2016 Volume 22 Issue 3 Pages 377-385
2016 Volume 22 Issue 3 Pages 377-385
Biological control is an alternative to synthetic fungicides to avoid postharvest diseases which limit the storage period and marketing life of fruit and vegetables. Present investigation is focused on the isolation, identification and characterization of Bacillus subtilis CF-3 from fermented bean curd through the analyses of phenotype, physiological and biochemical characterization, 16S rDNA gene sequence based on its biocontrol effect against pathogens (Monilinia fructicola, Cephalothecium, Rhizoctonia and Alternaria) causing peach decay. With treatment of CF-3, the ratio of good fruit was 65% which was higher than 30% in the control group significantly after 36d storage at 10°C and 65% compared with 50% in the control group after 7d storage at 25°C; The optimal growth temperature and pH of CF-3 were 37°C and 8; The results of antimicrobial stability indicated that CF-3's antibiotics had good UV, pH and thermal stability (except at 121°C). Findings of the present study suggested that the antifungal properties of CF-3 may have a potential to be developed as fungicide which can contribute to the postharvest preservation of peach fruit and provide a theoretical basis for the further study of biological control.
Postharvest loss of agricultural industry is a worldwide major problem because of postharvest diseases which is primarily controlled by application of synthetic fungicides (Weinberger et al., 2008). Reported postharvest losses in developed countries were 15 – 20% of the total harvest, comparably, the losses in China are even worse due to the neglect of postharvest preservation and prevention. Presently, chemical control still plays a major role in postharvest treatment. However, the long term use of chemical reagents not only induces tolerance but also causes a series of negative consequences on food safety and the ecological environment (Wilson and Wisniewski, 1989, Holmes and Eckert, 1999 and Ongena et al., 2005). Biological control is an alternative method avoiding indiscriminate use of fungicides and pesticides that utilizes microorganisms to inhibit the mycelial growth on fruit surfaces, prevent fruit rot and achieve postharvest preservation. This method not only defends against postharvest diseases but also does not induce tolerance and pollution to environment which makes sustainable development in the longer term (Chen et al., 1995). In previous researches, some Bacillus subtilis strains can effectively control Penicillium and green mold of citrus, brown rot of stone fruits and cherries (Pusey and Wilson, 1984, Utkhede and Sholberg, 1986, Benbow and Sugar, 1999). Some Pseudomonas syringae strains can effectively control green mold and grey mold of pears (Janisiewicz and Marchi, 1992). Some Penicillum sp. strains can control Penicillium of pineapple fruit (Lim and Rohrbach, 1980). However, the success of biocontrol remains limited and there still exists many problems including the inconsistency, variability of the efficacy under commercial conditions, limited tolerance of fluctuating environmental conditions and difficulties in production of shelf-stable formulations (Essghaier et al., 2009, Palaniyandi et al., 2011, Meng et al., 2012, Yánez-Mendizábal et al., 2012 and Singh et al., 2013). Thus more attention is being given to the isolation and screening of stable, effective and broad spectrum biocontrol bacteria.
Biocontrol bacteria isolated from food, plant roots, soil, etc are much safer than from other sources (Hammami et al., 2013 and Mitter et al., 2013). Fermented bean curd, the most typical traditional Chinese fermented soy food, is made by microbial fermentation beginning with soybean as raw material and is favored by many people for its unique taste and nutritional value (Juan et al., 2014). During the process of fermentation, some types of yeast, lactic acid bacteria, Bacillus, Streptococcus and other microorganisms are involved which can make fermented bean curd more nutritious and extend its shelf life (Halasza et al., 1994). However, most of the recent researches focus on the study of the commercial attributes of the beneficial bacteria from fermented bean curd, few information of biological control can be obtained. Present study deals with the isolating, screening, identifying and charactering biocontrol bacteria from fermented bean curd to control the incidence of postharvest diseases during the storage and transportation of peaches. The findings can be contributed to the postharvest preservation of peach fruit and provide a theoretical basis for the further research of biological control agents with stable properties.
Experimental materials Twenty samples of fermented bean curd purchased from retail stores in Shanghai, China and Peach fruits collected from an orchard in Shanghai, China were used in this study. Peach fruits were homogeneous in size and color and without any visible damage or molds were selected. Fermented bean curd and the harvested fruits were treated immediately. The pathogens (Monilinia fructicola, Cephalothecium, Rhizoctonia and Alternaria) were isolated from peach fruit and preserved by Laboratory of Food Safety and Quality Control, School of Life Sciences, Shanghai University, which were inoculated and incubated on potato dextrose agar (PDA) (Cunha et al., 2014).
Isolation and screening of biocontrol bacteria All of the bacteria isolated from fermented bean curds. Each fermented bean curd sample (10 g) was placed in conical flasks containing 100 mL sterile water. After oscillating for 10 minutes, serially diluted the mixture to 10−6. Then mixed 1 mL suspension of different concentration with NA medium (peptone 10 g, beef extract 3 g, NaCl 5 g, agar 15 g, distilled water 1000 mL, pH 7.2 – 7.4) in a petri dish, respectively, and inverted them in an incubator at 30°C for 24h (Hu et al., 2008). Single colonies on each plate were streaked onto other fresh plates to check for purity.
After purification, isolated bacteria strains were tested for their antagonistic ability against pathogens in vitro. Agar plugs of pathogens (6 mm in diameter) taken by punch was placed on the middle of the NA medium plate and the same kind of isolated bacteria strains (6 mm in diameter) were placed at both sides of the pathogen (2 cm away). The diameters of pathogens were evaluated 7d later. The strains which have inhibitory effect on all the pathogens were selected and passed to the secondary screening which was carried out according to the effect on the growth rate of pathogen mycelia (Nishi et al., 2013 and Yang et al., 2013). Subsequently, strains were cultured in liquid NA medium at 30°C for 7d with shaking at 100 rpm in an incubator shaker. The culture was centrifuged at 10000 rpm for 10 min and the filtrate was prepared by filtering centrifuged culture of the isolates through a 0.22 µm microfiltration membrane. And then the filtrate was mixed with PDA medium (V/V=1:5). Respectively, the same amount of sterile water was added to the control group. After cooling, pathogen was set on the middle of the plate and the diameter of pathogen mycelia was measured daily.
Phenotypic identification of the biocontrol bacteria Cell morphology was observed by using a light microscope and gram staining (Claus, 1992). To observe colony characteristics, bacteria were incubated on NA medium plate at 30°C for 48h. Biochemical characterization tests were carried out as described in Bergey's Manual (Holt et al., 1994) and the Manual for the Identification of Medical Bacteria (Cowan and Steel, 1965). The tests included salt tolerance determination, aerobic and anaerobic determination, utilization of citrate, starch hydrolysis, V-P (Voges Proskauer) experiment, gelatin liquefaction test and contact reaction.
Genetic identification of the biocontrol bacteria Genomic DNA of the tested bacteria was extracted using a reagent kit produced by Takara (Takara Code: D304). PCR amplification was performed with a universal set of primers for bacterial 16S rDNA gene (Weisburg et al., 1991). The forward primer was GAGCGGATAACAAT TTCACACAGG and the reverse primer was CGCCAGG GTTTTCCCAGTCACGAC (Takara Code: D301). This pair of primers could amplify an approximately 1.5 kb sequence of 16S rDNA gene. The PCR procedure is showed as follows: an initial denaturation at 94°C for 5min; followed by 30 cycles, each comprising heating for 1 min at 94°C, 1 min at 50 – 55°C and 1.5 min at 72°C; and a final extension at 72°C for 5 min. The amplified products were subjected to 1.0% agarose gel electrophoresis and then sequenced in Sangon Biotech Co., Ltd. (Shanghai, China).
A phylogenetic tree was finished by DNAMAN software. Homology comparison of the 16S rDNA gene sequence was performed with the reference sequences available in the Nucleotide Database of the National Center for Biotechnology Information (NCBI) using the basic local alignment search tool (BLAST). Phylogenetic analysis was performed with DNAMAN software (Yang et al., 2014).
Biocontrol tests on pathogens of peach The antimicrobial ability against pathogens (Monilinia fructicola, Cephalothecium, Rhizoctonia and Alternaria) of peach fruit of biocontrol bacteria were tested through dual culture method. A mycelial agar plug (6 mm in diameter) was taken from the margin of the actively growing mycelium of pathogens using a sterile cork borer and placed centrally on NA medium test plates (9 cm in diameter), biocontrol bacteria were streaked 2 cm away from the pathogen plug. As a control group, NA medium plates without biocontrol bacteria were cultivated under the same conditions. The mycelial diameter was measured after 7d at 30°C to contrast treatment effects and antagonistic activity. Three replications of each treatment were performed.
Biocontrol bacteria were also evaluated for its potential in integrated control of postharvest diseases of peach fruit. Each peach fruit was sprayed with 2 mL suspension of biocontrol bacteria (108 cfu mL−1) on the surface. After treatment, peach fruits were placed on plastic trays at 10°C for 36d and at 25°C for 7d, respectively. The effect on integrated control of postharvest diseases was evaluated by the ratio of good fruit. As a control, twenty peach fruits sprayed with distilled water were cultivated under the same conditions. Each treatment was performed using twenty peach fruits and the experiment was repeated three times.
Characterization of the Biocontrol Bacteria 0.5 mL culture of biocontrol bacteria (108 cfu mL−1) was added to 50 mL NA culture and cultivated respectively at 20°C, 28°C, 30°C, 37°C, 40°C, 45°C and 50°C. Absorbance value was measured under 625 nm 48h later. The NA culture without biocontrol bacteria was set as the control group respectively.
The pH of NA culture was adjusted to 4, 5, 6, 7, 8, 9 and 10 with 1 mol L−1 HCl and NaOH. 0.5 mL culture of biocontrol bacteria (108 cfu mL−1) was added to 50 mL NA culture of different pH value and then cultivated in a rotary shaker (100 rpm) at 30°C. Absorbance value was measured under 625 nm 48h later. The untreated NA culture was set as the control group. All experiments were repeated three times.
Antimicrobial stability of the antibiotics Biocontrol bacteria was cultivated in liquid NA medium at 30°C, 100 rpm for 7d. In order to study the influence of external factors on the antimicrobial stability of antibiotics, the culture was centrifuged at 10000 rpm for 10 min and the germfree filtrate was prepared by filtering centrifuged cultural supernatants of the isolates through a 0.22 µm microfiltration membrane. Subsequently, germfree filtrate of biocontrol bacteria was treated as follows: pH=5, 6, 7, 8, 9; ultraviolet-C (UV-C) radiation periods: 15 min, 30 min, 45 min, 60 min, 75 min; temperature: 25°C, 60°C, 100°C, 121°C. Treated germfree filtrate and PDA medium were mixed and poured into plates. A pathogen agar plug (6 mm in diameter) which was taken from actively growing mycelium of pathogens were placed on the middle of the plates respectively and incubated at 30°C. The PDA medium plates without germfree filtrate were set as the control group. Diameters of the pathogens were measured 7d later.
Statistical analyses The data were analyzed by PASW Statistics 18. The phylogenetic tree was made by DNAMAN software. When the number of means in each group is three, standard deviation is applied for sample separation. The statistical significance in this experiment is all applied at the level P < 0.05 (Sanchez et al., 2013).
Isolation and screening of biocontrol bacteria 37 strains which have inhibitory effect on all the pathogens were selected preliminary. Among the 37 strains, 14 strains were screened which the inhibition rate against pathogen mycelia growth is over 50% and second selection results was shown as Fig. 1. Compared with other strains, the suppressive effect of CF-3 on the four pathogens was not significantly different from them all, but still significantly lower than that in the control treatment (P < 0.05) and the most stable antifungal effect.

The size of colonies of the growth of pathogen mycelium.
Phenotypic identification of the biocontrol bacteria After routine culture on NA medium for 48h, strain CF-3 formed rough, canary yellow, uplifted, rod colonies with irregular edges. Furthermore, strain CF-3 was gram-positive based on the gram staining.
Strain CF-3 is aerobic, motile and can grow under a concentration of NaCl from 2% to 7%. It utilizes citrate, starch and liquefied gelatin, and produces acid from glucose. It is tested positive for Voges-Proskauer reaction, methyl red and contact reaction (Table 1). The results showed that strain CF-3 had the same properties with Bacillus subtilis and made a foundation for the identification by searching for sequence homology among published reference sequences with the BLAST tool.
| Tested items | CF-3 | Bacillus subtilis |
|---|---|---|
| Salt tolerance | ||
| 2% NaCl | + | + |
| 5% NaCl | + | + |
| 7% NaCl | + | + |
| 10% NaCl | − | − |
| Amylolysis | + | + |
| Gelatin liquefaction | + | + |
| Facultative anaerobic | − | − |
| Glucose oxidation | + | + |
| Utilization of citrate | + | + |
| V–P test | + | + |
| Contact reaction | + | + |
“+” represents positive or growing and “−” represents negative or not growing.
Genetic identification of the biocontrol bacteria The 16S rDNA gene sequence of strain CF-3 is 1503bp through the sequencing and electrophoresis of the products of PCR amplification of CF-3's 16S rDNA gene. The accession number is KU531732 in DNA GenBank. Homology comparison was made between CF-3 and other Bacillus subtilis strains and comparing the sequences to those from the GenBank databases, the sequence of 16S rDNA allows identification at the species level with an identity from 97% to 100% (Ouoba et al., 2004). The genetic distance scale is the references of each branch length in phylogenetic tree (Fig. 2). The location of the nodes represents the genetic relationship. The shorter the branch length between two end nodes means the relationship is closer. Strain CF-3 shows 99% similarity with Bacillus subtilis subsp. subtilis PPB11 as the phylogenetic tree showed. Based on the consistency between the results of the 16S rDNA sequence analysis and the physiological characterization, the results indicated that strain CF-3 is a strain of Bacillus subtilis.
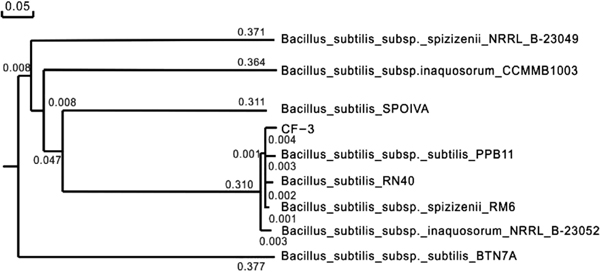
The phylogenetic tree of CF-3.
Biocontrol tests on pathogens The mycelial growth of all tested pathogens (Monilinia fructicola, Cephalothecium, Rhizoctonia and Alternaria) of peach fruit were effectively inhibited by CF-3 compared to control group (Fig. 3). In vivo test, CF-3 also showed great potential in integrated control to postharvest diseases inducing the decay development of peach fruit (Fig. 4–5).

Inhibitory effect of CF-3 on the four pathogenic fungi in vitro. The above was the control group and the below was the experimental group.

Effect of CF-3 on peaches stored at 10°C for 36 days. a: the control group; b: the experimental group.

Effect of CF-3 on peaches stored at 25°C for 7 days. a: the control group; b: the experimental group.
The observations from in vivo fruit trails showed significant differences in disease inhibition between the control and treated fruits. The ratio of good fruit was 65% which was higher than 30% in the control group significantly after 36 d storage at 10°C and 65% compared with 50% in the control group after 7 d storage at 25°C (Fig. 6–7). These results indicated that Bacillus subtilis CF-3 has good potential in integrated control of postharvest diseases of peach fruit.

Good fruit ratio at 10°C for 36 days

Good fruit ratio at 25°C for 7 days.
Characterization of CF-3 Absorbance values of CF-3 were measured after cultivated at different temperatures (Fig. 8). Absorbance value increased when temperature was below 37°C and achieved the maximum at 37°C. Afterwards, it declined gradually when temperature was above 37°C. The results indicated that the most suitable growth temperature of CF-3 was 37°C.

The absorbance value of CF-3 under different temperature.
a-Monilinia fructicola; b-Cephalothecium; c-Rhizoctonia; d-Alternaria.
After cultured at different pH levels, the absorbance value was also measured (Fig. 9). The absorbance values increased when pH values were between 4 and 8, achieved the maximum when pH was 8 and declined sharply when pH was above 9. The results demonstrated that it was not performable to the growth of CF-3 when pH was too low or too high. The optimal pH for CF-3 was 8.

The absorbance value of CF-3 under different pH.
Antimicrobial stability of CF-3's antibiotics Biological control as an alternative to chemical control is usually not as effective and stable as chemical fungicides. The inhibition rates of strain CF-3 to pathogens under different periods of ultraviolet-C, pH levels and temperatures can reflect its antibiotics' ultraviolet-C, pH and thermal stability.
a) Ultraviolet-C stability of CF-3's antibiotics
The difference among the inhibition rates under different periods of ultraviolet-C was not significantly except the inhibition rate to Cephalothecium under 60 min ultraviolet-C was significantly less than others (P < 0.05) (Fig. 10).

Inhibition rate under different periods of ultraviolet-C. CK means the absence of treatments of ultraviolet-C.
b) pH stability of CF-3's antibiotics
The results indicated that there was no apparent difference among the inhibition rates to Rhizoctonia under different pH levels (Fig. 11). When pH is 8, the inhibition rate to Monilinia fructicola is significantly lower than other levels of pH (P < 0.05). The inhibition rates to the four different fungi are all over 70% during various condition of pH.

Inhibition rate under different pH.CK means no treatment control group.
c) Thermal stability of CF-3's antibiotics
The difference among the inhibition rates was not significant when the temperature was below 100°C. However, the inhibitive rate flopped sharply when CF-3 was treated at 121°C (Fig. 12).

Inhibition rate under different temperature. CK represents blank control group.
The antagonistic effects to the four different fungi of the metabolites that generated by CF-3 showed good stability when the sterile culture filtrate of CF-3 was processed in different ultraviolet-C, pH and thermal conditions. ?
The observed inhibitory effect of B. subtilis on pathogens of postharvest diseases under different storing temperatures, and stability of CF-3's antibiotics under different ultraviolet-C radiation periods, temperatures and pH values supplied concrete evidence of the high efficacy and potential of B. subtilis for use in biocontrol. B. subtilis has been extensively studied in the biocontrol of postharvest fungal diseases. For example, Bacillus subtilis QM3 isolated from Qinghai yak dung was testified to have inhibitory effect on plant pathogens (Hu et al., 2008), Bacillus subtilis CM1–CM5 isolated from cowdung microflora have inhibitory effect on Fusarium oxysporum and Botryodiplodia theobromae isolated from the postharvest rots of yam tubers (Swain and Ray, 2009). Bacillus subtilis L194 isolated from Tunisian soil can effectively inhibit mycelial growth of Phoma medicaginis, pathogenic fungi of Medicago truncatula seedlings (Ben et al., 2012). In this paper, the determination of phylogenetic relationship of potential isolate which species it belongs to was validated by genetic characterization based on 16S rDNA partial sequence analysis. The genetic relationships given by the phylogenetic tree reflected the genetic diversity of strains of Bacillus. A 3% cut off for 16S rDNA divergence has been calibrated to yield the species (Gaibhiye et al., 2010). Bacillus subtilis CF-3, a biocontrol strain isolated from food source (fermented bean curd), was identified and also comfirmed that it is effective in controlling postharvest diseases of peach fruit.
It also showed high mycelial growth suppression and reduced disease incidence of brown rot on peach fruit by two strains Bacillus sp. C06 and Bacillus sp. T03-c (Zhou et al., 2008) and the efficacy of Bacillus subtilis SM21 on controlling Rhizopus rot caused by Rhizopus stolonifer in postharvest peach fruit was investigated (Wang et al., 2013). As to Bacillus subtilis CF-3, the results demonstrated that it could effectively control pathogens (Monilinia fructicola, Cephalothecium, Rhizoctonia and Alternaria) of peach and had great potential in integrated control of postharvest diseases of peach fruit. CF-3 strain used in this research is the first report on an Bacillus subtilis strain isolated from fermented bean curd having inhibitory effecton pathogens of peach fruit. Additionally, on account of its broad antimicrobial spectrum, Bacillus subtilis CF-3 can contribute to the postharvest preservation of peach fruit.
The effects of temperature and pH on stability of B. firmus CAS 7 purified protease were studied (Annamalai et al., 2014). Thermal stability studies revealed that the purified protease was 100% stable up to 70°C and it retained 84% of its original activity at 80°C and 55% at 95°C. The purified protease of B. firmus CAS 7 was active over wide ranges of pH between 5 and 12, the optimal pH is at 9.0 and was 100% stable at pH 9 – 10.
The effects of temperature and pH on stability of Bacillus sp. B001 protease AprB were researched (Deng et al., 2010). Thermal stability of AprB was evaluated by incubation at temperatures from 40°C to 60°C for 120 min. Ca2+ increased thermal stability of AprB since the enzyme retained about 93% and 70% of the original activity after incubation at 60°C for 30 and 60 min, respectively. AprB was stable between pH 5 and 12 and retained about 90% of its activity after incubation at 30°C for 6 h. In Gegeckas's study (Gegeckas et al., 2014), the thermal stability of the keratinolytic proteinase from Bacillus thuringiensis AD-12 (BtKER) was evaluated by incubating the purified BtKER at different temperatures (between 30°C and 70°C) for 4 h at pH 7. It was stable at 40°C and also retained more than 80% of the initial activity after 4 h of incubation at that temperature and its half-life time was 2 h at 50°C. Additionally, BtKER retained 22% and 13% residual activity after 1h incubation at 60°C and 70°C, respectively. The pH stability profile indicated that the purified BtKER was highly stable in the pH range from 6 to 8. The treatment of keratinolytic proteinase at acidic (pH 5) and alkaline (pH 11) conditions reduced relative activity to 48% and 49%, respectively.
In our study, thermal stability of CF-3's antibiotics ranged from 58% to 92% between 25°C and 100°C, and it demonstrated that thermal stability was relatively stable except at 121°C; CF-3's antibiotics were stable over ranges of pH between 5 and 9 and it retained about 80% of its activity at different pH. Additionally, CF-3's antibiotics were stable over ranges of ultraviolet-C between 15 min and 75 min and the inhibition rates to other three pathogens (Monilinia fructicola, Rhizoctonia and Alternaria) were more than 78% (about 92% of the maximum activity) except the inhibition rate to Cephalothecium. The results demonstrated that CF-3's antibiotics have good ultraviolet-C, pH and thermal stability on the whole and the stability of CF-3's antibiotics under extremely high temperature (121°C) was not ideal, this should be further studied to improve the biocontrol effect.
Results obtained by Bais indicated that upon root colonization, Bacillus subtilis 6051 forms a stable, extensive biofilm and secretes surfactin which act together to protect plants against attack by pathogenic bacteria (Bais et al., 2008). Asaka and Shoda found that the antibiotics iturin A and surfactin produced by Bacillus subtilis RB14 play a major role in the suppression of damping-off caused by pathogens persisted in soil (Asaka and Shoda, 1996). Two antibiotics produced by Bacillus subtilis CL27, with activity against A. brassicicola, were identified as peptides (Leifert et al., 1995). Bacilopeptins, new iturin-group antifungal antibiotics, were isolated from the culture broth of Bacillus subtilis FR-2 isolated from the rhizosphere of garlic suffering from the basal rot caused by Fusarium oxysporum (Kajimura et al., 1995). In addition, further study is still needed to illustrate the antibacterial mechanism of Bacillus subtilis CF-3 against postharvest fungal pathogens and discover the active compounds.
Acknowledgements This study was supported by the National Natural Science Foundation of China (No.31401539) and the National Science-technology Support Plan Project (No.2015BAD16B02). This study was also supported by the project of Food Science Discipline Construction of Shanghai University.