2016 Volume 22 Issue 5 Pages 575-582
2016 Volume 22 Issue 5 Pages 575-582
Thirty-five predominant lactic acid bacteria (LAB) were isolated from six samples of traditional Mongolian Airag (fermented mare's milk) collected in four provinces near Ulaanbaatar, and the bacteria were classified into ten types of LAB by 16S rDNA sequencing. Two types of yeast were also isolated and identified by 26S rDNA sequencing. Two supernatants produced by Lactobacillus hilgardii (Uvu-21) and L. diolivorans (Tuv-33) were found to have potent antibacterial and proteolytic activities, with high thermal and wide pH range stabilities. The activities disappeared after protease treatment, suggesting that the biological activity originated from peptides produced by the LAB. In addition, the fermentation ability of the yeasts was weaker than that of K7 yeast used in sake brewing. These results provide useful information about Airag as a healthy daily beverage in Mongolia.
Mongolian Airag is a traditional beverage that is made by the fermentation of mare's milk with lactic acid bacteria (LAB) and yeast in a large cowhide bag, and contains 2 – 4 w/w% ethanol (Cagno et al., 2004; Watanabe, 2011). Mare's milk contains a higher proportion of proteins and lower proportion of fatty acids than human and cow's milk, and mare's milk has a remarkably high concentration of unsaturated fatty acids (Malacarne et al., 2002; Potocnik et al., 2011). Therefore, in Mongolia and Inner Mongolia of China, Airag is consumed daily to improve physical strength, promote digestion, and provide nourishment. There are several reports on the identification of LAB in Airag and investigation of the characteristics of Airag as a beverage.
Two antibacterial peptides with molecular weights of 5206 and 5218 Da were isolated from Mongolian Airag samples and characterized, revealing similarity with enteriocins L50A, L50B, and I, which were isolated from the supernatant of LAB Enterococcus faecium and Ent. faecium 6T1a (Batdorj et al., 2006). These two peptides had potent antibacterial activity in a wide pH range on both gram-positive Lactobacillus, Enterococcus, pathogenic Listeria, and Staphylococcus strains, and gram-negative E. coli bacteria (Batdorj et al., 2006). Batdorj also found an antibacterial peptide from L. delbrueckii subsp. lactis strain T3 in Tarag, a traditional Mongolian yoghurt produced by the fermentation of cow's milk with LAB. That peptide had a potent inhibitory effect on E. coli and Listeria innocua strains (Batdorj et al., 2007).
Watanabe reported that several hundred LAB and yeasts had been isolated from 22 Airag and 31 Tarag samples in Mongolia and identified by culture- and molecular biology-based methods (Watanabe et al., 2008). In Airag, L. helveticus and L. kefiranofaciens were the main LAB, and L. delbrueckii subsp. bulgarius and Streptococcus thermophils were predominant in Tarag. The lactose-fermenting Kluyveromyces marxianus was the major yeast in Airag samples, and Saccharomyces cerevisiae, Issatchenkia orientalis, and Kazachstania unispora as glucose-fermenting yeast were predominant in Tarag samples. These results suggest that the most important factor in the diversity of microbial composition between livestock animal milks was the type of Mongolian traditional beverage rather than the geographical region (Watanabe et al., 2008; Oki et al., 2014).
A 3.3-kDa antibacterial peptide produced by the lactic acid bacterium Leuconostoc mesenteroides strain 406 in a Mongolian Airag sample was isolated and characterized by Miyamoto and coworkers (Wulijideligen et al., 2012). It was found that the supernatant of the LAB inhibited the growth of several LAB, pathogenic and food spoilage organisms. Although the supernatant was heat- and pH-stable, the inhibitory activity on bacteria was decreased by digestion by several proteases.
Miyamoto also isolated and characterized many LAB in samples of Inner Mongolian Chigee, a fermented mare's milk from China (Burentegusi et al., 2002). Many LAB were also isolated from Koumiss, which is a beverage composed of fermented livestock animal milks produced in Central Asian countries (Danova et al., 2005; Mechai et al., 2014).
Research on the classification and functionality of LAB in fermented milks is attracting attention because fermented beverages and foods are widely consumed in Asian countries to promote health. In the present study, we report on the isolation and identification of several LAB in Airag using 16S rDNA sequencing analysis. We found that the supernatants had potent antibacterial and proteolytic activities. We also isolated yeasts in Airag samples and investigated their ability to ferment glucose compared with that of K7 yeast.
Materials Six Airag samples were collected in four provinces of Mongolia near Ulaanbaatar, Arkhangai Khashaat (Ark), Bulgan Saikhan (Bul), Uvurkhangai Bayan- Undur (Uvu), and Tuv Delgerkhaan (Tuv), in July 2014. Each sample (50 mL) was stored at 4°C until use. For microbial enumeration, Airag samples (2 mL) were kept at −80°C in 20% glycerol until use. Escherichia coli (ATCC25922), Bacillus subtilis (NBRC 13722), L. acidophilus (NBRC 13951), L. paracasei (NBRC 15889), and L. plantarum (NBRC 101978) were obtained from the National Institute of Technology and Evolution (NITE) of Japan. MRS and M17 broths and fat-free dry skim milk were obtained from Oxoid Co. Ltd., Hampshire, England. α-Chymotrypsin, trypsin, and proteinase K were obtained from Sigma-Aldrich Co., 3050Spruce Street, St. Louis city, USA. Cycloheximide and chloramphenicol were purchased from Wako Pure Chemical Ind. Ltd., Oosaka city, Japan, and Anaeropack was purchased from Mitsubishi Gas Chemical Co. Ltd., Kanagawa prefecture 253 - 0084, Japan. YPG (yeast, peptone, and glucose) medium was prepared from 10 g/L of yeast extract, 20 g/L of peptone and 50 g/L of glucose in 1000 mL of deionized water at pH 5.0, and the medium was autoclaved for 20 min at 121°C before use. Kyokai No. 7 (K7) yeast (S. cerevisiae, NBRC 2347) for sake brewing was purchased from NITE and used as the standard yeast for fermentation.
Measurement The concentrations of D-lactose, D-glucose, and ethanol in Airag were measured using an aqueous HPLC system with a Tosoh TSK Amide-80 column (particle size: 2 mm, 7.6 mm × 150 mm), eluted with a mixed solution of acetonitrile-water (7:3 v/v) at a flow rate of 0.5 mL/min, and a Tosoh RI-8020 detector, as well as a Shimadzu GC-8A gas chromatograph equipped with a capillary column (SE-30, 3.2 mm × 30 m) at 60°C for hydrogen flame ionization detection and 130°C for column and injection temperatures. The lactic acid and protein concentrations were determined by a titrimetric method according to the Association of Official Analytical Chemists (AOAC) instructions (AOAC, 1996). Amplification of DNA regions was carried out using a Bio-Rad Mycycler and MJ Research Peltier PTC-200 thermal cyclers. Electrophoresis of the PCR products was performed by a Shimadzu MCE-202 MultiNA spectrophotometer. The PCR conditions were heating for 5 min at 95°C as the initial denaturation step and then 35 cycles of 30 sec at 95°C for denaturation, 1 min at 58°C for annealing, and 2 min at 72°C for elongation. A final extension step for 10 min at 72°C was performed at the last cycle. The sequence analysis of the DNA regions was conducted using an Applied Biosystems AB 3130 genetic analyzer with a 36 cm capillary column. Catalase activity (Batdorj et al., 2006) and Gram staining (Bartholomew et al., 1952) for the identification of LAB in Airag were performed according to the methods described in the literature. Turbidity was detected at an absorbance of 600 nm using a Hitachi U0080D spectrophotometer, Tokyo, Japan
Isolation of LAB and yeasts in Airag samples LAB and yeasts were isolated from Airag samples according to the direct plating method (Coventry et al., 1997). For the isolation of LAB, Airag samples (1 mL) were diluted in water (6 mL) and plated on MRS agar plates with cycloheximide (25 µg/mL) as the antibiotic, and the plates were incubated under anaerobic conditions for 3 d at 37°C using an Anaeropack. After incubation, the obtained colonies were purified by restreaking on a new agar plate and then identified by the 16S rDNA method. Yeasts in Airag were also isolated by the same method using YPG agar plates with the antibiotic chloramphenicol (50 µg/mL) and incubating the plates for 3 d at 30°C. Yeasts isolated were identified by the 26S rDNA sequencing method. All purified colonies were suspended in 20% glycerol (w/v) in sterilized tubes and kept at −80°C.
Identification of LAB and yeasts LAB and yeasts in Airag were identified by 16S rDNA and 26S rDNA analyses, respectively, using universal primers 9F (5′-GAGTTTGATCCTGGCTCAG-3′) and 802R (5′-TACCAGGGTATCTAATCC-3′), and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) and NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′). The results are shown in Table 2.
| Name | Source province | Catalase activityb | Gram stainingb | Identified speciesc | Identity % |
|---|---|---|---|---|---|
| Ark-1 | Arkhangai Khashaat | − | + | Enterococcus hirae | 100 |
| Ark-2 | Arkhangai Khashaat | − | + | Enterococcus faecalis | 98 |
| Bul-3 | Bulgan Saikhan-I | − | + | Enterococcus hirae | 99 |
| BuI-4 | Bulgan Saikhan-I | − | + | Enterococcus durans | 99 |
| BuI-5 | Bulgan Saikhan-I | − | + | Lactobacillus helveticus | 100 |
| BuI-6 | Bulgan Saikhan-I | − | + | Enterococcus faecium | 100 |
| Bul-7 | Bulgan Saikhan-I | − | + | Lactobacillus kefiri | 98 |
| BuI-8 | Bulgan Saikhan-II | − | + | Enterococcus faecium | 100 |
| BuI-9 | Bulgan Saikhan-II | − | + | Lactobacillus sp. | 98 |
| BuI-10 | Bulgan Saikhan-II | − | + | Lactobacillus casei | 99 |
| BuI-11 | Bulgan Saikhan-II | − | + | Lactobacillus casei | 100 |
| BuI-12 | Bulgan Saikhan-II | − | + | Lactobacillus helveticus | 100 |
| BuI-13 | Bulgan Saikhan-II | − | + | Lactobacillus kefiri | 99 |
| BuI-14 | Bulgan Saikhan-III | − | + | Lactobacillus casei | 99 |
| Bul-15 | Bulgan Saikhan-III | − | + | Lactobacillus casei | 100 |
| BuI-16 | Bulgan Saikhan-III | − | + | Lactobacillus helveticus | 99 |
| Bul-17 | Bulgan Saikhan-III | − | + | Lactobacillus kefiri | 98 |
| Bul-18 | Bulgan Saikhan-III | − | + | Lactobacillus paracasei | 98 |
| Bul-19 | Bulgan Saikhan-III | − | + | Lactobacillus paracasei | 99 |
| BuI-20 | Bulgan Saikhan-III | − | + | Lactobacillus hilgardii | 100 |
| Uvu-21 | Uvurkhangai BU | − | + | Lactobacillus hilgardii | 100 |
| Uvu-22 | Uvurkhangai BU | − | + | Lactobacillus hilgardii | 99 |
| Uvu-23 | Uvurkhangai BU | − | + | Lactobacillus hilgardii | 98 |
| Uvu-24 | Uvurkhangai BU | − | + | Lactobacillus diolivorans | 100 |
| Uvu-25 | Uvurkhangai BU | − | + | Lactobacillus diolivorans | 99 |
| Uvu-26 | Uvurkhangai BU | − | + | Lactobacillus paracasei | 99 |
| Uvu-27 | Uvurkhangai BU | − | + | Lactobacillus helveticus | 98 |
| Uvu-28 | Uvurkhangai BU | − | + | Lactobacillus helveticus | 100 |
| Uvu-29 | Uvurkhangai BU | − | + | Enterococcus durans | 100 |
| Uvu-30 | Uvurkhangai BU | − | + | Enterococcus sp. | 100 |
| Tuv-31 | Tuv Delgerkhaan | − | + | Lactobacillus casei | 99 |
| Tuv-32 | Tuv Delgerkhaan | − | + | Lactobacillus casei | 100 |
| Tuv-33 | Tuv Delgerkhaan | − | + | Lactobacillus diolivorans | 99 |
| Tuv-34 | Tuv Delgerkhaan | − | + | Lactobacillus paracasei | 98 |
| Tuv-35 | Tuv Delgerkhaan | − | + | Lactobacillus kefiri | 98 |
Antibacterial and proteolytic activities of LAB supernatants The antibacterial activity of supernatants was determined by comparison with that of standard LAB according to the agar well diffusion method (Jivka et al., 2014). LAB (8×106 cells/mL) isolated from Airag was grown in 3 mL MRS broth under anaerobic conditions for 24 h at 37°C. The culture was centrifuged for 10 min at 8000G and then the supernatant was adjusted to pH 6.5 with 0.5 N aqueous NaOH. The supernatant (50 µL) was placed on E. coli and B. subtilis agar plates and incubated overnight at 37°C. The antibacterial activity was determined by the diameter of each inhibited circle around the wells on the agar plate and expressed as an arbitrary unit (AU) per mL. One AU was defined by the reciprocal of the highest serial 2-fold dilution (Hernández et al., 2005).
The proteolytic activity of the supernatant was assayed using agar plates with 2.5% skim milk, and the activity was determined by the diameter of the hydrolyzed clear circle around the LAB (Mechai et al., 2014; Guessas et al., 2012). The supernatant (50 µL) was placed on an agar plate containing 2.5% skim milk and the plate was incubated for 16 h at 37°C. The proteolytic activity was determined by the diameter of each inhibition circle around the wells and represented as units per mL. One unit of protease activity was determined by the amount of the supernatant (1 mL) that released 1 µg of tyrosine per 1 min under the above conditions (Jivka et al., 2014; Wildeboer et al., 2009).
The antibacterial and proteolytic activities were compared with those of three standard LAB, L. acidophilus, L. paracasei, and L. plantarum.
Time course of antibacterial activity of LAB supernatants toward E. coli A typical procedure for the antibacterial activity of LAB supernatants on E. coli and B. subtilis was as follows. The LAB L. diolivorans (Tuv-33) (1×108 cells/mL) was added to MRS medium (3 mL) and the mixture was then cultured for 24 h at 37°C. After centrifugation, E. coli (8×106 cells/mL) and LB broth (1.5 mL) were added to the supernatant (1.5 mL) and the mixture was gently stirred for 24 h at 37°C. For determination of antibacterial activity, the turbidity of the solution was measured by the absorbance at 600 nm at each prescribed time. The results are presented in Figure 1. The antibacterial activity toward B. subtilis was also examined by the same procedure as above.

Time course of the antibacterial activity of LAB supernatants.
The supernatant was incubated with (A) E. coli and (B) B. subtilis for 24 h at 37°C and the turbidity was measured by the absorbance at 600 nm.
Thermal stability of LAB supernatant The supernatant (1.5 mL) described in 2.6 was heated at 100°C for 15, 30, 60 and 180 min, respectively. After cooling, E. coli (8×106 cells/mL) and LB broth (1.5 mL) were added to the solution and then the mixture was cultured for 24 h at 37°C. The turbidity was measured by the absorbance at 600 nm. The results are shown in Figure 3.
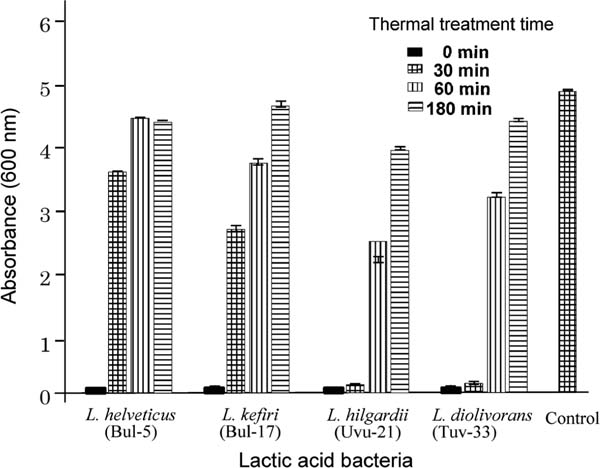
Thermal stability of LAB supernatants.
The supernatants were heated for fixed times at 100°C and then incubated with E. coli for 24 h at 37°C. Turbidity was measured absorbance at 600 nm.
Effect of pH on biological activity of LAB The pH dependence of the supernatant was performed as follows. LAB were incubated in MRS broth under anaerobic conditions for 24 h at 37°C, and then the pH of the supernatant was adjusted to the desired pH shown in Figure 2. The supernatant was stirred for a further 2 h and readjusted to a pH of 6.5. The supernatant was subjected to determination of pH dependence using E. coli and B. subtilis.

Effect of pH of L. hilgardii (Uvu-21) and L. dilolivorans (Tuv-33) supernatants on antibacterial activity toward (A) E. coli and (B) B. subtilis.
The relationship between the pH of the LAB supernatant and its biological activity was elucidated by changing the pH of the supernatant and using the same procedure as described in section 2.5.
Ethanol fermentation of yeasts A typical fermentation of D-glucose using K. unispora and Kazachstania sp. yeasts isolated from Airag was carried out as follows. Glucose (2.0 g) was fermented with 1×108 cells/mL of K. unispora in deionized water at 30°C and pH 5.0. The ethanol produced and the glucose remaining were determined after 72 h fermentation by gas chromatography (GC) and HPLC, respectively. Ethanol conversion was calculated from the theoretical concentration of ethanol produced by glucose in the feed. K7 yeast was also fermented using the same conditions as above for the control.
Isolation of LAB in Airag samples Airag is a health-promoting beverage that is typically prepared by stirring and fermenting fresh mare's milk with a small amount of previously fermented Airag containing LAB and yeasts. Several papers have reported on the isolation and identification of LAB from Mongolian fermented milks, in addition to characterizing them as beverages. However, few papers have reported on the exact functionality or biological activities of LAB in Airag. In this study, six Airag samples were collected in four provinces of Mongolia, and LAB and yeasts in the samples were isolated and characterized to reveal their functionality. Table 1 shows the results of component analysis of Airag samples, such as lactic acid (1.1 – 1.2 w/w%), lactose (3.5 – 5.0 w/w%), glucose (0 – 0.18 w/w%), ethanol (2.7 – 3.1 w/w%), and proteins. The density was around 1.02 g/cm3. Airag samples collected in Bulgan Saikhan-I (Bul-I) and Tuv Delgerkhaan (Tuv) had higher ethanol and lactose concentrations, respectively.
| Source province of Airag sample | Lactic acid w/w% | Lactose w/w% | Glucose w/w% | Ethanol w/w% | Total protein w/w% | Density g/cm3 |
|---|---|---|---|---|---|---|
| Arkhangai Khashaat | 1.1±0.01 | 4.3±0.01 | 0.13±0.01 | 3.2±0.20 | 3.0±0.01 | 1.02±0.01 |
| Bulgan Saikhan-I | 1.2±0.02 | 3.9±0.01 | nd | 3.5±0.10 | 3.1±0.02 | 1.02±0.02 |
| Bulgan Saikhan-II | 1.2±0.02 | 3.5±0.01 | nd | 2.9±0.01 | 2.8±0.10 | 1.02±0 |
| Bulgan Saikhan-III | 1.2±0.10 | 3.6±0.03 | nd | 3.0±0.02 | 2.7±0.20 | 1.02±0 |
| Uvurkhangai BU | 1.1±0.01 | 4.6±0.01 | 0.17±0.01 | 2.1±0.01 | 2.8±0.30 | 1.02±0.01 |
| Tuv Delgerkhaan | 1.1±0.01 | 5.0±0.02 | 0.18±0.02 | 2.3±0.01 | 2.9±0.10 | 1 |
Table 2 presents the isolation and identification of thirty-five LAB from Airag samples. LAB were isolated by the direct plating method using MRS agar plates with cycloheximide. Each Airag sample contained several kinds of bacteria. The isolated bacterial strains did not have catalase activity (Batdorj et al., 2006) and were gram positive (Bartholomew et al., 1952), indicating that all isolated bacteria were LAB (Batdorj et al., 2006). LAB were identified by 16S rDNA analysis, and comparison of DNA sequences with those previously reported indicated more than 98% sequence similarity.
Antibacterial and proteolytic activities of LAB All LAB were examined for antibacterial activity toward E. coli and B. subtilis and proteolytic activity toward skim milk, and the biological activities were compared with those of the standard LAB, L. acidophilus, L. paracasei, and L. plantarum. The results are shown in Table 3. Among the LAB in Table 2, the supernatants of ten LAB were found to have one or both biological activities, as shown in Table 3. Results showed that LAB with high antibacterial activity were contained in each Airag sample. Three LAB, L. kefiri (Bul-17), L. hilgardii (Uvu-21), and L. diolivorans (Tuv-33), produced a zone of inhibition (> 5 mm diameter) on the E. coli agar plate and were found to have potent antibacterial activity as high as 1600 AU/mL. Seven LAB in Table 3 had proteolytic activity on the 2.5% skim milk agar plates. Among them, L. diolivorans (Tuv-33) had the highest proteolytic activity of more than 3.9 units/mL. These results suggest that L. diolivorans (Tuv-33) had the most potent biological activity. In general, the antibacterial activity of LAB originates from the peptides produced. Therefore, the cell-free supernatants of two LAB with potent antibacterial activity, L. hilgardii (Uvu-21) and L. diolivorans (Tuv-33), were digested by proteases at pH 6.5. After treatment of the supernatant with α-chymotrypsin, trypsin, and proteinase K, respectively, the supernatant was incubated with E. coli; the antibacterial activity of E. coli disappeared, suggesting that the antibacterial activity is attributable to the peptides produced by LAB. The isolation and structural determination of these peptides are under investigation.
| Name | Classification | Antibacterial activity | Proteolytic activitya | ||
|---|---|---|---|---|---|
| E. coli | B. subtilis | ||||
| % | AU/mL | AU/mL | unit/mL | ||
| Ar-1 | Enterococcus hirae | 100 | 200 | 200 | 0 |
| Ar-2 | Enterococcus faecalis | 98 | 200 | 200 | 0 |
| Bul-5 | Lactobacillus helveticus | 100 | 400 | 400 | 2.1 |
| Bul-6 | Enterococcus faecium | 100 | 0 | 200 | 0 |
| Bul-9 | Lactobacillus sp. | 98 | 200 | 0 | 0.5 |
| Bul-11 | Lactobacillus casei | 100 | 800 | 800 | 0.9 |
| Bul-17 | Lactobacillus kefiri | 98 | 1600 | 1600 | 0 |
| Uvu-21 | Lactobacillus hilgardii | 100 | 1600 | 1600 | 0.8 |
| Tuv-33 | Lactobacillus diolivorans | 99 | 1600 | 1600 | 3.9 |
| Tuv-34 | Lactobacillus paracasei | 98 | 400 | 400 | 0 |
| NBRC 13951 | Lactobacillus acidophilus | 800 | 800 | 0 | |
| NBRC 15889 | Lactobacillus paracasei | 200 | 0 | 3.2 | |
| NBRC 101978 | Lactobacillus plantarum | 0 | 0 | 0.4 | |
Figure 1 shows the time course of the antibacterial activity of LAB supernatants toward E. coli. and B. subtilis as gram-positive and -negative strains, respectively. In Figures 1A and 1B, the supernatant was incubated with E. coli and B. subtilis for 24 h at 37°C, respectively, and the turbidity was measured by absorbance at 600 nm. The control showed increased turbidity because E. coli multiplied in the absence of the LAB supernatant. The turbidity of the supernatants from L. casei (Bul-11) and L. paracasei (Tuv-34) increased slightly in an incubation time-dependent manner, respectively. However, it was found that the supernatants of L. helveticus (Bul-5), L. kefiri (Bul-17), L. hilgardii (Uvu-21), and L. diolivorans (Tuv-33) had potent antibacterial activity toward both bacteria; 24 h incubation produced clear solutions, indicating that E. coli and B. subtilis did not multiply.
Figure 2 presents the effect of LAB supernatant pH on antibacterial activity. The supernatants of L. hilgardii (Uvu-21) and L. diolivorans (Tuv-33) showed potent antibacterial activity between pH 4 and 8, and also showed higher antibacterial activity against B. subtilis than E. coli over a wide pH range. The activity was reduced with decreasing and increasing pH, and disappeared at pH 2 and 10.
Figure 3 shows the thermal stability of the LAB. Mixtures of each LAB supernatant and MRS medium were heated for 30, 60, or 90 min at 100°C, and the thermal stability was evaluated by measuring the turbidity of the supernatant (absorbance at 600 nm) after incubation with E. coli for 24 h at 37°C. Although the antibacterial activity of the supernatants produced by L. helvetic (Bul-5) and L. kefiri (Bul-17) disappeared after heating for 30 min at 100°C, the supernatants of L. hilgardii (Uvu-21) and L. diolivorans (Tuv-33) showed high thermal stability. After heating for 30 min at 100°C, the antibacterial activity of supernatants showed the same potency as before heating. However, the antibacterial activity of all supernatants decreased after heating for more than 60 min, suggesting that the peptides in the supernatants were denatured by heating. Therefore, the biological activity likely originates from the peptides produced by the LAB.
Isolation and fermentation of yeasts in Airag samples Yeasts in Airag were isolated by incubation with chloramphenicol and identified by 26S rDNA sequencing to give two yeasts, K. unispora and Kazachstania sp. After the two yeasts were cultured on YPG agar plates, the fermentation ability was compared with that of K7 yeast, which is used for sake brewing. Table 4 shows the results of the yeast identification and fermentation. Ethanol fermentation of glucose (2.0 g) was carried out with the yeasts (1×108 cells/mL) in deionized water for 72 h at 30°C and pH 5.0. Ethanol fermentation with a mixture of the two yeasts (1×104 cells/mL of each yeast) was also performed. While the K7 yeast produced ethanol at a concentration of 5.4 g/L, yeasts isolated from Airag produced concentrations of 2.1 – 3.0 g/L, respectively. The ethanol produced using a mixture of the two yeasts gave almost the same result of 2.3 g/L. Thus, the fermentation ability of yeasts isolated from Airag was lower than that of K7 yeast. After 72 h fermentation, glucose was still detected for K. unisporia and Kazachstania sp. isolated from Arkhangai Khashaat and Bulgan Saikhan Airag samples, but no glucose remained in the fermented solution obtained using K. unisporia isolated from a Tuv Delgerkhaan Airag sample. These results are in agreement with the low ethanol concentration of the Airag samples used here, as shown in Table 1.
| No | Source Province | Classificationa | Identitya % | Ethanolb g/L | Glucose remaining g/L |
|---|---|---|---|---|---|
| 1 | Arkhangai Khashaat | Kazachstania unispora | 97 | 2.0 | 6.2 |
| 2 | Bulgan Saikhan | Kazachstania unispora | 99 | 2.1 | 6.0 |
| 3 | Uvurkhangai BU | Kazachstania sp | 99 | 2.6 | 2.1 |
| 4 | Tuv Delgerkhaan | Kazachstania unispora | 100 | 3.0 | 0 |
| 5c | Uvurkhangai BU | Kazachstania sp | 2.3 | 2.9 | |
| Tuv Delgerkhaan | Kazachstania unispora | ||||
| K7d | 5.4 | 0 |
In conclusion, thirty-five LAB were isolated from six Airag samples collected in four provinces of Mongolia. Among the LAB, the supernatants of ten LAB were found to have potent antibacterial activity toward both E. coli and B. subtilis, and proteolytic activity on 2.5% skim milk. The supernatants of four LAB, L. helveticus (Bul-5), L. kefiri (Bul-17), L. hilgardii (Uvu-21), and L. diolivorans (Tuv-33), completely inhibited the increase of pathogenic E. coli and/or B. subtilis for 24 h at 37°C; turbidity measurements (absorbance at 600 nm) were near 0 and the solutions were clear. The supernatants of two LAB, L. hilgardii (Uvu-21) and L. diolivorans (Tuv-33), showed a wide range of thermal and pH stabilities in their antibacterial activity. The antibacterial activity of LAB likely originates from the peptides they produce. The isolation and identification of these antibacterial peptides is under investigation and structure-activity relationships will be elucidated. In addition, two yeasts were isolated, and their ability to ferment glucose was compared with that of K7 yeast used in sake brewing. The fermentation ability of the two yeasts was weaker than that of K7 yeast, producing 2.1 – 3.0 g/L of ethanol. These results are in agreement with the low ethanol concentrations of the Airag samples.
Acknowledgments This research was partly supported by a Grant-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science 2012-2014 (No. 24550129) and 2015-2017 (No. 15K05617).