2016 Volume 22 Issue 5 Pages 679-686
2016 Volume 22 Issue 5 Pages 679-686
Ripened sweet rice wines (mirins) are stored at room temperature for several years to produce a well-balanced sweet flavor. We performed reverse-phase HPLC quantitative analysis of d-amino acids in eight ripened mirins by using derivatization reagents, and investigated the influence of the Maillard reaction on amino acid racemization in mirin during maturation. The relative quantities of d-enantiomers (%d) of Asp, Glu, and Ser in mirins matured for seven years or more were higher than in non-ripened mirins. Based on the calculated correlation coefficient between %D and the amount of Amadori rearrangement products (ARPs) analyzed by LC-MS in ripened mirins for each amino acid, Asp showed a statistically significant strong correlation. Finally, we conducted heating experiments using synthetic ARPs. Our results revealed for the first time that Asp was racemized via ARPs under the influence of pH during mirin maturation.
d-amino acids (d-AAs) are found naturally in various perishable foods such as vegetables, fruits, and milk (Gogami et al., 2006; Palla et al., 1989). Fermented foods were reported to have higher levels of d-AAs than perishable foods. In soy sauces, d-Ala, d-Asp, and d-Glu were detected at concentrations of 1.6, 0.9, and 1.0 mM, respectively, with corresponding levels in cheese of 5.8, 4.4, and 4.8 mM, respectively (all numbers are average values for eight products, Inoue et al., 2014). Balsamic vinegar matured for twenty-five years contained a high amount of d-Pro (1.7 mM, Erbe et al., 1998). Other fermented foods, including soybean paste (Inoue et al., 2014), black vinegar (Okada et al., 2011), and sake (Japanese alcoholic beverage, Gogami et al., 2011), were reported to contain several d-AAs. d-AAs are thought to be produced by lactic acid bacteria in fermented foods (Gogami et al., 2012), with their racemase enzymes being responsible for the conversion of l-amino acids (l-AAs) into d-AAs during the fermentation process.
In addition to perishable and fermented foods, processed plant syrup, roasted cocoa beans, and thermally-treated bee honey were reported to have high relative amounts of d-AAs compared to the total d+l amino acid content (Pätzold and Brückner, 2005; Pätzold and Brückner, 2006a, 2006b). The racemization during thermal processing was attributed to the Maillard reaction. Brückner et al. heated aqueous solutions of l-AAs with/without excess saccharides for 96 h at pH 7.0 and 100°C, and showed that saccharides induced racemization of some l-AAs (Brückner et al., 2001). They proposed that d-AAs were generated from Amadori rearrangement products (ARPs), which are stable intermediates formed in the course of the Maillard reaction.
Sweet rice wines (mirins) are alcoholic condiments high in glucose that are used as a seasoning in Japanese cuisine, and those matured for longer periods are considered to be key ingredients for top-level cuisine. Mirin is produced from glutinous rice, koji malt (non-glutinous rice malted using Aspergillus oryzae), and distilled spirit, and is saccharified at room temperature for around two months (conversion of starch into glucose). After filtration, some mirins are immediately made into seasoning products (usually by adding liquid sugar), whereas others are stored at room temperature for long periods to ripen in the presence of high concentrations of alcohol and glucose, thereby developing a well-balanced sweet flavor (Kawabe and Morita, 1998). Several reactions occur between the components during maturation, including the Maillard reaction. In fact, the amounts of 3-deoxyglucosone, which is one of the sugar degradation products, and browning compounds, which absorb light at 430 nm indicating the progress of the Maillard reaction were reported to be high in ripened mirins (Takahashi and Takemura, 2011). In addition, some ARPs (fructosyl histidine and fructosyl methionine) were identified as the compounds exhibiting antioxidative activities in mirins (Ishizaki et al., 2006). Few studies, however, were concerned with the chirality of amino acids in non-ripened and ripened mirins.
Thus, we performed quantitative analysis of l- and d-AAs in mirins, and investigated the influence of the Maillard reaction on amino acid racemization during mirin maturation.
Reagents l- and d-Ala were purchased from Peptide Institute, Inc. (Osaka, Japan). l- and d-Asp were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). d-glucose was purchased from Kishida Chemical Co., Ltd. (Osaka, Japan). Pentafluoropropionic acid and o-phthalaldehyde were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). N-isobutyryl-l-cysteine was purchased from Sigma Aldrich Japan (Tokyo, Japan). All other chemicals were analytical reagent grade.
Materials All mirins were commercial products obtained from randomly chosen manufacturers in Japan. Three non-ripened mirins (NRM1 – NRM3) were produced from glutinous rice, koji malt, distilled spirit, and liquid sugar; eight ripened mirins (RM1 – RM8) did not contain liquid sugar. The maturation times for RM1 – RM2, RM3 – RM5, RM6, RM7, and RM8 were one, three, seven, ten, and thirteen years, respectively. Non-ripened mirin that did not contain liquid sugar (NRMT) was used in the heating experiments. All mirins contained around 14% alcohol.
Measurement of l- and d-AA contents in mirins The contents of twelve l- and d-AAs (Ala, Asp, Glu, Ile, Leu, Lys, Phe, Ser, Thr, Trp, Tyr, and Val) and Gly in the mirins were determined by HPLC using a previously reported procedure employing derivatization reagents (Brückner et al., 1994). The twelve amino acids were selected due to their reported high concentrations (d+l) in non-ripened mirin (Morita and Tanabe, 1970). Each mirin was diluted with 2% sulfosalicylic acid, and the resulting solution was filtered after storing for 12 h at 5°C. The filtered sample was diluted (1:9) with 100 mM borate buffer (pH 9.5). An aliquot of the above solution (900 µL) and the derivatization reagent (prepared from N-isobutyryl-l-cysteine (3 mg) and o-phthalaldehyde (2 mg) in 100 µL of methanol) were mixed and left for 2 min at room temperature prior to being analyzed. HPLC analysis was performed using an Agilent HPLC 1100 series instrument with a fluorescence detector (Agilent Technology, Santa Clara, USA) and an Inertsil ODS-4 column (250 × 4.6 mm inner diameter; GL Science Inc., Tokyo, Japan). The mobile phase consisted of 30 mM sodium acetate buffer (pH 6.0, solvent A) and methanol/acetonitrile (12:1 v/v, solvent B). The following linear gradient of solvent B (min/%B) was used: (0/0.5), (75/60), (80/100). The injection volume was 5 µL, the flow rate was 0.8 mL/min, and the column temperature was 40°C. Fluorescence detection was performed using excitation and emission wavelengths of 230 and 445 nm, respectively. Quantitation was performed by the external standard method. All analyses were performed in duplicate. The relative content of amino acid enantiomers was calculated according to the equation %d = 100% × d/(d + l), where %d indicates the relative amount of d-AAs, and d and l represent the amount of each d- or l-enantiomer determined by HPLC.
Measurement of ARP content in ripened mirins The glucose-derived ARPs of various amino acids (N-(1-deoxy-d-fructos-1-yl)-amino acids) are named fructosyl amino acids. In this study, the content of nine ARPs (fructosyl alanine (Fru-Ala), fructosyl aspartic acid (Fru-Asp), fructosyl glutamic acid (Fru-Glu), fructosyl isoleucine (Fru-Ile), fructosyl leucine (Fru-Leu), fructosyl phenylalanine (Fru-Phe), fructosyl serine (Fru-Ser), fructosyl tyrosine (Fru-Tyr), and fructosyl valine (Fru-Val)) in mirins was analyzed by LC-MS using a previously reported ion-pairing reagent (Troise et al., 2015). LC-MS analysis was performed on an Agilent HPLC 1200 series instrument and an Agilent G6520A Q-TOF mass spectrometer (Agilent Technologies, Inc.) using electrospray ionization (ESI) in positive ion mode and a ZORBAX Eclipse Plus C18 column (100 × 3.0 mm inner diameter; Agilent Technologies, Inc.). The mobile phase consisted of 5 mM pentafluoropropionic acid (solvent A) and acetonitrile (solvent B). The following linear gradient of solvent B (min/%B) was used: (0/0), (30/20). The injection volume was 2 µL, the flow rate was 0.2 mL/min, and the column temperature was 40°C. The interface parameters were as follows: capillary voltage 4 kV, fragmentor 100 V, gas temperature 325°C. Reference samples were prepared using a previously reported method (Penndorf et al., 2007). Each amino acid (0.2 mmol; l-Ala, l-Asp, l-Glu, l-Ile, l-Leu, l-Phe, l-Ser, l-Tyr, and l-Val) and 1.2 mmol of d-glucose were dissolved in 8.4 mL of methanol and refluxed at 90°C for 9 h. The solvent was removed in vacuo, and the dry residues were dissolved in 2 mL of water. After membrane filtration (0.45 µm), these solutions, containing the original amino acids and the corresponding ARPs, were used as reference samples. Each ARP mass (m/z value) and retention time were determined based on the previously reported data (Troise et al., 2015). The determined masses ([M+H]+) were as follows: 252.1168 (Fru-Ala), 296.1055 (Fru-Asp), 310.1144 (Fru-Glu), 294.1618 (Fru-Ile), 294.1618 (Fru-Leu), 328.1430 (Fru-Phe), 268.1185 (Fru-Ser), 344.1344 (Fru-Tyr), and 280.1641 (Fru-Val). NRM1 and ripened mirins were diluted in water and analyzed. Chromatograms for the mass of each ARP were obtained using extracted ion chromatogram (EIC) mode, and the relative peak area of each compound was normalized to NRM1. All analyses were performed in duplicate.
The correlation coefficients (r) between %d and the relative amount of ARPs in eight ripened mirins were calculated for each amino acid by Pearson's correlation coefficient test using SPSS 7.5.1 (SPSS, Chicago, USA).
Synthesis of ARPs for heating experiments Fru-l-Ala and Fru-l-Asp were synthesized according to a previously reported method (Anet, 1959). l-Ala or l-Asp (0.05 mol) and d-glucose (0.2 mol) were dissolved in 5 mL of 10 M sodium pyrosulfite at 100°C with stirring for 90 (l-Ala) or 40 min (l-Asp), and then 175 mL of water and 345 mL of de-aerated ethanol were added to the mixture. The solution was subjected to cation exchange column chromatography (IR120BH, H+-form (Organo Corp., Kanagawa, Japan)). After washing with 70% ethanol and water, ARPs were eluted from the column with 0.5 M aqueous ammonia using a fraction collector. The fractions were analyzed using an amino acid analyzer (L-8800A; Hitachi High-Technologies Co., Tokyo, Japan), and the ones not containing unreacted amino acids were combined and dried under vacuum. The residues were dissolved in 70% ethanol and cooled to −20°C. Dropwise addition of 100% ethanol yielded the target compounds as hygroscopic solids. The above-mentioned recrystallization was repeated three times, and the product was freeze-dried, yielding Fru-l-Ala and Fru-l-Asp as white powders with respective purities of 98 and 99%, determined by the above LC-MS method.
Heating experiments The heating experiments were conducted to clarify the influence of ARPs on amino acid racemization in mirin. NRMT, which was used in the heating experiments, contained 640 µM l-Asp, 39 µM d-Asp, 800 µM l-Ala, and 40 µM d-Ala. The NRMT was membrane filtered (0.2 µm) prior to use. First, 2.5 mM solutions of Fru-l-Asp or l-Asp in filtered NRMT were prepared, and 900 µL of each solution was heated at 60°C for 8, 16, and 24 days in a 1 mL Pyrex screw-cap vial with a PTFE-faced septum (Experiment 1). Second, a 2.5 mM solution of Fru-l-Ala in filtered NRMT was prepared, and 900 µL of this solution was heated at 60°C for 24 days in the same way (Experiment 2). Third, a 2.5 mM solution of Fru-l-Asp in 1 M sodium acetate buffer (pH 4.0 or 5.6) was prepared, and 900 µL of this solution was heated at 60°C for 24 days in the same way (Experiment 3). All experiments were performed in duplicate.
Other analyses The pH values were measured at 25°C using an F-52 pH meter (Horiba, Kyoto, Japan). Absorbance was measured at 430 nm using a U-2900 UV-vis spectrophotometer (Hitachi High-Technologies Co.). The glucose content in the mirins was determined by HILIC-HPLC. The HPLC analysis was performed using an L-2000 series instrument with an l-2490 RI detector (Hitachi High-Technologies Co.) and an Asahipak NH2P-50 4E column (250 × 4.6 mm inner diameter; Showa Denko K.K., Tokyo, Japan). The mobile phases consisted of 75% (v/v) acetonitrile in water. The flow rate was 1 mL/min, and the column temperature was 40°C.
Quantities of l- and d-AAs in mirins The glucose content, pH value, and absorbance (430 nm) of the samples are shown in Table 1. The absorbance values of ripened mirins were higher than those of non-ripened mirins. Longer maturation periods resulted in lower pH values. l- and d-AA quantities and relative content are shown in Tables 2 and 3, respectively. All twelve l-AAs were detected in mirins, with the content of l-Ala (510 – 2200 µM), l-Leu (410 – 2200 µM), l-Ser (390 – 2800 µM), and l-Asp (350 – 3500 µM) mostly exceeding that of the other l-AAs. Although the content of l-Glu was also high for most mirins, it was very low for mirins matured for seven years or more (RM6 – RM8): NRM1–NRM3, 450 – 1900 µM; RM1 – RM5, 2000 – 4600 µM; RM6 – RM8, 51 – 630 µM. Ripened mirins showed a higher l-AA content than non-ripened mirins, with the exception of l-Glu.
| Sample | Maturation time | Glucose% | pH | Abs430 |
|---|---|---|---|---|
| NRM1 | - | 34.1 | 5.4 | 0.01 |
| NRM2 | - | 39.3 | 5.6 | 0.01 |
| NRM3 | - | 29.5 | 5.6 | 0.02 |
| RM1 | 1 year | 42.1 | 5.0 | 0.27 |
| RM2 | 1 year | 37.4 | 4.9 | 0.49 |
| RM3 | 3 years | 39.5 | 4.7 | 0.71 |
| RM4 | 3 years | 36.5 | 4.9 | 0.38 |
| RM5 | 3 years | 45.8 | 4.9 | 0.15 |
| RM6 | 7 years | 33.1 | 4.5 | 0.66 |
| RM7 | 10 years | 35.6 | 4.5 | 0.66 |
| RM8 | 13 years | 31.0 | 3.9 | 4.90 |
| L-AA (µM) | NRM1 | NRM2 | NRM3 | RM1 | RM2 | RM3 | RM4 | RM5 | RM6 | RM7 | RM8 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ala | 880 | 510 | 1800 | 1600 | 2200 | 1900 | 2000 | 1800 | 1400 | 880 | 1800 |
| Asp | 560 | 350 | 1200 | 1900 | 3500 | 2700 | 3000 | 1600 | 1200 | 750 | 1800 |
| Gu | 800 | 450 | 1900 | 2400 | 4600 | 2700 | 3900 | 2000 | 630 | 51 | 89 |
| lie | 270 | 160 | 760 | 790 | 1600 | 1000 | 1200 | 510 | 500 | 340 | 1100 |
| Leu | 750 | 410 | 1600 | 1300 | 2200 | 1600 | 1800 | 1200 | 910 | 580 | 1500 |
| Lys | 120 | 91 | 280 | 800 | 1400 | 1100 | 1100 | 44 | 110 | 140 | 860 |
| Phe | 310 | 210 | 870 | 370 | 700 | 430 | 530 | 510 | 380 | 240 | 440 |
| Ser | 600 | 390 | 1400 | 1900 | 2800 | 2400 | 2300 | 1200 | 870 | 470 | 960 |
| Thr | 330 | 180 | 750 | 740 | 1200 | 960 | 990 | 510 | 410 | 280 | 700 |
| Tip | 26 | 39 | 160 | 8 | 17 | n.d. | 5 | 16 | n.d. | n.d. | n.d. |
| Tyr | 280 | 220 | 780 | 1200 | 1600 | 1500 | 1400 | 630 | 480 | 390 | 1200 |
| Val | 440 | 270 | 1200 | 1400 | 2200 | 1800 | 1900 | 870 | 830 | 580 | 1600 |
| Gly* | 530 | 1800 | 1400 | 1600 | 2700 | 2000 | 2100 | 1100 | 1500 | 830 | 1000 |
| NRM1 | NRM2 | NRM3 | RM1 | RM2 | RM3 | RM4 | RM5 | RM6 | RM7 | RM8 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-AA | %D | D-AA | %D | D-AA | %D | D-AA | %D | D-AA | %D | D-AA | %D | D-AA | %D | D-AA | %D | D-AA | %D | D-AA | %D | D-AA | %D | |
| (µM) | (%) | (µM) | (%) | (µM) | (%) | (µM) | (%) | (µM) | (%) | (µM) | (%) | (µM) | (%) | (µM) | (%) | (µM) | (%) | (µM) | (%) | (µM) | (%) | |
| Ala | 35 | 3.8 | 12 | 2.4 | 50 | 2.7 | 35 | 2.1 | 390 | 15.1 | 75 | 3.8 | 43 | 2.1 | 120 | 6.3 | 180 | 11.4 | 30 | 3.3 | 180 | 9.1 |
| Asp | 14 | 2.4 | 8 | 2.3 | 30 | 2.5 | 12 | 0.6 | 21 | 0.6 | 22 | 0.8 | 9 | 0.3 | 21 | 1.3 | 91 | 7.0 | 32 | 4.0 | 220 | 10.9 |
| Glu | n.d. | - | 4 | 1.0 | 26 | 1.4 | 17 | 0.7 | 30 | 0.6 | 37 | 1.4 | 14 | 0.4 | 27 | 1.3 | 29 | 4.3 | 60 | 54.2 | 33 | 27.1 |
| Ile | n.d. | - | n.d. | - | 26 | 3.3 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 2 | 0.4 | n.d. | - | n.d. | - | 9 | 0.8 |
| Leu | 5 | 0.6 | 3 | 0.6 | 29 | 1.8 | 5 | 0.4 | 9 | 0.4 | 8 | 0.5 | 4 | 0.2 | 7 | 0.6 | 8 | 0.9 | 8 | 1.3 | 55 | 3.5 |
| Lys | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| Phe | 9 | 2.7 | 10 | 4.5 | 17 | 1.9 | 5 | 1.3 | 13 | 1.9 | 7 | 1.7 | 4 | 0.7 | 4 | 0.8 | 12 | 2.9 | 10 | 3.9 | 62 | 12.4 |
| Ser | 6 | 1.0 | n.d. | - | 22 | 1.6 | 21 | 1.1 | 18 | 0.6 | 53 | 2.2 | 8 | 0.3 | 15 | 1.3 | 37 | 4.1 | 26 | 5.2 | 89 | 8.5 |
| Thr | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| Trp | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| Tyr | n.d. | - | n.d. | - | 13 | 1.6 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 15 | 3.0 | n.d. | - | 49 | 3.9 |
| Val | n.d. | - | n.d. | - | 35 | 2.8 | 3 | 0.2 | 5 | 0.2 | 4 | 0.2 | n.d. | - | 4 | 0.5 | 3 | 0.4 | 3 | 0.6 | 8 | 0.5 |
d-Ala, d-Asp, d-Leu, and d-Phe were detected in all samples analyzed including non-ripened mirins. d-Lys, d-Thr, and d-Trp were not detected in any samples. The concentrations of d-Ala (12 – 50 µM) and d-Asp (8 – 30 µM) in non-ripened mirins, and d-Ala (30 – 390 µM), d-Asp (9 – 220 µM), and d-Ser (8 – 89 µM) in ripened mirins were mostly higher than those of the other d-AAs.
The %d values for Asp, Glu, and Ser in mirins matured for seven years or more (RM6 – RM8) were higher than in non-ripened mirins due to the high d-Asp and d-Ser contents and the low l-Glu content, respectively ((amino acid: minimum-maximum of %d in NRM1–NRM3, those in RM6 – RM8): (Asp: 2.3 – 2.5%, 4.0 – 10.6%; Glu: n.d. – 1.4%, 4.3 – 54.2%; Ser: n.d. – 1.6%, 4.1 – 8.5%)). On the other hand, the %d value for Ala depended on the sample tested: compared to non-ripened mirins (2.4 – 3.8%), the value for RM7 (3.3%) was almost the same, and the values for RM2 (15.1%) and RM5 (6.3%) were higher.
Calculation of the correlation coefficient between %d and the amount of ARPs in ripened mirins Semi-quantities of nine glucose-derived ARPs for d-AAs contained in ripened mirins are shown in Table 4, with the corresponding values for NRM1 set equal to unity. All nine ARPs were detected in all ripened mirins and NRM1. While the contents of Fru-Ala (2.0 – 6.5), Fru-Asp (2.0 – 8.8), Fru-Ile (3.2 – 12.2), Fru-Leu (3.4 – 12.6), Fru-Phe (2.2 – 5.7), Fru-Ser (2.4 – 4.7), Fru-Tyr (5.7 – 14.3), and Fru-Val (2.7 – 7.9) in all ripened mirins were higher than in NRM1, that of Fru-Glu (0.2 – 1.2) was mostly lower, except for in RM3.
| NRM1 | RM1 | RM2 | RM3 | RM4 | RM5 | RM6 | RM7 | RM8 | |
|---|---|---|---|---|---|---|---|---|---|
| Fru-Ala | 1.0 | 2.7 | 3.4 | 4.8 | 3.0 | 2.0 | 3.0 | 3.5 | 6.5 |
| Fru-Asp | 1.0 | 2.0 | 2.6 | 3.5 | 2.6 | 2.7 | 3.5 | 6.4 | 8.8 |
| Fru-Qu | 1.0 | 0.7 | 0.9 | 1.2 | 0.4 | 0.4 | 0.2 | 0.2 | 0.3 |
| Fru-Ile | 1.0 | 5.0 | 6.7 | 7.8 | 5.3 | 3.2 | 5.7 | 6.6 | 12.2 |
| Fru-Leu | 1.0 | 5.3 | 7.7 | 9.3 | 6.7 | 3.4 | 7.0 | 6.7 | 12.6 |
| Fru-Phe | 1.0 | 2.2 | 2.6 | 3.8 | 3.7 | 2.9 | 5.0 | 4.8 | 5.7 |
| Fru-Ser | 1.0 | 2.4 | 3.3 | 4.7 | 3.4 | 3.4 | 3.0 | 3.5 | 3.0 |
| Fru-Tyr | 1.0 | 14.3 | 10.3 | 14.0 | 9.5 | 5.7 | 8.7 | 9.1 | 11.1 |
| Fru-Val | 1.0 | 4.3 | 4.8 | 7.2 | 4.8 | 2.7 | 4.8 | 4.9 | 7.9 |
Correlation coefficients between %d and the relative amount of ARPs in eight ripened mirins were calculated for six amino acids that were detected in all ripened samples (Ala, Asp, Glu, Leu, Phe, and Ser), with the results shown in Table 5. As shown, Asp exhibited a statistically significant strong correlation (r = 0.84). On the other hand, Ser and Ala, having high d-enantiomer content in ripened mirins, did not exhibit statistically significant correlations (r = −0.10 and 0.15, respectively).
(Pearson's correlation coefficient test; n=8)
Racemization via ARPs Heating experiments were conducted with NRMT containing synthetic Fru-l-Asp or Fru-l-Ala, taking the above correlation coefficients into account (Asp: strong correlation, Ala: no correlation). In Experiment 1, the amounts of l- and d-Asp increased with time after heating NRMT containing 2.5 mM of added Fru-l-Asp (after 24 days: 2.0 mM l-Asp, 0.24 mM d-Asp, Fig. 1), and the %d of Asp also increased (initial: 5.8%, after 24 days: 10.6%, Fig. 2). On the other hand, the amount of d-Asp did not increase after heating NRMT containing added l-Asp; therefore, the %d of Asp was unchanged (Fig. 2). The results suggest that Asp was racemized via ARPs during thermal treatment.
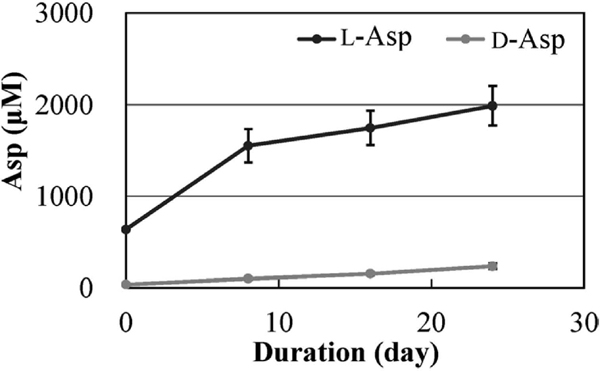
Asp concentration after heating NRMT containing added Fru-l-Asp (in duplicate; bars, S.E.)

%d of Asp after heating NRMT containing added Fru-l-Asp or l-Asp (in duplicate; bars, S.E.)
In contrast, the amount of d-Ala did not increase, while that of l-Ala increased after heating NRMT containing added Fru-l-Ala (Experiment 2).
As shown in Table 1, longer maturation periods resulted in lower pH values. The pH decreased after heating NRMT containing added Fru-l-Asp (initial: 5.1, after 24 days: 3.9). Thus, heating experiments were conducted in sodium acetate buffer (pH 4.0 or 5.6) containing synthetic Fru-l-Asp, in order to investigate the influence of solution pH on the generation of d-Asp via ARPs during thermal treatment. As a result, the %d of Asp at pH 4.0 was higher than at pH 5.6 (pH 4.0: 11.3%, pH 5.6: 5.1%, Fig. 3). This finding revealed that the proportion of d-Asp released from Fru-l-Asp was influenced by pH.

%d of Asp after heating for 24 days each buffer containing added Fru-l-Asp (in duplicate; bars, S.E.)
In the course of the Maillard reaction, sugars were reported to degrade into dicarbonyl compounds and organic acids, which are acidic compounds (Parker, 2013). The low pH and the high absorbance (430 nm) of ripened mirins were attributed to the degradation of sugars and the formation of melanoidin in the course of the reaction, respectively. In addition, high amounts of the dicarbonyl compound (3-deoxyglucosone) were also observed in ripened mirins (data not shown).
The content of l-AAs in ripened mirins was generally higher than in non-ripened mirins, since the latter were mixed with liquid sugar and thus diluted. However, the content of l-Glu in long-ripened mirins (RM6 – RM8) was very low. The disappearance rate of l-Glu during non-ripened mirin storage at various temperatures (Morita and Tanabe, 1970) was reported to be the fastest among the amino acids, supporting the HYPOTHESIS that free l-AAs would be consumed in the Maillard reaction during maturation.
All non-ripened mirins contained some d-AAs. Most of these were derived from the rice, which is the raw material for mirin, and is reported to contain d-AAs (Gogami et al., 2009). NRM3 showed higher l-, d-AA and lower glucose contents than NRM1 and NRM2. This is because NRM3 would probably be produced while the peptidase activity in koji malt was controlled to maintain a high level. The %d values for each amino acid in three non-ripened mirins did not differ greatly (Ala, Asp, and Phe), since d-AAs in non-ripened samples, including NRM3, were mainly derived from the rice.
In contrast, the correlation between %d and the amount of ARPs (Table 5), and the results of the heating experiments (Figs. 1 and 2), suggest that Asp would be racemized via ARPs during maturation. Brückner et al. reported that among twelve amino acids, the racemization of l-Asp was most strongly induced by glucose in model experiments (%d without glucose: 3.0%, %d with glucose: 21.2%, Brückner et al., 2001). These results indicate that racemization during mirin maturation would occur similarly to the model experiments.
Glucose-induced racemization of l-Phe, l-Leu, and l-Ala was also reported, but the corresponding racemization degree was very low (about one-tenth of that of Asp). Brückner's proposed racemization mechanism is as follows (Fig. 4): the reaction of amino acids with glucose starts with the formation of ARPs, which easily undergo enolization. Enolization favors proton abstraction from the Cα atom of the bonded amino acid with the formation of an intermediate sp3-hybridized carbanion. These steps might also be favored by the formation of intramolecular hydrogen bridges. Re-protonation can take place at both sides of the more or less planar carbanion, thus generating a partially racemized amino acid. The degree of racemization would depend on the steric and electronic properties of the amino acid side chains (Brückner et al., 2001; Pätzold and Brückner, 2006b).

Proposed mechanisms of amino acid racemization via Amadori rearrangement products (ARPs). (1)ARPs, (2a) and (3a) carbanion of 2,3-enols and those re-protonation, (2b) and (3b) carbanion of 1,2-enols and those re-protonation, and (4) l-,d-amino acids. R refers to amino acid side chain. (from Pätzold and Brückner, 2006b)
While the degree of racemization was reported to be influenced by the solution pH in model experiments with l-AAs, it was unknown whether this was caused by the change in the ARP amounts formed or by the change in the proportion of d-AA released from ARPs. In heating experiments using sodium acetate buffer containing synthetic Fru-l-Asp, the %d of Asp at pH 4.0 was higher than at pH 5.6. This is the first report describing the effect of solution pH on the proportion of d-AA released from ARPs. The effect is thought to be caused by the enolization pathway change at different pH values. The ARPs are known to undergo mainly 1,2-enolization under acidic conditions and mainly 2,3-enolization under neutral conditions (Pätzold and Brückner, 2006b; Henryk, 2012). All enols (1,2- and 2,3-enols) can form respective carbanions (Fig. 4). The structural difference between the two carbanions is presumed to influence the racemization degree. Since the pH decreased with the increase of the mirin aging period, the proportion of d-Asp released from Fru-l-Asp would change throughout the maturation.
The results of the heating experiments suggested that the amino acid that was mainly racemized via ARPs during maturation showed a strong correlation between %d and the amount of ARPs in ripened mirins. Leu and Phe also exhibited statistically significant correlations in ripened mirins (r = 0.75 and 0.74, respectively), with racemization of l-Leu and l-Phe reported to be induced by glucose as described above. Leu and Phe may be slowly racemized via ARPs during mirin maturation, and future studies using synthetic ARPs are necessary to clarify the pathway details.
In contrast, the amino acid with a high d-enantiomer content in ripened mirins, showing no correlation between %d and the ARP amount, would be racemized via a pathway not involving the Maillard reaction (Ser and Ala). While the %d of Ser in mirins matured for seven years or more was high, the correlation between them was not statistically significant (r = −0.10). l-Ser was reported to undergo easy racemization without participation of the sugar due to its ability to form a cyclic structure (self-racemization, Erbe and Brückner, 2000). d-Ser is presumed to form via this pathway during mirin maturation. Ala also showed no correlation between them, since the %d value for Ala varied for each sample (r = 0.15). Alanine racemase is reported to exist in microorganisms generally (Yoshimura, 2008). Thus, the biochemical reaction may occur during mirin maturation.
Furthermore, d-AAs were reported to have an effect on the food flavor profile. We previously showed that d-Asp significantly suppressed sourness and bitterness in basic taste solutions (citric acid and caffeine solutions, respectively) compared to the l-enantiomer (Inoue et al., 2014). Okada et al. reported an effect of d-Asp (34 µM), d-Glu (33 µM), and d-Ala (100 µM) on “a strong taste (Nojun)” using principal component analysis of the relationship between d-AA concentration and the taste of 141 bottles of sake (Okada et al., 2013). Sake has much in common with mirin, for example, the raw material (rice), alcohol content, and production using microorganisms (sake: koji malt, yeast, and lactic acid bacteria). Therefore, similar effects of d-AAs on taste are expected for mirin, especially long-ripened mirin.
In summary, we showed for the first time that the %d of Asp, Glu, and Ser in mirins matured for seven years or more was higher than in non-ripened mirins. Furthermore, we suggest that Asp is racemized via ARPs under the influence of pH during mirin maturation.