2016 年 22 巻 2 号 p. 227-234
2016 年 22 巻 2 号 p. 227-234
The anti-inflammatory effects of fucoxanthin (Fx) were investigated in vivo and in vitro. When mouse ear swelling was induced by three sensitizers, arachidonic acid, 12-O-tetradecanoylphorbol-13-acetate and oxazolone, both percutaneous and oral administration of 150 nmol/mouse of Fx suppressed the effects, similar to a typical natural inhibitor, epigallocatechin gallate (EGCG). The inhibitory effects of Fx, and its metabolite fucoxanthinol (Fxol), on inflammation-related enzymatic activities [phospholipase A2 (PLA2), cyclooxygenase (COX)-2, lipoxygenase, and hyaluronidase (HA)] were evaluated to elucidate the mechanism of suppression of mouse ear swelling. Both Fx and Fxol inhibited PLA2, COX-2, and HA activities to a greater extent than EGCG. Moreover, they suppressed mRNA expressions of PLA2 and COX-2 in rat basophilic leukemia-2H3 cells. These results suggest that the suppressive effects of Fx and Fxol on mouse ear swelling were due to the inhibition of enzymatic activities and mRNA expression.
Fucoxanthin (Fx) is believed to account for >10% of total carotenoids among marine products and is the most predominant in commonly eaten brown algae such as Laminaria japonica, Sargassum fusiforme, S. horneri and Undaria pinnatifida. In particular, S. horneri contains 0.37% (dry weight) of Fx, which is the highest among brown algae. Edible brown algae are the best sources of Fx (Kim and Pangestuti, 2011; Peng et al., 2011). In recent years, interest has focused on the functional benefits of Fx, with the discovery of antioxidative (Sachindra et al., 2007), anticancer (Kotake-Nara et al., 2005; Sugawara et al., 2006) and antiobesity (Maeda et al., 2005) effects. Among the functions of Fx, antiobesity effects have been well established. Fx derived from U. pinnatifida suppressed excess fat accumulation by increasing mitochondrial uncoupling protein 1 expression and downregulating leptin and tumor necrosis factor (TNF)-α in mouse abdominal white adipose tissue (Maeda et al., 2005; 2007). Fx derived from S. fusiforme and U. pinnatifida and its metabolite, fucoxanthinol (Fxol), also inhibited adipocyte differentiation of 3T3-L1 cells via the downregulation of peroxisome proliferator-activated receptor γ (Maeda et al., 2006). Moreover, the antiobesity effect of Fx was demonstrated in obese human female volunteers in a double-blind, randomized, placebo-controlled study (Avidov et al., 2010).
Recently, anti-inflammatory and immunomodulatory effects of Fx have also been reported. In lipopolysaccharide (LPS)-stimulated murine macrophage RAW 264.7 cells, Fx and its derivatives suppressed the mRNA expression of cyclooxygenase (COX)-2 and inducible nitric oxide synthase, protein expression of COX-2, TNF-α and interleukin (IL)-6, and production of nitric oxide and prostaglandin E2 (Heo et al., 2012). The suppression of inflammatory reactions by Fx is attributed to the inhibition of mitogen-activated protein kinase phosphorylation, suppression of the cytoplasmic degradation of B (IκB)-α inhibitors, and decrease of nuclear factor κB (Kim et al., 2010). Fx also suppressed degranulation in rat basophilic leukemia (RBL)-2H3 cells via high-affinity IgE receptor (FcεRI)-mediated intracellular signaling, such as the phosphorylation of Lyn kinase and Fyn kinase (Sakai et al., 2009). In in vivo experiments, intravenously or orally administered Fx suppressed footpad inflammation induced by LPS and the infiltration of inflammatory cells in rat and mouse ear swelling induced by 2,4-dinitrofluorobenzene (DNFB) (Shiratori et al., 2005; Sakai et al., 2011). Fx also exerted an immunomodulatory effect by suppressing T cell differentiation into Th17 cells, leading to the inhibition of inflammatory disease associated with Th17 cells (Kawashima, 2011).
As described above, diverse mechanisms of the anti-inflammatory effects by Fx have been discovered. However, although the anti-inflammatory effect of Fx on DNFB-induced mouse ear swelling has been reported (Sakai et al., 2011), studies of different inflammation types (rapid or delayed) have not been performed. In addition, although inflammation in mouse ear swelling is attributed to reactions of the arachidonic acid (AA) cascade (Meurer et al., 1988; Rao et al., 1993), further studies on the involvement of the cascade in Fx-mediated anti-inflammatory reactions are currently lacking. With respect to the inhibitory effects of Fx on enzymes [phospholipase A2 (PLA2), COX-2, lipoxygenase (LOX), and hyaluronidase (HA)] involved in the AA cascade (Funk, 2001) and topical inflammatory reaction (Sakamoto et al., 1980), the suppressive effect of Fx on mRNA or protein expression of COX-2 has been demonstrated (Heo et al., 2012). However, the inhibitory effects of Fx on the enzymatic reactions of PLA2, COX-2, LOX and HA have not been investigated. Furthermore, when Fx as a food component is absorbed and metabolized in vivo, Fxol is generated from Fx (Sugawara et al., 2002). The inhibitory effects of Fxol on the enzymatic activities have also not been examined.
The purpose of the present study was to resolve the above-mentioned issues. Fx was percutaneously or orally administered to ICR-strain mice, and inhibitory effects on mouse ear swelling induced by three types of sensitizers [rapid type, AA and 12-O-tetradecanoylphorbol-13-acetate (TPA); delayed type, oxazolone (OXA)] (Meurer et al., 1988; Rao et al., 1993) in vivo were investigated. To determine whether the anti-inflammatory effects of Fx act through the AA pathway and a topical tissue, in vitro enzymatic activities of PLA2, COX-2, LOX, and HA were assessed. To assess the efficacy of Fx after absorption in the body, the inhibitory effects of Fxol on AA cascade enzymatic activities were also investigated. Finally, given that Fx and Fxol inhibit mRNA expression of COX-2 (Heo et al., 2012; Maeda et al., 2015), we investigated whether the anti-inflammatory effects of Fx and Fxol are also attributed to the inhibition of mRNA expression of PLA2 and COX-2 in RBL-2H3 cells as a cultured mast cell model (Bursumian et al., 1981).
Materials Fx and Fxol were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Epigallocatechin gallate (EGCG; Sigma-Aldrich), a tea catechin, was used as a typical natural inhibitor, as EGCG is well known to exert anti-allergic and anti-inflammatory effects via various inhibition mechanisms (Yoshino et al., 2010; Tachibana, 2011).
Antiinflammatory effects on mouse ear swelling
Animals As an ear-swelling model, ICR-strain mice were used. The mice (male, 4 weeks old) were purchased from Kyudo Co., Ltd. (Tosu, Saga, Japan) and were housed in individual cages at 23°C–26°C under a 12-h light-dark cycle until tested. An MF diet (KBT Oriental Co., Ltd., Tosu, Saga, Japan) and tap water were freely available. All of the animal experiments were performed with permission from the Committee for Use and Care of Laboratory Animals of the National Fisheries University and in compliance with the Guideline for Animal Experiments in Research Institutes under the jurisdiction of the Ministry of Agriculture, Forestry, and Fisheries (approval number 13 – 7; March 22, 2013).
Arachidonic acid AA-induced ear swelling was conducted using the method described by Young et al. (1984) with some modifications. Ten microliters of AA (12.5 mg/mL in acetone, stored at −20°C until used; Wako Pure Chemical Industries, Ltd., Osaka, Japan) and 5 µL of test sample dissolved in methanol were mixed and spread on a mouse ear. One hour later, ear swelling was determined with a thickness gauge (547 series; Mitsutoyo Corporation, Kawasaki, Kanagawa, Japan).
Eighteen hours after oral administration of a test sample dissolved in 400 µL of 10% Tween 60 solution using a stainless steel feeding needle, 10 µL of AA was spread on the mouse ear. One hour later, ear swelling was determined with a thickness gauge. The inhibition ratio of the ear swelling was calculated according to the following formula:
 |
12-O-tetradecanoylphorbol-13-acetate TPA-induced ear swelling was conducted using the method described by Young et al. (1984) with some modifications. Immediately before use, a stock solution of TPA (800 µg/mL in acetone, stored at −20°C until used; Wako) was diluted to 80 µg/mL with acetone. Ten microliters of TPA and 5 µL of test sample were mixed and spread on the mouse ear. Four hours later, ear swelling was determined with a thickness gauge.
For oral administration, 400 µL of test sample was administered 3 and 21 h prior to spreading TPA (10 µL) on a mouse ear. Four hours after spreading the TPA, ear swelling was determined with a thickness gauge. The inhibition ratio of the ear swelling was calculated as described above.
Oxazolone OXA-induced ear swelling was conducted using the method described by Yoshino et al. (2010) with some modifications. The OXA solution (Sigma-Aldrich) was stored at −20°C until used. Fifty microliters of 1% OXA dissolved in ethanol was spread on the cleanly shaven abdominal region of a mouse. Five days later, 10 µL of 0.5% OXA dissolved in acetone and 5 µL of test sample were mixed and spread on a mouse ear. After 24 h, ear swelling was determined with a thickness gauge.
When mice were orally administered test samples, the examination was performed according to the procedure described by Suzuki et al. (2000) with some modifications. Four-hundred microliters of test sample was orally administered 1 h prior to the spreading of 1% OXA (50 µL) on the abdominal region of a mouse as described above. Five days later, 400 µL of the test sample was orally administered once again 1 h prior to the spreading of 0.5% OXA (10 µL) on the mouse ear. Twenty-four hours after spreading the OXA, ear swelling was determined with a thickness gauge. The inhibition ratio of the ear swelling was calculated as described above.
Inhibitory effects on enzymatic activities
Phospholipase A2 Assays for inhibitory effects of test samples on PLA2 activity were conducted according to Sugiura et al. (2009). Diheptanoyl thio-PC (Cayman Chemical Co., Ann Arbor, MI, USA) dissolved to 33.2 mM in dimethyl sulfoxide (DMSO) was diluted with 25 mM Tris-HCl buffer (pH 7.5) to 1.66 mM. One-hundred microliters of diheptanoyl thio-PC solution and 5 µL of sample or DMSO (solvent control) were mixed, and 5 µL of 5,5′-dithio-bis-(2-nitrobenzoic acid) (50 mM; Sigma-Aldrich) dissolved in Tris-HCl buffer was added. After incubating the reaction mixture at room temperature for 5 min, background absorbance at 415 nm (OD415) was measured with a 96-well microplate reader (MTP-120; Corona Electric Co., Ltd., Hitachinaka, Ibaraki, Japan). To start the enzymatic reaction, 5 µL of porcine pancreatic PLA2 (25 units; Sigma-Aldrich) diluted with Tris-HCl buffer was added. Seventy-five minutes after addition of PLA2, the increase in OD415 by 5-thio-2-nitrobenzoic acid produced from the reaction was measured. The inhibition ratio of the PLA2 activity was calculated according to the following formula:
 |
Cyclooxygenase-2 Assay for the inhibitory effects of Fx and Fxol on COX-2 activity, and ELISA for PGs produced from the assay, were performed using a kit for COX inhibitor screening (Cayman). The assay for inhibitory effects and ELISA for PGs were performed according to the protocols recommended by the manufacturer.
Lipoxygenase Soybean lipoxygenase (SBL, Type I-B; Sigma-Aldrich) was used as an alternative to human 5-LOX (Komoda et al., 1995). The inhibitory effects of test samples on SBL activity were evaluated according to the method of Komoda et al. (1995). After 2 mL of SBL (100 units) in 0.2 M borate buffer (pH 9.0) and 20 µL of test samples or methanol (solvent control) were mixed, the mixture was incubated at room temperature for 5 min. After 25 µL of linoleic acid was diluted with ethanol to 0.4 mM as the substrate was added, optical density at 234 nm (OD234) was measured with a spectrophotometer (U-1800; Hitachi High Technologies Corp., Tokyo, Japan). After 20 min, the increase in OD234 due to conjugated diene production from linoleic acid by SBL activity was determined. The inhibition ratio of the SBL activity was calculated according to the following formula:
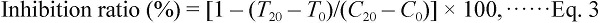 |
Hyaluronidase Measurement of the inhibitory effect on HA activity was conducted following Kakegawa et al. (1984). HA (from bovine testes; Sigma-Aldrich), compound 48/80 (Sigma-Aldrich), and potassium hyaluronate (Nacalai Tesque Inc., Kyoto, Japan) were dissolved in 0.1 M acetate buffer (pH 4.0). HA (12.5 µL, 0.8 mg/mL) and 25 µL of test sample were mixed and incubated at 37°C for 20 min. After 25 µL of compound 48/80 (0.1 mg/mL) was added and the mixture was incubated at 37°C for 20 min to activate HA, 62.5 µL of potassium hyaluronate (0.16 mg/mL) was added and incubated at 37°C for 40 min. To stop the enzymatic reaction, after 25 µL of 0.4 M sodium hydrate and 0.8 M potassium borate were added, the reaction mixture was boiled for 3 min and cooled in tap water. After the addition of 750 µL of p-dimethylaminobenzaldehyde (10 mg/mL; Wako) dissolved in acetate, OD585 was measured with a spectrophotometer. The inhibition ratio of HA activity was calculated as described below:
 |
Quantitation of sPLA2 and COX-2 mRNAs
Cell culture RBL-2H3 cells provided by the Health Science Research Resources Bank (JCRB0023; Sennan, Osaka, Japan) were cultured in Eagle's Modified Essential Medium (EMEM; Sigma-Aldrich) supplemented with fetal bovine serum (final, 10%; Lot, AWD12939; HyClone Laboratories, Inc., South-Logan, UT, USA), penicillin (final, 105 U/L; Wako) and streptomycin (final, 100 mg/L; Wako). The cells were transferred every 3 or 4 days by trypsinization until they attained confluence.
Cell stimulation The RBL cells were stimulated with a calcium ionophore, A23187 (Sigma-Aldrich), according to the method of Matsui et al. (2009) with some modifications. The RBL cells suspended in EMEM were inoculated in each well of a 6-well plate (1 × 106 cells/well) and precultured overnight. The cells were exposed to samples (100 or 200 µM) for 30 min and then stimulated with A23187 (2 µM). After being cultured for 3 h, the cell suspension was collected with a cell scraper. Because the induction of cell death by Fx and Fxol was suspected from the mRNA expression in RBL cells at 200 µM, induction was tested by Trypan blue exclusion. The survival rates of Fx- and Fxol-treated cells were 96.4% and 98.8%, respectively. Thus, cell death caused by the extract was not observed (data not shown).
Quantitative real-time PCR Since mRNA expression of type II A sPLA2 is closely linked to COX-2-dependent PGD2 generation (Murakami et al., 1997), in the present study, mRNA expression of type II A sPLA2 in RBL cells was evaluated. Total RNA was isolated from RBL cells with ISOGEN reagent (Nippon Gene Co., Ltd., Tokyo, Japan) according to the manufacturer's instructions, and quantified by spectrophotometry at 260 nm. Single-strand cDNA was synthesized using an oligo (dT) primer and High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA.). The cDNA was used for real-time quantitative polymerase chain reaction (qPCR) using SYBR Premix Ex TaqTM II (Tli RNaseH Plus) (Takara Bio, Inc., Otsu, Shiga, Japan) with the TP870 Thermal Cycler Dice Real Time System (Takara Bio). Relative mRNA levels were calculated by the comparative threshold cycle method. The following specific forward and reverse primers were used for real-time qPCR: type II A sPLA2, sense, 5′-ATG AAG GTC CTC CTC CTG CTA G-3′ and antisense, 5′-TCA GCA TTT GGG CTT CTT CC-3′; COX-2, sense, 5′-CCC ATG TCA AAA CCG TGG TG-3′ and antisense, 5′-CTG TGT TTG GGG TGG GCT TC-3′; and GAPDH, sense, 5′-TGT GTC CGT CGT GGA TCT GA-3′ and antisense, 5′-CCT GCT TCA CCA CCT TCT TGA T-3′.
Statistical analysis Data are expressed as mean ± SD. In the study of mouse ear swelling, statistical analyses were performed using Student′s t test. P < 0.05 was considered statistically significant. In the study of inhibition of enzymatic activity and quantitation of sPLA2 and COX-2 mRNAs, after reproducibility was confirmed by repeated runs, multiple comparisons between groups were performed with Dunnett's test using the software Excel Statistics 2015 (Social Survey Research Information Co., Ltd., Tokyo, Japan). P < 0.05 was considered statistically significant.
Anti-inflammatory effects on mouse ear swelling As shown in Table 1, Fx percutaneously administered to ICR mice suppressed mouse ear swelling induced by each of the three sensitizers, AA, TPA and OXA, with effects similar to those of EGCG (P > 0.05). At a concentration of 150 nmol, suppression ratios were over 50%, except for OXA-induced ear swelling in the Fx administration group. In particular, Fx and EGCG expressed the strongest inhibition of TPA-induced ear swelling. Fx not only suppressed rapid inflammation induced by AA and TPA but also delayed inflammation induced by OXA. As shown in Table 2, when 150 nmol of Fx and EGCG were orally administered to ICR mice, the inflammation in mouse ear swelling induced by AA, TPA and OXA was suppressed by 20% – 28%. The suppression of Fx was similar to that of EGCG (P > 0.05).
| Sensitizer | AA | TPA | OXA | ||||
|---|---|---|---|---|---|---|---|
| Sample dose (nmol/ear) | 15 | 150 | 15 | 150 | 15 | 150 | |
| Suppression ratio (%) | Fx | 32.9 ± 6.2 | 54.8 ± 8.0 | 37.4 ± 7.8 | 68.1 ± 28.1 | 12.0 ± 9.6 | 41.4 ± 9.6 |
| EGCG | 29.8 ± 9.1 | 51.3 ± 7.6 | 30.1 ± 6.7 | 58.8 ± 10.1 | 21.5 ± 19.0 | 54.7 ± 6.1 | |
Fx, fucoxanthin; EGCG, epigallocate chin gallate; AA, arachidonic acid; TPA, 12-O-te trade canoylphorbol-13-acetate; OXA, oxazolone. Data are expres sedas mean ± SD (n = 4).
| Sensitizer | AA | TPA | OXA | |
|---|---|---|---|---|
| Suppression ratio (%) | Fx | 20.1 ± 5.3 | 25.3 ± 11.0 | 24.1 ± 4.9 |
| EGCG | 22.6 ± 1.6 | 26.8 ± 7.3 | 27.5 ± 2.0 |
Fx, fucoxanthin; EGCG, epigallocate chin gallate; AA, arachidonic acid; TPA, 12-O-tetrade canoylphorbol-13-acetate; OXA, oxazolone. The sample dose was 150 nmol/mouse. Data are expres sedas mean ± SD (n = 4).
Inhibitory effects on enzymatic activities As shown by the IC50 values (Table 3), the inhibitory effects of Fx and Fxol on PLA2 and COX-2 activities were significantly stronger than those of EGCG (P < 0.01). In a comparison between Fx and Fxol, the effects of Fx tended to be stronger than those of Fxol. In contrast, EGCG inhibited SBL activity (IC50 value, 486.3 µM), whereas Fx and Fxol had no inhibitory effects on enzymatic activity. In the assay for HA activity, Fx and Fxol inhibited enzymatic activity with IC50 values of 409.0 µM and 385.7 µM, respectively. The inhibitory effect of EGCG on HA activity was significantly stronger than those of Fx and Fxol (P < 0.05). In view of this finding, given that Fx and Fxol inhibited enzymatic activities that are involved in not only the AA cascade (Funk, 2001) but also topical inflammatory reactions (Sakamoto et al., 1980), Fx and Fxol, like EGCG, may be highly effective against inflammatory reactions.
| Enzymes | IC50 (µM) | ||
|---|---|---|---|
| Fx | Fxol | EGCG | |
| PLA2 | 439.3* ± 137.4 | 699.7* ± 197.9 | 1403.7 ± 185.7 |
| COX-2 | 278.0* ± 19.8 | 367.0* ± 2.8 | 781.3 ± 19.9 |
| SBL | – | – | 486.3 ± 102.1 |
| HA | 409.0 ± 7.1 | 385.7 ± 58.0 | 242.3 ± 6.5 |
Fx, fucoxanthin; Fxol, fucoxanthinol; EGCG, epigallocatechin gallate; PLA2, phospholipase A2; COX-2, cyclooxygenase-2; SBL, soybean lipoxygenase; HA, hyaluronidase. The inhibition ratio was calculated from the results of triplicate experiments. Data are expressed as mean ± SD. The reproducibility was confirmed by repeated runs. Asterisks indicate that IC50 values of Fx or Fxol are significantly lower than those of EGCG (*P < 0.01).
Suppressive effects on mRNA expression of PLA2 and COX-2 IC50 values described above are higher than for PLA2 (IC50, < 7 µM; Singer et al., 2002) or COX-2 (IC50, 0.04 µM; Penning et al., 1997) inhibitor and there are reports of inhibition of COX-2 mRNA expression by Fx or Fxol (Heo et al., 2012; Maeda et al., 2015). For these reasons, the suppressive effects of Fx and Fxol on mouse ear swelling were suspected to be due to the inhibition of enzymatic activities and mRNA expression. As shown in Fig. 1, Fx and Fxol at 100 µM drastically suppressed the mRNA expression of PLA2 in RBL cells, similar to EGCG. As shown in Fig. 2, Fx and Fxol at 200 µM significantly suppressed the mRNA expression of COX-2 in RBL cells. However, no suppression by EGCG was detected at either concentration. Thus, given that Fx and Fxol also suppressed mRNA expression of the enzyme, they might suppress the mouse ear swelling more effectively than EGCG.

The suppressive effects of Fx and Fxol on mRNA expression of type II A sPLA2 in RBL cells stimulated by A23187. The concentration of the samples was 100 µM. Values were calculated from the results of triplicate experiments and are expressed as mean ± SD. Reproducibility was confirmed by repeated runs. Asterisks indicate statistical significance against PC (*P < 0.01). NC, negative control; PC, positive control; Fx, fucoxanthin; Fxol, fucoxanthinol; and EGCG, epigallocatechin gallate.

The suppressive effects of Fx and Fxol on mRNA expression of COX-2 in RBL cells stimulated by A23187. Values were calculated from the results of triplicate experiments and are expressed as mean ± SD. Reproducibility was confirmed by repeated runs. Asterisks indicate statistical significance against PC (*P < 0.01). NC, negative control; PC, positive control; Fx, fucoxanthin; Fxol, fucoxanthinol; and EGCG, epigallocatechin gallate.
The inflammatory enzymes PLA2, COX-2, and LOX act in the AA cascade of inflammatory lymphocytes, such as mast cells, and synthesize chemical mediators, such as leukotrienes (LTs) and PGs. The chemical mediators are released by the inflammatory lymphocytes and then induce inflammatory reactions. Among the three enzymes, PLA2 produces AA from the phospholipids of cell membranes. COX-2, induced by allergic responses, produces PGs, such as PGD2 and PGE2, from AA. LOX produces LTs, such as LTB4 and LTC4, from AA (Dennis, 1987; Funk, 2001). The enzymatic activities generate inflammatory reactions in mouse ear swelling induced by AA, TPA and OXA (Meurer et al., 1988; Rao et al., 1993).
In this study, percutaneously administered Fx suppressed mouse ear swelling induced by AA, TPA and OXA (Table 1). Fx also inhibited the activities of the inflammatory enzymes PLA2 and COX-2 (Table 3). AA and TPA induce rapid-type inflammation, reaching maximum inflammation within 4 h. AA causes inflammatory reactions as an origin of chemical mediators (LTs and PGs) resulting from a preferential induction of COX-2 activity (Young et al., 1984), whereas TPA causes inflammatory reactions resulting from preferential induction of LOX activity and COX-2 protein expression (Kujubu et al., 1991). As shown in Table 3, Fx inhibited PLA2 and COX-2 activities. Thus, the inhibitory effects of Fx on mouse ear swelling induced by AA and TPA could be attributed to the inhibition of COX-2 activity with the inhibition of PLA2 activity to generate AA. Given that Fx suppresses COX-2 mRNA expression in RAW 264.7 cells, a cultured mouse macrophage cell line (Heo et al., 2012), and PLA2 or COX-2 mRNA expression in RBL cells (Fig. 1 and 2) at a lower concentration than needed for the inhibition of enzymatic activities, the inhibitory effect of Fx on TPA-induced ear swelling may more plausibly be attributed to the suppression of PLA2 or COX-2 mRNA expression.
OXA acts as a hapten and causes inflammatory cytokine production as IL-4 and TNF-α via the activation and differentiation of T lymphocytes or macrophages, with the synthesis of chemical mediators (Ferreri et al., 1991; Xu et al., 1996). Owing to such a delayed-type allergic reaction reaching maximum inflammation over 24 h, chemical mediators synthesized by COX-2 and LOX are released from inflammatory lymphocytes (Meurer et al., 1988; Ferreri et al., 1991). It has also been reported that Fx regulates the differentiation of T-lymphocytes (Kawashima, 2011). Thus, the suppressive effect of Fx may have resulted from the suppression of cytokine or chemical mediator production via the regulation of T-lymphocyte differentiation. Further studies on T-lymphocyte reactions are required for a better understanding of this effect.
Although HA is not involved in the AA cascade, inflammation in the topical tissue is attributed to HA activity (Sakamoto et al., 1980). HA is activated by a chemical mediator such as histamine degranulated by mast cells (Kakegawa et al., 1984). Degranulation in RBL-2H3 cells to release chemical mediators was suppressed by Fx (Sakai et al., 2009). In the present study, Fx inhibited HA activity (Table 3). Thus, the suppression of mouse ear swelling by percutaneously administered Fx may also have resulted from the inhibition of inflammation in topical tissue via HA activity and degranulation of chemical mediators.
Orally administered Fx also suppressed mouse ear swelling (Table 2). Because orally administered Fx is absorbed by the gut and metabolized to Fxol (Sugawara et al., 2002) and Fxol suppressed COX-2 mRNA expression in RAW 264.7 cells (Maeda et al., 2015), we also assessed the inhibitory effects of Fxol on enzymatic (PLA2, COX-2, LOX and HA) activities and PLA2 or COX-2 mRNA expression in RBL cells. The results showed that Fxol suppressed mRNA expression of both enzymes (Figs. 1 and 2), and inhibited enzymatic activities similar to those of Fx, although it did not inhibit LOX (SBL) activity (Table 3). Thus, Fxol may also reduce the chemical mediators produced by the enzymes and exert anti-inflammatory effects. When Fx was orally administered to mice, Fxol may have inhibited the enzymatic activity and mRNA expression of PLA2 or COX-2 and alleviated the inflammatory reactions. In topical inflammatory tissues, Fxol suppressed ear swelling to inhibit HA activity. Moreover, given that Fxol suppressed degranulation in RBL-2H3 cells leading to the release of chemical mediators (Manabe et al., 2014) and COX-2 mRNA expression in RAW 264.7 cells (Maeda et al., 2015), its effect may also contribute to the suppression of mouse ear swelling. Comparing Tables 1 and 2, the inhibitory effects of orally administered Fx on mouse ear swelling (Table 2; 20%–28% inhibition) were apparently weaker than those of percutaneously administered Fx (Table 1; 40%–70% inhibition) at a concentration of 150 nmol. Given that the plasma level of Fx metabolites (Fxol) in mice orally administered 200 nM of Fx was 50 pM after 2 h (Asai et al., 2004), these results (Table 2) are to be expected, given that orally administered Fx must be absorbed by the small intestine (Sugawara et al., 2002; Asai et al., 2004) and that the absorbed Fx, converted to Fxol, would be expected to exert the inhibitory effect in the ear via the bloodstream, whereas percutaneous Fx is administered directly to the site where inflammation is assessed. Thus, our study suggests that the inhibitory effects of orally administered Fx on mouse ear swelling resulted partly from the effects of Fxol metabolized from Fx in mice.
As shown in Table 3, Fx and Fxol, in contrast to EGCG, did not inhibit LOX (SBL) activity. However, suspecting that Fx and Fxol suppress the mRNA expression of 5-LOX, we sought to determine whether they suppress it in RBL cells. Unfortunately, because 5-LOX mRNA indicated only slight expression in RBL cells stimulated by A23187, the suppressive effects of Fx and Fxol on expression could not be determined. Further studies of LOX mRNA expression and LT generation are required.
Percutaneously or orally administered Fx suppressed mouse ear swelling induced by three sensitizers, AA, TPA and OXA. Fx and its metabolite, Fxol, inhibited the enzymatic activities of PLA2, COX-2, and HA involved in inflammatory reactions. These results suggest that percutaneously administered Fx topically suppressed the inflammatory reactions by the inhibition of enzymatic activities. Orally administered Fx was metabolized to Fxol, which systemically suppressed the inflammatory reactions, an action attributed to the inhibitory effects of Fxol on enzymatic activities. Fx and Fxol also suppressed the mRNA expression of PLA2 and COX-2 in RBL cells. Thus, given that Fx by both percutaneous and oral administration suppressed mouse ear swelling, it could be a very useful ingredient in health foods for the purpose of alleviating inflammation and allergy.
Acknowledgements This work was supported by JSPS KAKENHI Grant Number 25850099. We thank Mr. R. Ota, Mr. R. Taniguchi and Mr. K. Furuya (National Fisheries University) for technical assistance, as well as Dr. D.A. Coury (Texasta, Inc., Gilmer, TX, USA) for helpful comments on the manuscript.