the fact that a major portion of latent heat was removed from the samples when the phase was changed from frozen form to non-frozen one (Ramaswamy and Tung, 1981; Becker and Fricke, 1999; Tocci and Mascheron, 2008). Therefore, the position of thawing peak (−1.54 ± 0.07°C) was defined as initial freezing point of fresh Pacific white shrimp samples in the heat capacity versus temperature plot.
Considering that it is important to prevent temperature fluctuations that result in the thawing and re-freezing of shrimp samples during controlled freezing-point storage, −0.8°C was chosen as the storage temperature of this work combined with the function and characteristics of equipment.
Characteristics of fresh shrimp In this research, microbiological, physicochemical and sensory analysis of all samples were done before the gas mixture application in order to effectively study the effects of different gas mixture ratios on these results and in the meantime keep the pace of all data. At the time of packaging, low mesophilic counts (4.6 ± 0.2 log CFU/g, n > 6) and psychrotrophic counts (4.0 ± 0.5 log CFU/g, n > 6) for fresh Pacific white shrimp (Litopenaeus vannamei) indicated acceptable quality. Given that for newly caught shrimp, initial mesophilic bacterial counts and psychrotrophic bacterial counts are between 2 and 6 log CFU/g, depending on environment, temperature and species. Similar conclusions were reported for shrimp (Penaeus semisulcatus), freshly procured from the local fish market of India (Lakshmanan et al., 2002). The odor and appearance scores of fresh raw shrimp were 9.4 ± 0.4, 9.2 ± 0.2 (n > 6), respectively, which were in agreement with the relatively low microbial flora. In addition, TVB-N contents (7.8 ± 0.4, n > 6) were also lower as compared to those of most of fishes.
Microbiological analyses Though the microbiological counts in raw shrimp depended on environment, temperature and species at the time of packaging, the microbiology varied with initial gas mixtures during the whole periods of storage once packaged. The initial mesophilic bacteria in fresh shrimp was 4.6 ± 0.2 log CFU/g but decreased by about 0.5 – 1 log CFU/g within 1–2 days in most of packaging treatment, and later steadily increased on controlled freezing-point storage at −0.8°C, whereas the initial psychrotrophic bacteria of fresh shrimp (4.0 ± 0.5 log CFU/g), which was ca. 0.6 log CFU/g lower than the mesophilic bacteria because of the effect of cold shock, might adapt the environment gradually, recover and exceed mesophilic counts in all packaging treatment from day 2 on. Similar observations were made earlier by Lakshmanan et al. (2002), Thepnuan et al. (2008) and Wang et al. (2010). This increase in counts can be due to the growth of psychrotrophic bacteria in Pacific white shrimp at −0.8°C.
Regardless of packaging conditions, mesophilic and psychrotrophic bacterial counts were notably inhibited (P < 0.05) by increased CO2 levels after 2 days of storage and the highest inhibitory effects on them were observed in Pacific white shrimp packaged with initial gas mix of 100 mL CO2 per 100 mL atmosphere (G4), which may be attributed to the inhibitory effect created by the presence of CO2 on microbial growth (Table 3 and Fig. 3). It is well established that under CO2, a bacteriostatic effect is exerted on aerobic flora growth (Skandamis and Nychas, 2002; Laursen et al., 2006). Besides, the solubilization of CO2 in the water present in samples tends to create weakly acidic conditions and to stress the more strict aerobic microorganisms (Huss, 1995). Moreover the effect of O2 increases was noticeable in raw shrimp after 2, 4 and 6 days of storage and the worst microbiological counts were developed in pure N2 atmosphere (G6). But interestingly in our study, shrimp samples packaged under 100% O2 atmosphere (G5) had lower mesophilic and psychrotrophic bacteria counts as compared to those under 100% N2 atmosphere (G6) (P < 0.05) (Table 3 and Fig. 3). This phenomenon might be considered that the proliferation of microbial flora under O2 atmosphere was slower than that under N2.

Psychrotrophic bacteria in Pacific white shrimp as affected by initial gas mixture after 2, 4 and 6 days of storage under controlled freezing-point temperature at −0.8°C
| Runs | Storage Time(d) | Response Variables | ||||||
|---|---|---|---|---|---|---|---|---|
| Odor | Appearance | Exudate | TVB-N | Mesophilic | Psychrotrophic | pH | ||
| G0 (n = 3) | 2 | 8.2 ± 0.3 | 7.5 ± 0.3 | 4.10 ± 0.12 | 13.82 ± 2.20 | 3.8 ± 0.1 | 4.4 ± 0.3 | 7.21 ± 0.08 |
| 4 | 7.8 ± 0.2 | 7.0 ± 0.2 | 3.83 ± 0.17 | 18.14 ± 1.94 | 4.1 ± 0.2 | 4.8 ± 0.1 | 7.46 ± 0.13 | |
| 6 | 7.6 ± 0.2 | 6.8 ± 0.1 | 5.51 ± 0.24 | 28.27 ± 2.46 | 4.7 ± 0.2 | 5.2 ± 0.2 | 7.51 ± 0.16 | |
| G1 | 2 | 8.2 | 8.2 | 3.03 | 18.34 | 3.6 | 4.3 | 7.21 |
| 4 | 8.0 | 7.6 | 3.49 | 16.42 | 4.1 | 4.8 | 7.58 | |
| 6 | 7.6 | 7.1 | 2.86 | 26.78 | 4.5 | 5.1 | 7.52 | |
| G2 | 2 | 8.0 | 7.1 | 4.82 | 17.43 | 4.0 | 4.6 | 7.10 |
| 4 | 7.5 | 6.3 | 3.64 | 25.46 | 4.6 | 5.0 | 7.55 | |
| 6 | 7.2 | 5.0 | 6.28 | 31.10 | 5.0 | 5.2 | 7.83 | |
| G3 | 2 | 7.5 | 7.6 | 2.20 | 17.52 | 4.2 | 4.6 | 7.18 |
| 4 | 7.0 | 7.1 | 2.38 | 20.73 | 4.9 | 5.2 | 7.47 | |
| 6 | 6.4 | 6.6 | 5.37 | 25.05 | 5.1 | 5.4 | 7.67 | |
| G4 | 2 | 8.0 | 8.5 | 6.97 | 17.87 | 3.2 | 3.9 | 7.31 |
| 4 | 7.7 | 8.5 | 5.36 | 22.05 | 3.9 | 4.6 | 7.40 | |
| 6 | 7.7 | 8.1 | 9.52 | 25.53 | 4.3 | 4.9 | 7.38 | |
| G5 | 2 | 8.1 | 6.6 | 3.58 | 17.14 | 4.2 | 4.6 | 7.37 |
| 4 | 7.7 | 5.0 | 5.67 | 22.46 | 4.8 | 5.0 | 7.55 | |
| 6 | 7.1 | 4.6 | 4.96 | 29.68 | 5.1 | 5.3 | 7.49 | |
| G6 | 2 | 7.0 | 8.2 | 2.87 | 16.28 | 4.7 | 5.1 | 7.22 |
| 4 | 6.5 | 8.1 | 2.12 | 20.73 | 5.1 | 5.2 | 7.41 | |
| 6 | 6.1 | 7.6 | 4.03 | 28.79 | 5.3 | 5.7 | 7.51 | |
| G7 | 2 | 8.5 | 7.9 | 3.56 | 19.06 | 3.7 | 4.0 | 7.30 |
| 4 | 8.0 | 7.4 | 2.59 | 25.05 | 4.3 | 4.8 | 7.59 | |
| 6 | 7.8 | 6.8 | 4.79 | 26.18 | 4.9 | 5.1 | 7.56 | |
| G8 | 2 | 8.0 | 8.5 | 3.63 | 17.83 | 3.4 | 4.2 | 7.42 |
| 4 | 7.9 | 8.3 | 5.40 | 29.40 | 4.1 | 4.7 | 7.67 | |
| 6 | 7.3 | 7.6 | 7.61 | 22.70 | 4.5 | 5.1 | 7.58 | |
| G9 | 2 | 8.1 | 6.9 | 3.05 | 18.02 | 3.8 | 4.2 | 7.16 |
| 4 | 7.8 | 5.0 | 4.07 | 23.05 | 4.5 | 4.7 | 7.46 | |
| 6 | 7.5 | 4.8 | 5.20 | 27.33 | 4.9 | 5.2 | 7.66 | |
Physicochemical analyses The pH value is frequently used to complement the shrimp spoilage analysis. In this research, the initial value of pH in fresh shrimp at the time of packaging was found to be 7.28 ± 0.24 indicating the freshness of shrimp samples, which pH values of fresh or lively shrimps are generally under 7.2 or so. As displayed in Table 3, samples packaged with higher CO2 levels (i.e. 100% CO2, 67% CO2 and 50% CO2) had no statistically significant changes (P > 0.05) in pH values after 4 and 6 days of storage, which may be attributed to the dissolution of CO2 in the shrimp samples, acidifying it via the formation of carbonic acid (Banks et al., 1980). However, during the storage period at −0.8°C, pH values of the samples with higher O2 and N2 concentration were significantly (P < 0.05) higher than those of all the rest (Table 3), and the progressive increase of pH was postulated to be due to the rapid spoilage of the products and the formation of alkaline compounds of autolysis and bacterial metabolites such as ammonia, dimethylamine, trimethylamine, as well as other biogenic amines (Ruiz-Capillas and Moral, 2001; Laursen et al., 2006).
MAP treatment had practical implications on the exudates for MA-packaged samples, especially the higher the content of CO2 in MAP, more exudates was formed (Goulas and Kontominas, 2007). As depicted in Table 3 and Fig. 4, 100%CO2 atmosphere (G4) had the most significant effects (P < 0.01) on the exudates of shrimp samples, in the range from 5.36 g/100 g to 9.52 g/100 g. Because CO2 is a weak acid, accordingly the pH and water holding capacity of shrimp meat are definitively altered due to the solubilization of CO2 in water, and moreover different CO2 concentrations in the packaging gas can influence exudates formation from MA packaged products (Fernández et al., 2009). Besides, Sivertsvik et al. (2002) reported that the relatively low storage temperature favored the dissolution of CO2 in both the aqueous and fatty phase of fish. Given that the lower the temperature, the higher the solubility of CO2. The results in our study supported findings of increased exudates by increasing CO2 levels and storage time. On the contrary, the lower exudates were detected in the samples packaged with low CO2 levels, and moreover the notable exudates (2.12 – 4.03 g/100 g) were found in these packages.

pH values and Exudates in Pacific white shrimp as affected by initial gas mixture after 6 days of storage under controlled freezing-point temperature at −0.8°C
It was reported by Oehlenschläger (1997) that TVB-N contents in freshly caught marine fish investigated immediately after hauling was normally found to be on a very constant level. Similarly in our study, TVB-N contents of fresh Pacific white shrimp (Litopenaeus vannamei) were also kept in a steady range of 7.41–8.15 mg N/100 g shrimp meat at the initial. But TVB-N, as a considerably important freshness index, is responsible for the odor encountered in shrimp once having past the initial phase of freshness. As the storage time increased, it was observed that atmosphere with higher CO2 concentration notably inhibited (P < 0.05) the increase of TVB-N contents which were between 17.83 mg N/100 g and 26.78 mg N/100 g after 2 and 6 days of storage, respectively (Table 3). Especially samples with 100% CO2 (G4) and 67%CO2/17%O2/17%N2 (G1) had least changes in TVB-N contents after 4 and 6 days of storage, but it was also interesting to note that within the same period of storage, the steepest changes (P < 0.05) were found in samples packaged with 100%O2, 100%N2 and 50%O2/50%N2 atmosphere (Table 3 and Fig. 5), which was related to the activity of spoilage bacteria and endogenous enzymes that resulted in the formation of compounds including ammonia and primary, secondary and tertiary amines imparting characteristic off-flavors to shrimp (Debevere and Boskou, 1996; Ruiz-Capillas and Moral, 2001).

TVB-N contents in Pacific white shrimp as affected by initial gas mixture after 2, 4 and 6 days of storage under controlled freezing-point temperature at −0.8°C
Sensory evaluation The results of sensory evaluation (odor and appearance) of Pacific white shrimp under all packaging are presented in Table 3. On each sampling day, the spoilage patterns described by the panelists were similar in all packaging treatment and guaranteed no significant differences (P > 0.05) among the evaluation by analysis of variance, and score 6.0 was considered to be lower limit of sensory acceptance. According to the test panel, sensory scores showed a significant decline (P < 0.05) in all the samples with increasing storage period, in which mean odor scores were above 6.0 during the whole period of storage regardless of gas compositions whereas for shrimp appearance most of samples were receiving a score of higher than 6.0 except samples with high O2 atmosphere (100%O2, 17%CO2/67%O2/17%N2 and 50%O2/50%N2) (Table 3 and Fig. 6).

Odor scores and Appearance scores in Pacific white shrimp as affected by initial gas mixture after 6 days of storage under controlled freezing-point temperature at −0.8°C
However, of all the packaging treatments examined in the present study, it was found that none of the samples became putrid during the 6 days of storage because the samples that became spoiled during storage had a distinct old-shrimp-like odor usually with a sign of leaked liquid, softening shell and inflexible muscle, which also correlated rather well with microbiological counts and TVB-N contents. As expected, the best sensory scores (odor and appearance) after 6 days of storage were given to shrimp samples stored in pure CO2 and 50%CO2/50%O2, in addition, better scores were also obtained in other gas compositions, i.e. 67%CO2/17%O2/17%N2, 50%CO2/50%N2 (Table 3 and Fig. 6). From these results it was obvious that high percentage of CO2 might reduce the oxidative browning (results not shown) and inhibited propagation of mesophilic and psychrotrophic bacteria in shrimp samples, which was in agreement with the conclusions of Lu (2009). In contrast increasing levels of N2 and O2 led to the worst development in odor scores (i.e. 100%N2, 17%CO2/17%O2 /67%N2) and appearance scores of shrimp (i.e. 100% O2, 17%CO2/67%O2/17%N2, 50%N2/50%O2), respectively (Table 3 and Fig. 6). Interestingly, since high sensory scores were obtained under 50%CO2/50%O2 atmosphere, this was similar to the often recommended and commercially used gas mixture of MA packaged shrimp of 40–60 mL CO2 balanced with N2 per 100 mL gas atmosphere, such as deepwater pink shrimp (Parapenaeus longirostris) (López-Caballero et al., 2002), cold water shrimp (Pandalus borealis) (Mejlholm et al., 2005), Chinese shrimp (Fenneropenaeus chinensis) (Lu, 2009) and Pacific white shrimp (Litopenaeus vannamei) (Wang et al., 2010).
Optimization and verification of parameters Minitab's response optimizer in Minitab's DOE package was employed to obtain the targeted pH value, minimum microbiological growth, formation of exudates and TVB-N contents and maximum odor and appearance scores. In this study to optimize the response variables the importance and target values and upper or lower boundaries were in increasing order: pH (target, 7.2–7.4); Exudates (minimum, target: upper, 1–2:5 g/100 g); TVB-N (minimum, target: upper, 0:20 mg N/100 g); mesophilic bacterial counts and psychrotrophic bacterial counts (minimum, target: upper 3.5 – 4.5:5 – 5.5 log CFU/g) and the most importance odor and appearance scores (maximum, target: lower 9:6). After optimization analysis, the un-weighted and weighted gas compositions were found with 58.4%CO2/17.8%O2/23.8%N2 and 74.2%CO2/9.7%O2/16.1%N2, respectively. In addition, some local solutions were also obtained through individual response analysis, i.e. 37.7%CO2/62.3%O2, 58.4%CO2/17.8%O2/23.8%N2, 89.7%CO2/10.3%O2. Obviously the middle mixture was the same as the un-weight results. Due to the existence of polyphenoloxidase (PPO) in shrimps, the optimum gas mixtures are different from those in fishes, i.e. 30 – 40%O2 (Boskou and Debevere, 1997) and 40%CO2/30%O2/30%N2 (Sivertsvik et al., 2002; Goulas and Kontominas, 2007). However, it is recognized that O2 could not only inhibit potential growth of anaerobes but maintain the better aroma of meat (Jayasingh et al., 2001; Sivertsvik, 2007; Pantazi et al., 2008).
Based on the optimized parameters, the un-weighted (MAP1, 58.4%CO2/17.8%O2/23.8%N2) and weighted (MAP2, 74.2%CO2/9.7%O2/16.1%N2) gas compositions were used to pack the fresh Pacific white shrimp (Litopenaeus vannamei) and all the response variables of the shrimp samples were analyzed. Then, the experimental values of each of the responses were compared with the control (MAP3, 40%CO2/30%O2/30%N2), and the results are depicted in Fig. 7. According to a period of 14 days' storage, it was found that MAP2 was more effective for inhibiting growth of mesophilic bacteria and psychrotrophic bacteria and decreasing TVB-N contents as compared to the control. The pH values of samples in MAP1 and MAP2, similar to MAP3, showed the similar changes during storage. However, pH values of samples under MAP2 were significantly (P < 0.05) lower than those under MAP1. Formation of exudates under MAP1, MAP2 and MAP3 increased during the storage with the increase of CO2 levels but the values were not significantly different (P > 0.05) between MAP1 and MAP2 samples. Sensory evaluation indicated that the shelf-life of fresh Pacific white shrimp got extended and reached 11 –12 days for the optimum MA-packaged under MAP2 and 9 – 10 days under MAP1 as compared to 9 days' shelf-life under empirical MAP3 (Fig. 7).

Effects of MAP1, MAP2, and MAP3 on Mesophilic bacteria (A), Psychrotrophic bacteria (B), pH values (C), TVB-N values (D), Exudates (E), Odor scores (F), Appearance scores (G) of Pacific white shrimp during controlled freezing-point storage at −0.8°C. Each point is the mean ± SD of two replicate experiments with three samples analyzed per replicate (n = 6)

Effects of MAP1, MAP2, and MAP3 on Mesophilic bacteria (A), Psychrotrophic bacteria (B), pH values (C), TVB-N values (D), Exudates (E), Odor scores (F), Appearance scores (G) of Pacific white shrimp during controlled freezing-point storage at −0.8°C. Each point is the mean ± SD of two replicate experiments with three samples analyzed per replicate (n = 6)
In the present research the optimization design is valid within the limits of experimental factors used (such as the constant gas to product ratio of 3:1 and storage temperature) and could be employed to find the optimum gas mixture for modified atmosphere packaged Pacific white shrimp. However, changes in this ratio might impact the dissolvement of CO2 into shrimp samples. Likewise, any changes in solubility and the development of microbial flora, with the possibility of a different optimal solution. However, it is worth noting that our study is first on reporting the use of mixture design on the optimization of modified atmosphere for packaging of Pacific white shrimp (Litopenaeus vannamei) under controlled freezing-point storage at −0.8°C through microbiological, physicochemical and sensory analyses. Moreover, the use of optimized gas composition effectively extended the shelf-life of Pacific white shrimp (∼12 days), which would be applicable values for exploration of aquatic food storage, especially shrimps and shrimp products.
The optimal modified atmosphere for packaging of Pacific white shrimp (Litopenaeus vannamei) under controlled freezing-point storage at −0.8°C was determined to be 75%CO2, 10%O2 and 15%N2. Microbiological growth, pH and TVB-N contents decreased and formation of exudates increased with increasing of CO2 levels. Reduction of odor and appearance scores, and bacterial counts, pH and TVB-N contents increased in the shrimp with decreasing CO2 and increasing N2 concentration. But increasingly, low O2 levels (∼15%) favored the odor of raw shrimp. The optimized gas composition could give the most suitable formation of exudates and lowest TVB-N and inhibit the growth of microbial flora; and at the same time maintain high odor and appearance scores in packaged Pacific white shrimp, and the shelf-life was extended to 11 – 12 days, which would be obviously beneficial for the exploitation of quality control, shelf-life extension and development of active packaging on shrimps.
Acknowledgements This research was supported by the National Key Technology R&D Program of China (2012BAD28B05); National Natural Science Foundation of China (31071613); International S&T Cooperation Projects of China (2010DFB33930).