2016 年 22 巻 6 号 p. 743-749
2016 年 22 巻 6 号 p. 743-749
Surimi wash-water (SWW) contains a high concentration of protein resulting in a negative environmental impact and high disposal costs. Isoelectric point precipitation has been used for protein recovery from SWW. Here, we show an improved protein recovery method combining pH shift and heat treatment for Alaska pollock SWW. In a laboratory-scale experiment, a pH shift from neutral to pH 5 precipitated 63% of the SWW protein and subsequent heat treatment at 60°C precipitated almost all of the remaining protein. In a factory-scale experiment, the optimized pH shift and heat treatment yielded a 21% protein pellet with a 79% moisture content. The SWW protein gel thus obtained showed suitable quality as a surimi product as determined by the similar whiteness and strength as gel prepared from low-grade surimi. The optimized method combining pH shift with heat treatment improves the protein yield from SWW and prevents environmental pollution.
Surimi product is a traditional food in Japanese cuisine (Park, 2013). The texture of surimi product is stabilized by myofibrillar protein networks of myosin and actin (Stone and Stanley, 1992), though organic impurities such as sarcoplasmic proteins, lipids, and blood components decrease the strength and whiteness of protein gels. Accordingly, conventional surimi technology involves repeated washing of minced fish muscle to remove undesirable organic compounds. The wastewater thus generated contains a high concentration of organic fish solids (Karayannakidis, et al., 2007), which have a marked impact on the environment due to their high chemical and biochemical oxygen demand (Islam, et al., 2004). Therefore, a demand exists for the development of a recovery method from surimi wash-water (SWW) to increase the yield of fish protein and to reduce the negative environmental impact (Park, et al., 2001).
Various SWW protein recovery methods have been developed for industrial applications, such as ultrafiltration (Lin, Park, and Morrissey, 1995; Montero and Gómez-Guillén, 1998), precipitation by flocculation using alginate and chitosan (Wibowo, et al., 2005, 2007), and ohmic heat treatment of well-denatured protein (Huang, et al., 1997; Kanjanapongkul, et al., 2008). The most versatile method for the industrial application of surimi from fish is pH shift because of its low cost (Okazaki, 1994; Nishioka and Shimizu, 1983; Niki, et al., 1985; Torres, et al., 2007). In particular, the pH shift method has been applied to isoelectric solubilization/precipitation, which is a high-efficiency protein isolation method that lacks time-consuming washing processes (Gehring, et al., 2011; Martín-Sánchez, et al., 2009; Nolsøe and Undeland, 2008). Briefly, all fish proteins can be easily solubilized at acidic and/or alkaline pH due to the increase in electrostatic repulsion between proteins. In contrast, co-aggregation of proteins occurs at the pH levels close to their isoelectric point. The driving force of protein aggregation at the isoelectric points of protein complexes is hydrophobic interactions between protein molecules. The pH shift method can be applied on an industrial scale for SWW protein recovery (Nishioka and Shimizu, 1983; Niki, et al., 1985). However, not all of SWW proteins can be recovered by the pH shift method because proteins have different isoelectric points.
In this study, we improved the recovery of SWW protein in the pH shift method by combination with heat treatment. Heat treatments induce protein aggregate formation by increasing hydrophobic interactions between thermally unfolded molecules (Hamada, et al., 2009; Shiraki, et al., 2016; Totosaus, et al., 2002). Proteins are prone to form aggregates when subjected to heat treatment around their isoelectric point (Tomita, et al., 2011). The optimized conditions outlined in this paper are useful for the recovery of protein from the waste fluid of Alaska pollock.
Discharge process of surimi wash-water Alaska pollock from the Bering Sea was processed (Maruha Nichiro Co. Ltd., City, Alaska) into surimi by the conventional method (Figure 1). Briefly, fish meat blocks with backbone and skin were obtained from raw fish by beheading, gutting and cutting. The fish meat block was minced using a meat grinder equipped with a 5.0-mm bore plate accompanied by removing the backbone and skin. Minced meat was washed with cold water, washed meat was subjected to refining with a 1.3-mm bore plate in order to remove small bones or skin and large tendons, and meat was dewatered using a screw-press dehydrator to obtain raw surimi. Myofibrillar proteins and skin were removed from the screw-press waste-water using a decanter. All steps were performed at a temperature below 10°C. Surimi wash-water (SWW) emitted on the steps of leaching, dewatering and washing in the factory was stored at −30°C until use in the laboratory-scale experiment for 2 months; however factory-scale experiments were performed using SWW not subjected to freezing.

Surimi manufacturing process.
Determination of protein solubility The solution pH of 20 mL SWW was adjusted 2.0 – 12.0 by dropping of 6 M HC1 or 5 M NaOH with stirring at 25°C. Maximum addition volume of HCl or NaOH solution to 20 mL SWW was 0.3 mL or 0.15 mL, respectively, with the result that changes in total volume of SWW with the HCl or NaOH addition could be disregarded. Immediately, aliquots of 80 µL were heated to 40°C, 60°C or 90°C (GeneAtlasG, Astec, Fukuoka, Japan), and then cooled at 4°C. Treated samples were centrifuged at 3,000×g for 1 min at 25°C (CF15RX; Hitachi, Ltd., Tokyo, Japan). The protein contents of the supernatant were determined spectrophotometrically (BioSpec-nano, Shimadzu Corp., Kyoto, Japan) following the Biuret method. Protein solubility was defined by dividing protein contents in the supernatant after centrifugation with total SWW protein contents of homogenate before centrifugation. Solubility was determined as the average of three experiments.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis Supernatants of SWW after pH shift and heat treatment were mixed with 125 mM Tris-HCl (pH 6.8) loading buffer solution containing 4% (w/v) sodium dodecyl sulfate (SDS), 10% (w/v) sucrose, 10% (v/v) β-mercaptoethanol, and 0.01% (w/v) bromophenol blue at ratio of 1:1. Samples were heated for 5 min in boiling water, and were then subjected to SDS polyacrylamide gel electrophoresis (SDS-PAGE) using a 5 – 20% gradient gel (e-PAGEL; ATTO Co., Tokyo, Japan) with a molecular weight marker (Precision Plus Protein Dual Xtra Standards; BIO-RAD, Hercules, CA). Samples were loaded at 5 µL/lane. Gels were then stained using silver nitrate.
Recovery of protein in surimi wash-water in the factory SWW was stored in batch tanks with a capacity of 4,560 L. Immediately, 3,800 L of storage solution was adjusted to pH 5 by adding an 11 L of 6 M HCl from the top with bubbling air from the bottom to agitate at a temperature below 10°C. Temperature of the storage solution was raised to 50°C – 70°C by steam injection. Then, the heated solution was processed in a centrifugal thickener (BDN034; Tomoe Engineering Co., Ltd., Tokyo, Japan) at a rate of 10,500 L/h at 3,200×g for 3 min. The collected recovery was then dried and the water content was defined as % moisture, as measured by an electronic moisture balance (MOC-120H; Shimadzu, Tokyo, Japan). Solid contents were estimated by subtracting the moisture content from the total fraction weight. The yield of solids was calculated as the ratio of dry weight of the total recovered solids and that of the supplied SWW per unit of time.
Surimi gel preparation and analysis The SWW solution at pH 5 was heated at 60°C in the factory, and the recovered samples were combined with the original surimi (RA grade) at a rate of 0 – 30% (w/w), followed by mixing with 0.25% (w/w) sodium phosphate, 5% (w/w) sorbitol, 4% (w/w) sucrose, and 3% (w/w) sodium chloride for 3 min at 10°C using a cutter mixer (AP-12, Bibun Corp., Fukuyama, Japan). Mixtures were stuffed into 30-mm diameter tubes made of polyvinylidene chloride film, and both ends were bound tightly. “Direct gel” was prepared by heating for 30 min in a 90°C water bath. “Set gel” was prepared by holding tubes in a 30°C water bath for 60 min prior to heating for 30 min in the 90°C water bath. After heating, gels were equilibrated to room temperature (25°C), and were cut to 25 mm in height.
The central color of cut sections was determined using a colorimeter (ZE-6000, Nippon Denshoku Industries Co., Ltd., Tokyo, Japan), and lightness (L*), redness (a*) 100 − [(100 − L*)2 + a*2 + b*2]1/2 and yellowness (b*) values were recorded. Whiteness was calculated as and determined as the average of six samples.
Breaking force and strain were measured using a texture analyzer (CR-500DX, Sun Scientific Co., Tokyo, Japan) with a 5-mm diameter spherical probe at a depression speed of 60 mm/min. Breaking force and strain were determined as the average of 12 samples.
Protein precipitation by pH shift Thawed SWW contained some precipitation derived from myofibrillar proteins. The SWW pH was adjusted to 2 – 12 using small amounts of HCl or NaOH solution, irrespective of precipitation. Samples at various pH levels were centrifuged at 3000×g for 1 min to remove large particles. Aggregates were fully precipitated by the centrifugation. Concentrations of proteins in the supernatants were then measured using the conventional Biuret method (Gornall, et al., 1949). The acidic SWW mixed with biuret reagent increased the solution pH to 12; therefore, protein concentration was quantitatively determined under the alkaline pH without any correction or neutralization. Figure 2 shows the supernatant concentrations of proteins at various pH levels. Untreated SWW was approximately pH 7 and contained approximately 8 mg/mL proteins. Protein solubility remained constant at pH 7 and above. The alkaline SWW above pH 8 was apparently transparent with slightly dark color. Between pH 7 and 5, protein solubility decreased; minimum solubility was observed at pH 4 – 5. Further decreasing the pH of clouded samples around pH 5 increased the solubility of protein in the range of pH 2 – 4. The single valley shape of solubility curve indicated that the average isoelectric point of SWW proteins of Alaska pollock was approximately pH 5, which is similar to the value previously reported for other fishes, such as threadfin bream and Pacific whiting (Bourtoom, et al., 2009; DeWitt and Morrissey, 2002; Krasaechol, et al., 2008). However, not all SWW proteins can be recovered by the pH shift method because various proteins present in SWW have different isoelectric points.

Solubility of SWW proteins at various pH.
In order to identify the individual SWW proteins, SDS-PAGE was performed at various pH levels (Figure 3). The molecular weights of SWW proteins were distributed in a wide range from 10 kDa to 250 kDa. The solution at pH 5 contained the lowest number of bands. To more clearly demonstrate this, the pH dependence of protein contents was examined by SDS-PAGE. With increasing pH, the 100-kDa band was diminished above pH 10, and another emerged at 250 kDa. In contrast, at acidic pH, the bands at 75 and 40 kDa were diminished below pH 5, and another emerged at 30 kDa. Myofibrillar proteins, typically actin and myosin, are constituents in SWW, coupled with sarcoplasmic protein (Moosavi-Nasab, et al., 2005). Thus, bands at 40 kDa and 17 kDa may result from actin and myosin light chains, respectively. These data indicate that the composition of protein aggregates is affected by the pH of the solution. The pH shift method is favorable for recovering great quantities of SWW proteins due to the low cost and time saving.
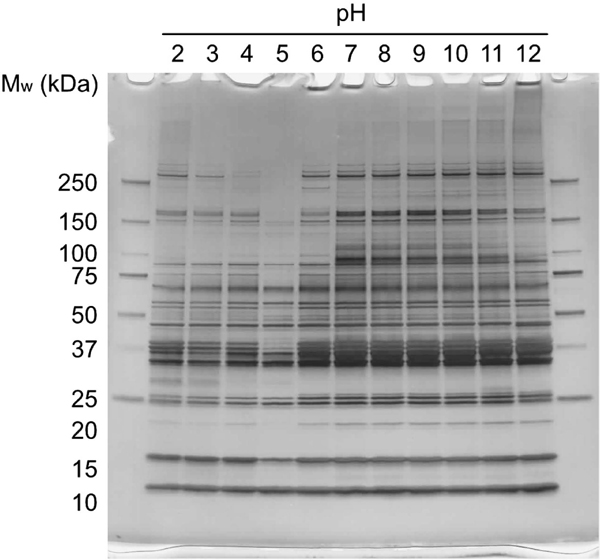
SDS-PAGE analyses of SWW at various pH.
Protein precipitation by heat treatment More than 37% of proteins remained solubilized in solution, even under optimal conditions at pH 5 (Figure 2). Figure 4 shows the residual concentration of proteins from SWW after heating to 40°C – 90°C at the respective pH levels. A solubility of 100% equals an identical protein concentration to stock SWW. Solubilities at pH 2, 9, and 12 remained constant after heat treatment at 40°C for 20 min.

Solubility of SWW proteins after the heat treatment at 40°C (circles), 60°C (squares), and 90°C (triangles) at pH 2 (a), 5 (b), 7 (c), 9 (d), and 12 (e).
Solubility at pH 5 at 40°C remained unchanged. In contrast, solubility at pH 5 at 60°C and 90°C decreased, possibly due to the hydrophobic associations between thermally unfolded molecules in the absence of electrostatic repulsion. A similar pattern was observed at pH 7, although the absolute values of solubility differed. These results indicate that the favorable conditions for recovery of SWW are at pH 5 at above 60°C. Note that the solubility at pH 2 before heating was 60%; thereafter, the solubility increased approximately 100% following heat treatment at 60°C and 90°C for several min. This may be because labile aggregates dissociated to monomeric or oligomeric states by incubation due to electrostatic repulsion.
Figure 5 shows SDS-PAGE analysis of the SWW protein composition after heat treatment at 60°C at pH 7 and 5. Heat treatment of either sample, even for 2 min, immediately precipitated an appreciable amount of protein. The residual bands in the samples at pH 7 were gradually diminished from 2 min to 20 min of treatment, except for three proteins at 12, 25 and 250 kDa. On the other hand, all of the bands in the samples at pH 5 were immediately diminished with 2 min of treatment. The bands at 40 kDa and 17 kDa disappeared after heat treatment; this is a piece of evidence that the bands was from heat-sensitive actin and myosin light chain. After 20 min of heat treatment, all proteins in the samples at pH 5 were precipitated. These data showed that all SWW proteins were completely recovered at the combination of pH 5 at 60°C for 20 min in the laboratory-scale experiment.

SDS-PAGE analyses of SWW after the heat treatment at 60°C for 0 – 20 min at pH 7 (a) and pH 5 (b).
Recovery of protein from SSW in factory On the basis of laboratory-scale experiments, we further investigated a factory-scale device for the recovery of SWW protein. The recovery yield of the factory-scale experiment was highest at pH 5, which is similar to the laboratory-scale experiment. Table 1 shows the composition and recovery yield obtained from the treated samples by pH shift and heat treatment. The solid and moisture contents of the 50°C-treated sample were 20.4% and 79.6%, while those of the 60°C-treated sample were below 21.1% and 78.9%. The solid-state content was further increased by increasing the treatment temperature to 70°C. It was noted that the rate of protein precipitation from the 60°C-treated sample on the laboratory scale was almost 85%, while the recovery yield on the factory scale was no more than 21.1% under the same pH and heat treatment conditions. This was probably due to the differences in centrifugation conditions: a batch tabletop centrifuge was used for laboratory-scale recovery of SWW protein, while a continuous decanter centrifuge was used for factory-scale recovery. Thus, it could be concluded that continuous decanter centrifugation in the factory cannot recover small and highly soluble protein aggregates. In fact, small particles can be recovered by alginate as a solvent additive to form a large coacervate (Wibowo, et al., 2005, 2007). The difference between laboratory- and factory-scale processes is another factor that may contribute to the destabilization of protein due to freezing SWW for laboratory-scale experiments. It has been reported that frozen storage made SWW protein of Alaska pollock prone to aggregation by heat treatment (Wang, et al., 2013).
| Temperature (°C) | Solid contenta (%) | Moisture contenta (%) | Yield ratioa (%) |
|---|---|---|---|
| 50 | 20.4 ± 2.1 | 79.6 ± 2.1 | 19.5 ± 1.5 |
| 60 | 21.1 ± 0.3 | 78.9 ± 0.3 | 21.5 ± 0.5 |
| 70 | 24.2 ± 0.3 | 75.9 ± 0.3 | 37.5 ± 0.5 |
Effect of recovered solid on the gel properties The factory-scale experiment showed that the recovery yield of protein from SWW increased with increasing heating temperature, though the sample heat-treated at 70°C showed browning due to the Maillard reaction. Browning is an unfavorable quality in surimi product. Thus, we further investigated the gelling properties of mixtures of the protein recovered from 60°C-treated samples of SWW into original surimi (RA grade). We chose a low-grade surimi (RA) for this prototype surimi because the excellent gel forming properties of high-grade surimi were markedly impaired by mixture with 60°C-treated samples of SWW. We prepared two-types of gel: “Direct gel” and “Set gel”. Table 2 shows the color properties and strength of direct and set gels. Both gels added to 60°C-treated samples demonstrated slight coloring. The gel strength of the set gel decreased with increasing recovery protein contents, while that of the direct gel retained constant. It is noteworthy that both pH and moisture content remained constant by the addition of recovered solid at 30% w/w. Therefore, these had little effect on the degradation of gel qualities by pH and moisture contents. Sarcoplasmic protein and protease included in SWW may impair gel properties, such as color and strength (Kim, et al., 2005; Velazquez et al., 2007; Visessanguan and An, 2000). Nevertheless, these data indicate that 60°C-treated samples can be sufficiently utilized as extenders of surimi products.
| Content of recovered solid (%) | Moisture contenta (%) | pHa | Direct gel | Set gel | ||||
|---|---|---|---|---|---|---|---|---|
| Whitenessb | Braking forcec (g) | Breaking strainc (mm) | Whitenessb | Braking forcec (g) | Breaking strainc (mm) | |||
| 0 | 71.2 | 7.0 | 34.99 ± 0.14 | 186.1 ± 5.8 | 6.0 ± 0.1 | 35.80 ± 0.14 | 248.3 ± 6.6 | 10.8 ± 0.7 |
| 10 | 70.9 | 7.0 | 32.40 ± 0.15 | 192.3 ± 3.6 | 7.5 ± 0.5 | 32.84 ± 0.13 | 214.3 ± 3.8 | 7.6 ± 0.5 |
| 20 | 70.4 | 6.8 | 32.43 ± 0.09 | 221.8 ± 3.8 | 6.2 ± 0.2 | 32.62 ± 0.08 | 231.8 ± 3.5 | 6.6 ± 0.5 |
| 30 | 71.8 | 6.8 | 32.94 ± 0.14 | 195.4 ± 3.1 | 5.9 ± 0.2 | 32.99 ± 0.39 | 211.8 ± 4.0 | 5.9 ± 0.2 |
For combination pH shift and heat treatment of recovered protein from Alaska pollock SWW, a two-step pH shift and two-step heat treatment was required (Niki et al., 1985). Here, we proposed an improved method using a one-step pH shift and one-step heat treatment, which provided a milder set of conditions to obtain a suitable recovery for additives to surimi products than the above method. In addition, the recovery yield of protein was higher using the decanter in this investigation than that using a conventional pressure flotation apparatus, although both samples contained equal salt concentrations. Consequently, the ash content of dried recovery was less using the decanter centrifugation. Therefore, surimi added to the recovery is suitable for use as food because of its low salt concentration.
In this study, we proposed an improved method of SWW protein recovery from Alaska pollock. Almost all SWW protein was successfully recovered at pH 5 with heat treatment above 60°C for 20 min in a laboratory-scale experiment. The use of a decanter in a factory-scale experiment decreased the recovery to 21% for the above conditions. The recovered protein from SWW mixed into surimi protein was slightly reduced in whiteness, but retained its breaking force and strain in the direct gel treatment. Therefore, it is concluded that pH shift and heat treatment successfully improve the recovery of protein from Alaska pollock for surimi products.
Acknowledgements We are grateful to Mr. Gai Ohashi for experimental advice and helpful discussion. This study was supported financially by Tsukuba University and Maruha Nichiro Co., Ltd.