2016 年 22 巻 4 号 p. 519-527
2016 年 22 巻 4 号 p. 519-527
With the predominance of producing γ-aminobutyric acid (GABA) in milk fermentation as basis, the objective of this study was to investigate the production of high-vitality starter cultures by Lactobacillus plantarum NDC 75017. The strain, isolated from traditional yogurt in Inner Mongolia of China, can produce γ-aminobutyric acid-enriched fermented milk and possesses beneficial properties. In this study, the strain was grown in optimized mMRS media in 3.7 L fully instrumented fermenter to produce biomass. Cells grown in the stationary phase were harvested and freeze dried. The viable cell count was up to 2.22 × 1011 CFU g−1 of freeze-dried product upon the addition of 8% w/v trehalose, 4% w/v maltodextrin, 4% w/v L-glutamate sodium salt, and 10% w/v skim milk as cryoprotectants. These freeze dried cells were stored at temperatures of 25°C and 4°C and periodically checked for viability and preparation of fermented milk, which showed a higher cell viability and stability after stored at 4µC in 210 days. Based on our result, the high-viability starter cultures could be easily and economically produced.
Currently, lactic acid bacteria (LAB) starter cultures are widely used in fermentated food field, such as yoghurt, cheese, butter, pickles, and fermented sausages (Edward, et al., 2011). The strains as starter should have good technical characteristics, including easy to reach on a large scale and able to withstand freeze drying and maintain the viability and function. The viability of probiotic is a critical parameter for the development of starter cultures (Lacroix et al., 2007; Vinderola, et al., 2012). In addition, the starter must have the ability of staying high survival rate during the storage period and can be used in food production. Although the minimum number of living microorganisms per gram or milliliter in the fermented product has no worldwide standards, generally, it should meet a suggested minimum of >106 CFU mL−1 or 106 CFU g−1 that might have health-related effects for the body (Mohammadi et al., 2012). However, many surveys showed that many commercial LAB products (probiotic dry powder, granules products) did not achieve the suggested minimum (Hongpattarakere et al., 2013). There are many important factors that determine the survival of probiotic powder and granule products, such as the resistance of strains, growth conditions, the kinds of cryoprotectants, drying methods, temperatures, atmosphere, and relative humidity of storage period (A. Carvalho, et al., 2003; A. S. Carvalho, et al., 2004; Mohammadi, et al., 2012; Oldenhof et al., 2005; Santivarangkna et al., 2008; Schoug et al., 2006; Vinderola, et al., 2012).
High cell-density cultivation and low-temperature vacuum drying are the critical technologies for the production of high-viability starter cultures (Bauer, Schneider et al., 2012; Racine et al., 2007). These technologies enable high-cell yield at large scale and probiotic viability in food field. High cell-density cultivation is greatly influenced by some fermentation conditions, such as pH, temperature, medium composition, and dissolved oxygen tension (Gilliland, 1985).The fed-batch culture, continuous cell recycle fermentation and immobilized cell biofilm-type fermentations are used in the high cell-density cultivation of probiotic (Aguirre-Ezkauriatza et al., 2010; Bomrungnok et al., 2012; Koutinas, et al., 2009; Racine et al., 2007; Xiong, et al., 2013). Freeze-drying is currently the most appropriate and widely-used technique for the production of lactic acid bacteria starter cultures (Jagannath et al., 2010; Mohammadi, et al., 2012; Santivarangkna, et al., 2008). During the low-temperature vaccuum drying the strain suffered from many challenges in order to obtain the high-viability. The addition of different cryoprotectants in freeze drying process was necessary. These cryoprotectants, such as monosodium glutamate, proline, fructose, sorbitol, betaine, carnitine, glucose, sucrose, trehalose, maltodextrin and bacterial cellulose, were capable of improving survival of lactic acid bacteria during freezing and dehydration process (Edward, et al., 2011; Hongpattarakere, et al., 2013; Jagannath, et al., 2010).
Lb. plantarum NDC 75017 was isolated from traditional yogurt in Inner Mongolia of China. Lactobacillus plantarum is one of several LAB species which could show gamma-aminobutyric acid (GABA) producing ability (Siragusa, et al., 2007) and our former study (Shan et al., 2015) has proved that this strain of NDC 75017 could produce high concentration of GABA at a maximum production of 3.1456 mg g−1 with a high-cell density cultivation. It also showed good anti-oxidation and memory injuries alleviation in our previous study (Peng, et al., 2014a; Peng, et al., 2014b). And this strain also has been applied in the manufacture of functional fermented milk products (Shan, et al., 2015). The NDC 75017 has potential to be applied in fermented dairy products. In this study we used the high cell-density cultivation and low-temperature vacuum drying as a simple but effective strategy to achieve the production of high-viability starter cultures of Lb. plantarum NDC 75017.
Microorganism and media Stock cultures of Lb. plantarum NDC 75017 were preserved in 25% v/v glycerol at −80°C. The modified Man, Rogosa, and Sharpe (mMRS) broth media according to our previous studies (Peng, et al., 2014) were composed of (g L−1): glucose (Sun, Tianjing, China) , 16.30; yeast extract (Oxid, Basingstoke, England), 7.50; tryptone (AoBoxing, Beijing, China), 15.00; beef extract (AoBoxing, Beijing, China), 7.50; diammonium hydrogen citrate (Solarbio, Beijing, China) , 3.52; ammonium acetate (Sun, Tianjing, China), 5.00; sodium acetate anhydrous (Zhiyuan, Tianjing, China), 5.00; magnesium sulfate (Tian Da, Tianjing, China), 0.87; manganese sulfate monohydrate (Kermel, Tianjing, China), 0.41; and Tween 80 (Rui jin te, Tianjing, China), 1.50 mL. The mMRS media were used as inoculum and fermentation media. Meanwhile, a new basic MRS culture medium was prepared as additives in the fermentation process. The media were sterilized in autoclave (121°C, 15 min) before used.
Preparation of seed cultures and high cell-density cultivation of Lb. plantarum NDC 75017 in fermenter To prepare the inoculum, stored vials for the selected strains were thawed as needed and used as seed inoculum. Two successive inoculations were performed: (1) the stock cultures were inoculated into 5-mL sterilized mMRS media at 5% v/v and incubated at 30°C for 12 h without agitation; (2) inoculated 5% v/v of the above cultures into 100-mL mMRS media in a 200-mL Erlenmeyer flask, then incubated at 30°C for 8 h without agitation (approx. 107 CFU mL−1); these media were used as the seed cultures and then transferred into a 3.7 L Biostat B (B. Braun Biotech International, Melsungen, Germany) fermenter (Edward, et al., 2011). The high cell-density cultivation of Lb. plantarum NDC 75017 was conducted in the 3.7 L fully instrumented fermenter equipped with a pH probe. 2 L of working media were sterilized in the fermenter at 121°C for 15 min; 100 mL of precultured seed cultures were inoculated into 2 L of mMRS media, and cooled before cultured at 30°C for 24 h. In the fermentation process, sterilized air was flowed through the entire media at a speed of 0.3 standard litres per minute (SLPM) for cell proliferation. Agitation was applied at a rate of 100 rpm, which was just enough to mix the pH control reagent and additional glucose with the media, simultaneously, to minimize mixing air into the medium; the temperature was adjusted to 30°C as optimum for Lb. plantarum NDC 75017 (Shan et al., 2015).
The influence of pH value, neutralizer, and substrate concentration on viable cell counts and survival rate were investigated by single factor experiments. Cells were cultivated in a inconsecutive way in different pH conditions (4.5, 5.0, 5.5, 6.0, and 6.5), different neutralizers (5 M NaOH, 5 M NH3H2O, 5 M Na2CO3, and 5 M Ca (OH)2), and different glucose concentrations (20, 30, 40 g L−1 and new basic MRS), respectively. A constant pH for fermentation was controlled by incessantly adding neutralizer into fermenter and the value was displayed by the pH probe. Different concentrations of glucose solution and new basic MRS solution were separately added into the 3.7 L fermenter in a continuous fed-batch culture method during 6 h of fermentation-approximately in the middle of log phase for cell growth. The new basic MRS culture medium was used for control experiment. Throughout the whole experiments, the culture media were sampled every 2 h for the determination of biomass, glucose concentration, viable cell counts (CFU mL−1), and the survival rate after freeze drying. Cell viability in the fermentation broth was assessed by plate counting method on mMRS agar, which was performed by transferring 0.1 mL of bacterial suspension to 0.9 mL 0.85% w/v physiological saline, diluting this sample in a ten-fold series, subsequently spread plating on mMRS agar, and incubating plates at 30°C for 48 h. The biomass was estimated by measuring the dry weight; the cells were harvested by centrifugating at 6, 000 rpm at 4°C for 15 min and washed twice with 0.85% physiological saline; the obtained pellet was dried at 80°C for circa (ca.) 4 h and weighed (Edward, et al., 2011). Glucose concentration was determined by using a glucose oxidase method according to Keilin et al. (1948). The determination of the survival rate was performed according to Bauer et al. (2012).
Low-temperature vacuum-drying in different cryoprotectants Fresh cultures were centrifuged at 6,000 rpm for 15 min at 4°C and washed twice with 0.85% physiological saline. Then the pellet was collected and weighed. The pellet was resuspended with different cryoprotectants; for the optimization experiments, an experimental design according to the orthogonal experimental design methodology was applied. Aliquots of 2 mL of the cell suspension were transferred to 10 mL glass vials with an inner diameter of 40 mm. The sample height was ca. 0.5 cm. All of the samples were pre-frozen at −40°C in a freezer and then dried in a freezing dryer. The applied shelf temperature, chamber pressure, and condenser temperature were 15°C, 25 Mpa, and −80°C, respectively. The low-temperature vacuum-drying process was stopped after 24 h; vials were sealed and stored at 25°C and 4°C respectively for further experiments. Moreover, residual moisture content can exhibited great influence on the survival rate of bacterial powder after 24 h of vacuum-drying process according to Bauer, et al. (2012). The residual moisture content was assessed gravimetrically in triplicate (80°C, ca. 4h).
Scanning electron microscopy Lb. plantarum NDC 75017 strain and dried-powder were scanned in different situations: (a) viable cells in fermenter before drying; (b) control of 0.85% physiological saline without cryoprotectant; (c) using 10% skim milk as single protectant; (d) using optimized cryoprotectants discussed above. The pretreatment method of freeze-dried starter cultures of Lb. plantarum NDC 75017 for morphology observation was performed as Vinderola's experiment (Vinderola, et al., 2012). Samples were observed in an S-3400N scanning electron microscope (SEM) in high-vacuum mode with a secondary electron detector at an acceleration voltage of 5 kV.
Stability of freeze-dried powders in storage All the freeze-dried samples produced by low-temperature vacuum-drying under optimal incubation coditions discussed above were divided into two groups and each group was stored at 4°C and 25°C (room temperature) respectively, and the viability of bacterial counts was assessed at 15-day intervals over a period of 210 days. The samples were resuspended to the original volume with 0.85% physiological saline, serially diluted in the physiological saline and spread on mMRS agar. The plates were incubated at 30°C for 48 h, and the number of colonies was counted.
Comparisons of Lb. plantarum NDC 75017 fermented milk with mixed-strain fermented milk. Two kinds of fermented milk were assessed in this experiment, including singly fermented milk samples by Lb. plantarum NDC 75017 (LPM) and equivalently mixed-strain fermented milk (MSM) by Streptococcus thermophilus and Lb. bulgaricus. Approximately 108 cfu/mL of inocula for each types were applied to inoculate separately into sterilized skim milk (Nestle Company, Heilongjiang, China), subsequently, incubation at 36°C were performed for preparation of fermented milk according to Shan, et al. (2015). The fermention procedure was terminated while the pH reached 4.5, which measured by Delta 320 pH meter (Mettler-Toledo Instruments Co., Ltd., CH) and then stored at 4°C for 12 h after a rapid cooling down to 4 to 6°C.Based on the properties of viscosity, water-holding capacity (WHC), firmness, viscosity index, and GABA production, different kinds of fermented milk were determined in triplicates in the experiment. The values of WHC were measured by the procedure according to Keogh and O'Kennedy (1998). Meanwhile, textual features-viscosity, firmness and viscosity index were evaluated by Texture Analyser TA DHI (Stable Micro System, UK) using a back extrusion cell methods (Patrignani et al., 2006). The determination of GABA production in different kinds of fermented milk was carried out by high-performance liquid chromatography (HPLC) according to Yang et al (2008).
Statistical analysis Experiments were replicated at least in triplicates in independent assays. Mean microbial cell counts were subjected to one-way analysis of variance (ANOVA) and Duncan's multiple range test (DMRT). Significant differences were indicated at p < 0.05. SPSS software 17.0 (USA) was used for data analysis.
High cell-density cultivation of Lb. plantarum NDC 75017 in fermenter The growth curves of Lb. plantarum NDC 75017 were determined in the batch culture without controlling the pH before optimization experiments, which included viable cell counts (CFU mL−1), glucose concentration (g L−1), and pH value (Fig.1). The microbial population curve in Fig.1 showed an exponential phase period from 4 to 8 h, while a stationary phase from 8 to 18 h. The pH value of the mMRS decreased from 6.0 to 3.4, and the glucose concentration (g L−1) decreased from 16.3 to 1.2 in 20 h during the fermentation with increased cell growth. Glucose depletion and pH value descent suggested that glucose was used mostly to support biomass growth and lactic acid production (Aguirre-Ezkauriatza, et al., 2010; Bomrungnok, et al., 2012; Xiong, et al., 2013). The decreased pH value was likely due to the production of large amount of lactic acid during the fermentation process. After the maximum viable cell counts of 6.48 × 1010 CFU mL−1 was achieved at 14 h, the cell growth decreased rapidly because of glucose depletion and the extremely low pH value (at 18 h). The rapidly-decreasing cell counts indicated that cell lysis and subsequent cell death were occurred. From the earlier observation, microbial population increased first and then kept steady with the decrease of pH value. Fig.1 also showed that the glucose depletion was another inhibition factor. Related research suggested that the glucose uptake and metabolism could maintain mitochondrial homeostasis and enable anabolic pathways required for cell growth (V. Heiden, et al., 2001). Hence, the cell growth period may be prolonged by proper control of pH and addition of glucose in the fermenter, and cell concentration might be increased (Aguirre-Ezkauriatza, et al., 2010).

The growth curves with batch cultivation of Lb. plantarum NDC 75017 at 30°C, 100 rpm.
Fig.2, 3, and 4 showed the effect of different pH, neutralizers, glucose concentration, and harvest time on cell concentration of Lb. plantarum NDC 75017. The results (Fig.2) suggested that the highest viable cell counts (CFU mL−1) obtained were 9.3 × 1010, 12.6 × 1010, 16.5 × 1010, 9.6 × 1010, and 5.6 × 1010 when the pH value was controlled at 6.5, 6.0, 5.5, 5.0, and 4.5, respectively. The viable cell counts of Lb. plantarum NDC 75017 at optimal 5.5 were siginificantly higher than at other pH values according to ANOVA statistic method. For the investigation of the influence of different neutralizers on viable cell counts, the most appropriate pH value was maintained during the entire fermentation by adding 5 M NaOH, 5 M NH3·H2O, 5 M Na2CO3, and 5 M Ca(OH)2, respectively. Fig. 3 showed that the most appropriate neutralizer for the growth of Lb. plantarum NDC 75017 was 5 M NH3H2O. It was obvious that the viable cell counts in the fermentation medium fed with neutralizer was higher (19.4 × 1010 CFU mL−1) than those without neutralizer (6.5 × 1010 CFU mL−1). Aguirre-Ezkauriatza et al. (2010) conducted experiments in batches, with continuous and fed-batch culture conditions in a 3.7 L fully instrumented fermenter, and results showed that high cell-density fed-batch strategies were the best. When Lb. plantarum NDC 75017 was cultivated in a fermenter at different glucose concentrations, the numbers of maximum viable cells were different. The bacterial cells were harvested at different growth phases (late log phase, mid stationary phase, and late stationary phase) and then freeze dried. The highest survival rate was significantly different (p < 0.05) at different glucose concentrations and growth phases (Fig.4). Fig. 4 showed that the highest survival for 30 g L−1, 40 g L−1 glucose and new basic MRS culture medium were 83.1% (at 14 h), 83.5% (at 14 h) and 83.4% (at 12 h) for each other, exhibiting no significantly difference. While, considering with the economic benefit and the simplicity of operation, glucose as single additives were chose in our experiment, then the optimal cell harvest time was confirmed at 14 h. Furthermore, under 14 h of fermentation time, the optimization of glucose concentration was at 30 g L−1, with the highest viable cell counts of 2.74 × 1011 CFU mL−1, 4.2 times higher than mMRS, increasing unit output of the mMRS medium. An interesting phenomenon showed that in Fig. 4, although the maximum of viable cell counts during the whole fermentation process was at 18 h of 40 g L−1, the survival rate at 18 h reached the lowest value for 40 g L−1 glucose. A possible reason was that cell activity decreased with culture time prolonged during stationary phase, and the decline of cell activity resulted in a lower survival rate after low-temperature freeze drying. The result was in accordance with previous research findings by Hongpattarakere et al. (2013) and Carvalho (De Carvalho, et al., 2004) which bacterial cells during the stationary phase have been through the complete development of their cell structure and physiology, including membrane composition, cell wall structure, and expression levels of stress-related proteins, and all of these resulted in the increase of resistance to freeze-drying.
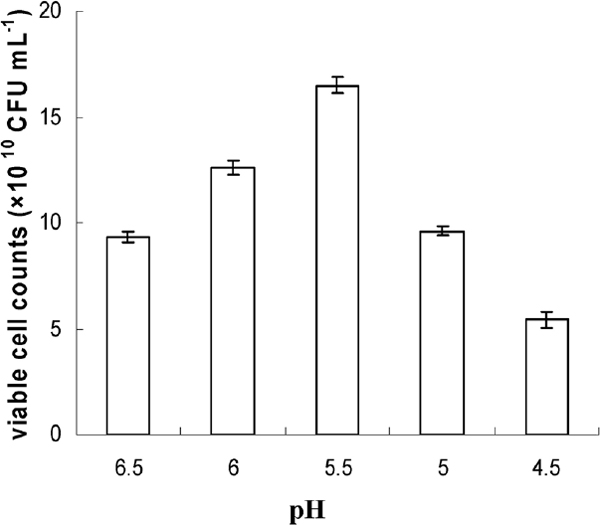
Effect of different pH-controlled conditions on viable cell counts of Lb. plantarum NDC 75017 at 30°C, 100 rpm.Culture conditions were in mMRS for 12 h with addition of 30 g L−1 glucose and a controlled pH by 5 M Na2CO3 as neutralizer.
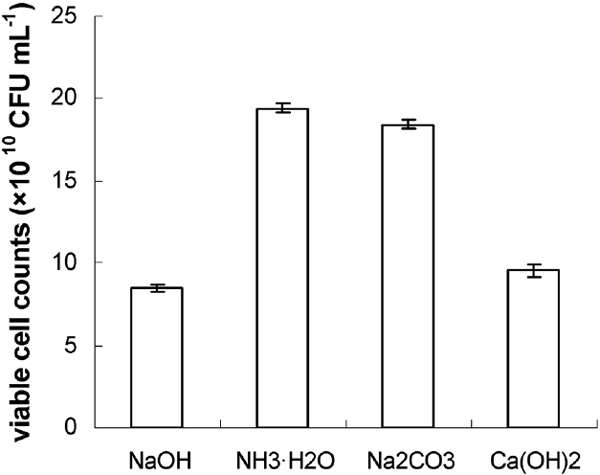
Effect of different neutralizers on viable cell counts of Lb. plantarum NDC 75017 at 30°C, 100 rpm.Culture conditions were in mMRS for 12 h with addition of 30 g L−1 glucose and a controlled pH at 5.5.
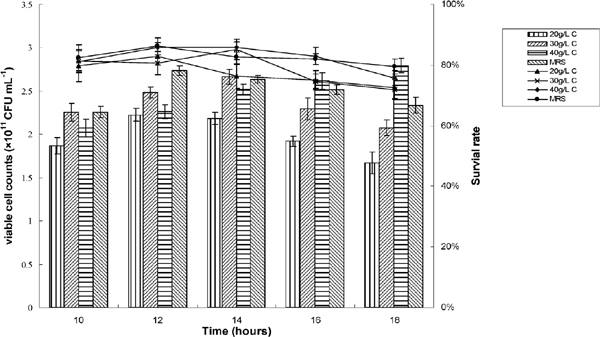
Effect of different glucose concentration and cell harvest time on viable cell counts and freeze-dried survival rate of Lb. plantarum NDC 75017 at 30°C, 100 rpm. Culture conditions were in mMRS with addition of 5 M NH3H2O as neutralizer and regulated pH at 5.5 for counting viable cells and survival rate values of freeze-dried powder were assessed after 45 days of storage at 4°C.The values of viable cell counts and survival rate were separately expressed by the bar-chart and line graph.
Effects of different cryoprotectant addition on the survival of Lb. plantarum NDC 75017 after low-temperature vacuum-drying Fresh cultures of Lb. plantarum NDC 75017 were freeze-dried in the presence of different cryoprotectants with coincident final concentration of 5% (w/v). The viable cells were detected after 45 days of storage at 4°C. The addition of trehalose, maltodextrin, L-glutamate sodium salt (L-GSS), and skim milk significantly (p < 0.05) enhanced the freeze-drying survival of Lb. plantarum NDC 75017 compared with glucose, betaine, ribitol, L-cysteine hydrochloride anhydrous (L-CHA), and vitamin C (VC) (Fig. 5). The addition of trehalose, maltodextrin, L-glutamate sodium salt, and skim milk resulted in a higher survival rate (49%, 52%, 52%, and 52%, respectively), whereas those added glucose, betaine, ribitol, L-cysteine hydrochloride anhydrous, and vitamin C survived at lower levels (7.22%, 6.08%, 5.32%, 5.32%, and 7.22% respectively). The number of viable counts decreased after low-temperature vacuum drying, and a similar result was obtained by Jagannath et al. (2010), who reported higher cell losses with 2–3 log cycle reductions after low-temperature vacuum drying in the CFU compared with the original cell suspension in the case of all lactic acid bacteria. However, the level of cell viability after low-temperature vacuum drying varied according to numerous factors including the strain and the efficacy of the protective agents used (Edward, et al., 2011; Hongpattarakere, et al., 2013; Jagannath, et al., 2010; Vinderola, et al., 2012). According to the orthogonal experimental design methodology (Shown in Table 1), addition of 8% trehalose, 4% maltodextrin, 4% L-glutamate sodium salt, and 10% skim milk as cryoprotectants, the viable cell count was up to 2.22 × 1011 CFU g−1 in the freeze-dried product. An interesting phenomenon was noticed that the presence of L-glutamate sodium salt enhanced the survival rate (52.09%) of Lb. plantarum NDC 75017 after freeze drying. The results indicated that L-glutamate sodium salt could function as potential cryoprotectant to enhance the freezing survival of Lb. plantarum NDC 75017, which could produce GABA by metabolizing L-glutamate sodium salt. Indeed, L-glutamate sodium was broadly used as cryoprotectant during freeze-drying to increase the survival rate of both GABA producing and Non-GABA producing strains. Abadias et al.(2001a) claimed that monosodium glutamate (MSG) can effectively preserve Candida sake cells, which cannot produce GABA by metabolizing L-glutamate sodium salts, during lyophilization. Two possible rationales for protection by L-glutamate sodium salt during dry-freezing were claimed by Font de Valdez et al.(1983): (a) the amino group of the protectant reacted with the carboxyl groups of the microorganism proteins to improve the stabilisation of protein structure; (b) the ability of retaining amounts of residue moisture was improved during freeze-drying. Moreover, the final residual moisture content arranged from 5% to 8% calculated by gravimetrical method.
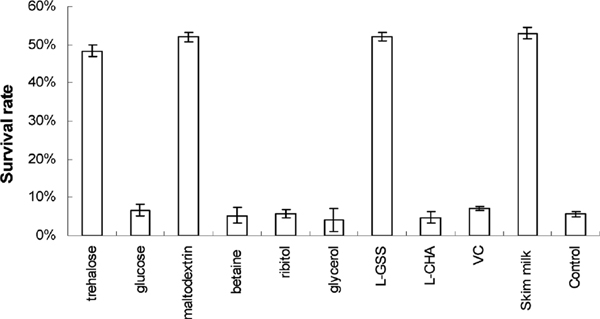
Effects of different cryoprotectants with equally final concentration of 5%(w/v) added in freeze-drying survival of Lb. plantarum NDC 75017. Culture conditions included the addition of 30 g L−1 glucose, 5 M NH3H2O and optimal pH at 5.5 with an incubation time of 14 h, the survival rates assessed after 45 days of storage at 4°C.
| Skim milk (%) | Trehalose (%) | Maltodextrin (%) | L-glutamate sodium salt (%) | Viable counts ×1011 CFU/mL) |
|---|---|---|---|---|
| 8 | 4 | 4 | 2 | 1.81 |
| 8 | 6 | 6 | 4 | 1.78 |
| 8 | 8 | 8 | 6 | 1.42 |
| 10 | 4 | 6 | 6 | 1.86 |
| 10 | 6 | 8 | 2 | 1.26 |
| 10 | 8 | 4 | 4 | 2.22 |
| 12 | 4 | 8 | 4 | 1.64 |
| 12 | 6 | 4 | 6 | 1.56 |
| 12 | 8 | 6 | 2 | 1.48 |
Indeed, both high cell density-fermented method and cryoprotectants addition were needed for high-vitality starter culture production of Lb. plantarum NDC 75017. But the synergy effects of using high cell density-fermenter method and cryoprotectants were quite limit due to different purposes, and both were belonging to two relatively independent processes. A starter culture with high production of GABA in our study was pursued, and obviously high cell density is required for effective synthesis of GABA, so the high cell density-fermented methods were created to produce maximum cell concentration. While, the purpose of adding cryoprotectants was to decrease cell damages during low-temperature vacuum drying procedure.
Scanning electron microscopy Based on the optimized conditions for cell cultivation, cells were incubated in mMRS for 14 h with addition of 30 g·L−1 glucose, 5 M NH3·H2O and a controlled pH at 5.5. Different cryoprotectants were added in subsequently low-temperature vacuum drying to produce freeze-dried starter cultures according to different situations discussed above. The scanning electron micrograph revealed the embedding of Lb. plantarum NDC 75017 to cryoprotectants (Fig. 6). Fig. 6a revealed the bacterial morphology of Lb. plantarum NDC 75017 before freeze-dried procedure under the SEM. While Fig.6b illustrated that lyophilized cells were tightly aggregating in large cell clusters and the surface of cells shrank when 0.85% physiological saline was used as sole cryoprotectant. Similar results had been reported by Jagannath (2010), which showed that water and saline were poor cryoprotectants. However, in the presence of optimal cryoprotectants (Fig. 6d) or skim milk (Fig. 6c), no cell shrinkage was found on the surface. It was clearly observed that the cells of Lb. plantarum NDC 75017 were under a thin layer (Fig. 6d). Such a protective coat did not appear in the presence of 0.85% physiological saline. It might explain the higher survival of Lb. plantarum NDC 75017 when trehalose, maltodextrin, L-glutamate sodium salt, and skim milk were added as cryoprotectants during the freeze-drying process. The present study has shown that trehalose, maltodextrin, glycerol, L-glutamate sodium sal t, and skim mi lk were effect ive cryoprotectants for Lb. plantarum NDC 75017 at low-temperature vacuum-drying process (Siaterlis et al,, 2009).
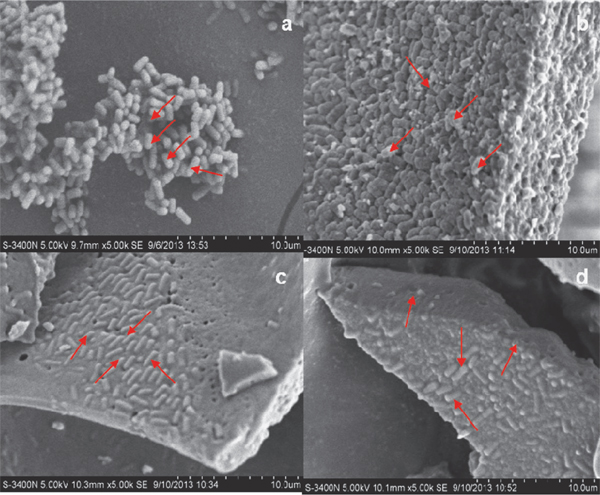
Morphological changes of Lb. plantarum NDC 75017: a. before drying; b. control of 0.85% physiological saline without cryoprotectant; c. protectant of skim milk; d. optimization cryoprotectants added: 8% trehalose, 4% maltodextrin, 4% L-glutamate sodium salt, 10% skim milk, w/v).
Stability of freeze-dried powder in storage Preparation for Lb. plantarum NDC 75017 dried-powder was conducted under optimal conditions discussed above. 30 g·L−1 glucose and 5 M NH3·H2O were added into mMRS for 14 h incubation with a controlled pH at 5.5, then 8% trehalose, 4% maltodextrin, 4% L-glutamate sodium salt, and 10% skim milk as cryoprotectants were added before low-temperature vacuum-drying procedure. All the freeze-dried samples were equivalently stored at 4°C and 25°C, and the viability of bacterial counts was assessed at 15-day intervals over a period of 210 days. Fig.7 showed the viability of Lb. plantarum NDC 75017 dried powder at the end of the 210 days of storage period at 4°C and 25°C, respectively. During the storage of dried powder, the cell counts decreased gradually and reached about 10.4 log CFU g−1 and 10.04 log CFU g−1 in 210 days of storage at 4°C and 25°C, respectively. The survival for 210 days of the freeze-dried Lb. plantarum NDC 75017 in 25°C was slightly lower than that kept at 4°C. Similar results have been reported by Hongpattarakere et al. (2013). Carvalho et al. (2004) have reported that there were multiple factors, such as oxygen content, high temperature, pH, and water activity, involved in the viability of dried powder in the storage period. Higher storage temperature (25°C) meant lower viabilities at the end of the 210 days of storage period.
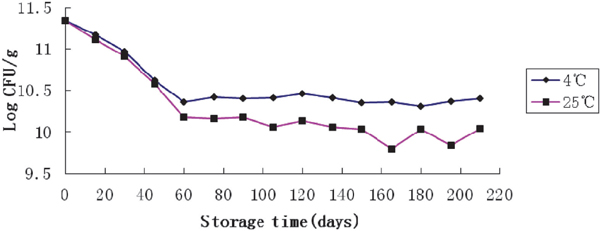
Viability of Lb. plantarum NDC 75017 dried starter cultures produced by low-temperature vacuum-drying after stored at 4 and 25°C.
Comparisons of different kinds of fermented milk by viscosity, water-holding capacity, firmness, and viscosity index properties Further experiments were conducted by using Lb. plantarum NDC 75017 as a single starter culture in fermented milk, meanwhile, mixed-strain (Str. thermophilus and Lb. bulgaricus) fermented milk as a comparison to assess its physical characteristics and GABA production.The results were summarized in Table 2, which showed that significantly differences were exerted in viscosity, WHC and firmness properties among two kinds of fermented milk. Meanwhile, LPM exhibited an mean value of 1351.8 ± 14.2 p·s, 55.7 ± 1.5(%) and 44.5 ± 0.9 g in viscosity, WHC and firmness respectively. The viscosity index between LPM and MSM showed no significant difference, with respective values of 147.3 ± 0.9 p·s and 146.8 ± 0.9 p·s. While, the production of GABA measured in LPM was 273.45 ± 1.56 mg/100 g, siginificantly higher than 134.76 ± 1.14 mg/100 g in MSM, which showed a better GABA-production ability of Lb. plantarum NDC 75017. In conclusion, the strain-Lb. plantarum NDC 75017 possesses benefical physical properties and potentialities in production of fermented milk as a high-vatality starter culture.
| Yogurt | Viscosity (p·s) | WHC (%) | Firmness (g) | Viscosity index (p·s) | GABA production (mg/100 g) |
|---|---|---|---|---|---|
| MSM | 1448 ± 9 ± 11 ± 6a | 71.7 ± 3 ± 2a | 77.3 ± 1 ± 7a | 146 ± 8 ± 0 ± 9a | 134 ± 76 ± 1 ± 14a |
| LPM | 1351 ± 8 ± 14 ± 2b | 55.7 ± 1 ± 5b | 44.5 ± 0 ± 9b | 147 ± 3 ± 0 ± 9a | 273 ± 45 ± 1 ± 56b |
Yogurts: MSM: mixed-strain fermented milk (Streptococcus thermophilus and Lactobacillus bulgaricus); LPM: Lactobacillus plantarum NDC 75017 fermented milk; WHC: water-holding capacity.
a+bMeans within a row with different superscripts differ (P < 0.05).
This study proposed a simple fermentation technology that offered a possibility for commercialization of a process for producing biomass and high-viability dried starter cultures by Lb. plantarum NDC 75017. The starter possessed a prospect to be applied in dairy and other health products with high nutritive values and functional properties. Furthermore, the viability and stability of strains upon freeze drying was strain dependent, and it is not a sufficient predictor for strain functionality in adverse conditions such as low pH, gastric acidity, bile tolerance, and adherence to gastrointestinal tract; thus, it must be studied before its further development as commercial starter culture. In addition, L-glutamate sodium salt added to mMRS broth as a cryoprotectant in the freeze-drying process could improve the freeze-drying tolerance of Lb. plantarum NDC 75017. However, further research and scale-up efforts for further studying the mechanism of glutamic acid sodium salt to improve the freeze-drying survival rate are required. Although our study was based on small-scale laboratory fermentations, it provided great insight into the benefit of using Lb. plantarum NDC 75017 freeze-drying powder to produce GABA-enriched fermented food.
Acknowledgments This study was supported by the Science Foundation for Distinguished Young Scholars of Heilongjiang Province (No. JC201415), the Promotion Program for Innovation of Science Research in Heilongjiang Province (No. YC13D005), the Nat ional Key Technology Support Program (No. 2013BAD18B11), and the “Academic Backbone” Project of Northeast Agricultural University (15XG26).