2016 Volume 22 Issue 6 Pages 801-810
2016 Volume 22 Issue 6 Pages 801-810
We performed random-centroid optimization to determine the optimum preparative method for glycation of chicken myofibrillar proteins (Mfs) with the strongest antioxidative activity against hydroxyl radicals (·OH) and with more than 60% solubility in low ion strength medium. Four factors, temperature, relative humidity (RH), reaction time, and quantity of maltose, were selected and 13 vertices were obtained. The examination was carried out according to each vertex and the optimum conditions were sought, resulting in 57.4°C, 37.3% RH, 37.2 h of reaction time, and a maltose mixing ratio of 5.43 for Mfs (w/w). The ·OH-averting capacity (HORAC) was measured as 7.8 ± 1.0 µmol of gallic acid (GA) equivalent/g of protein. Moreover, the optimized glycated chicken Mfs could form a gel by a 30-min heating at 90°C, and this gel maintained the antioxidant ability. Its HORAC was 4.4 ± 1.7 µmol of GA equivalent/g of protein.
Glycation via the Maillard reaction is an effective method to improve the functional properties of myofibrillar proteins (Mfs). The Maillard reaction with sugar has previously been used to improve the thermal stability and solubility in a low ionic strength medium of fish (Saeki and Inoue, 1997; Saeki and Tanabe, 1999; Sato et al., 2000, 2003) and shellfish (Katayama et al., 2002) Mfs. Previous investigations into functional alterations have used chicken Mfs instead of fish (Nishimura et al., 2011a, b; Isono et al., 2012; Nishimura et al., 2015), because fish muscular protein is thermally less stable (Connell, 1961) than that of other vertebrates, and a comparatively large amount of sugar is required in the glycation process to prevent denaturation of fish meat protein. Moreover, using a comparatively small amount of sugar brings about the same functional alterations to chicken Mfs, such as rising solubility in a low ionic strength medium and higher thermal stability (Nishimura et al., 2011a,b). Furthermore, we found that the Maillard reaction with maltose confers antioxidant abilities on chicken Mfs (Nishimura et al., 2011b). This antioxidant ability is attributed to the primary structure or specific peptide sequence of the glycated chicken Mfs, irrespective of the protein shape (Isono et al., 2012).
While preparing livestock or fish meat paste products such as sausage and kamaboko (boiled fish paste), a large amount of salt is required to solvate the Mfs of globulin protein, which may lead to high blood pressure and other lifestyle related diseases. Furthermore, meat paste products also need antioxidants as food additives to prevent deterioration during storage. However, when soluble glycated Mfs are used, excessive salt is unnecessary. Moreover, the amount of antioxidant added during storage can also be reduced if the gel formed by heating maintains antioxidant ability. Nishimura et al. (2015) found that glycated chicken Mfs mixed with maltose in a 1:5 ratio and then incubated at 65°C under 41% RH for 36 h possesses antioxidant ability and is more than 60% soluble in low ionic strength medium. We also observed that the glycated chicken Mfs could form a gel by heating at 90°C. These results indicated that the gel maintained its antioxidant ability.
In this study, the optimum preparative conditions to obtain chicken glycated Mfs, which are soluble in low ionic strength medium and exhibit the strongest antioxidant capacity, were investigated by random-centroid optimization (RCO) (Nishimura et al., 1997, 1998; Goto et al., 2000; Nishimura et al., 2001; Goto et al., 2004; Nakai et al., 2009). Thermal gel-formation of the optimized glycated chicken Mfs and their ability to maintain antioxidant activity were also investigated.
Materials and chemicals Chicken breast meat was purchased from a local poultry farm immediately after slaughter. Radical Catch, a kit for hydroxyl radical (·OH) measurement, was purchased from Hitachi Aloka Medical, Ltd. (Tokyo, Japan). Gallic acid (GA) was obtained from Chroma Dex, Inc. (Irvine, USA). All other chemicals were of reagent grade and were obtained from Nacalai Tesque, Inc. (Kyoto, Japan) or Wako Pure Chemicals Industries, Ltd. (Osaka, Japan).
Myofibril proteins (Mfs) preparation Fractions of chicken Mfs were prepared by following the modified method of Saeki (1997). Chicken breast meat was ground with a food chopper and suspended in 10 volumes of 0.1 M sodium phosphate buffer (pH 7.5), and the supernatant was removed by decantation. This step was repeated five times. The meat was then homogenized in a homogenizer (model AM-6; Nissei Co. Ltd., Tokyo, Japan) in 10 volumes (based on initial muscle weight) of 0.1 M sodium phosphate buffer (pH 7.5) for 0.5 min at 10,000 rpm. This step was repeated four times. After filtration through cotton gauze, 20% Triton X-100 solution was added to the filtrate to obtain a final concentration of 0.5% and stirred gently for 3 min. After standing for 10 min, the filtrate was centrifuged at 7,000 × g for 10 min to collect Mfs. The precipitate was resuspended in 50 mM NaCl and centrifuged. This procedure was performed five times. Purified chicken Mfs were obtained by filtering the solution through a nylon cloth. Part of this was dissolved in 0.5 M NaCl, 15 mM sodium phosphate buffer (pH 7.5) and used as the native chicken Mfs solution. All preparation steps were carried out on ice.
Random-centroid optimization (RCO) To determine the optimum preparative conditions for glycated chicken Mfs with solubility of more than 60% in a low ionic strength medium that exhibit the strongest antioxidant capacity, RCO (Nishimura et al., 1997, 1998; Goto et al., 2000; Nishimura et al., 2001; Goto et al., 2004; Nakai et al., 2009) was used. Glycated chicken Mfs were prepared according to 13 vertices from the four factors of preparative conditions. Among obtained glycated chicken Mfs, those with less than 60% solubility in a low ionic strength medium were evaluated as 100%. The vertex that provided the smallest residual ratio of ·OH was sought. Triplicates (at least) were independently assessed in the preparation of glycated chicken Mfs at each vertex. The experimental ranges of each factor were determined as follows: a temperature of 40 – 70°C, an RH of 25 – 45%, a reaction time of 24 – 48 h, and a maltose mixing ratio for Mfs (w/w) of 2 – 8.
Glycation of chicken Mfs The chicken Mfs were glycated by following the modified method of Saeki (1997). Chicken Mfs suspended in 50 mM NaCl were mixed with maltose at each weight ratio shown by the RCO. After adjusting the final protein concentration to 6.0 mg/mL, 5 mL of each Mfs-maltose solution was taken in a test tube (16 mm diameter), frozen at −80°C, and immediately lyophilized in a freeze-dryer (FD-1; Tokyo Rika Co., Ltd., Tokyo, Japan), destroying the sarcomeres and exposing Mfs. Lyophilization was stopped when the sample temperature reached 15 – 18°C. The lyophilized protein powder was immediately stored at −40°C and used within 30 days of preparation.
For the Maillard reaction, each lyophilized protein powder was incubated under 13 conditions shown in RCO. An incubator/humidity cabinet (L-200A; Tokyo Rikakikai Co., Ltd., Tokyo, Japan) was used to control the temperature and RH. Chicken Mfs without maltose (unmodified chicken Mfs) was also prepared.
Solubility of glycated chicken Mfs The solubility of the glycated Mfs was measured according to the modified method of Saeki and Inoue (1997). After incubating for glycation, the protein powder was immediately mixed with 0.1 M NaCl, 15 mM sodium phosphate buffer (pH 7.5) in an Ultra-turrax T25-S1 high-speed blender (IKA-Labotechnik, Staufen, Germany) at 13,500 rpm for 0.5 min, for a final protein concentration of 1.5 mg/mL. This homogenization procedure was conducted twice, followed by centrifugation at 32,000 × g for 30 min at 4°C. The amounts of protein before centrifugation and in the supernatant were determined by the Kjeldahl method (Kjeldahl, 1883). Total soluble Mfs was expressed as the percent protein concentration in the supernatant with respect to the total protein before centrifugation.
Preparation of sample for measurement of ·OH averting capacity (HORAC) After incubating for glycation, each protein powder was immediately stirred with 0.1 M NaCl, 15 mM sodium phosphate buffer (pH 7.5) and dialyzed in the same solution at 8 – 12°C for 2 days to remove the unreacted maltose. After centrifugation at 32,000 × g for 30 min at 4°C, the supernatant, containing of 7 – 9 mg of protein/mL, was used as the sample solution.
Measurement of HORAC HORAC was measured using an antioxidant potential measurement kit (Radical Catch; Aloka, Co., Ltd., Tokyo, Japan) and a chemiluminescence reader (AccuFLEX Lumi400, Aloka Co., Ltd., Tokyo, Japan) based on the Fenton reaction, in which antioxidants are trapped by luminol, resulting in light emission (Parejo et al., 2000). This is a procedure for assessing the ·OH scavenging activity using ferrous iron-induced luminol chemiluminescence (Yildiz and Demiryürek, 1998). Aliquots of sample solution above were harvested for measuring the antioxidant capacity. First, 50 µL of cobalt solution and 50 µL of luminol solution were mixed with 20 µL of diluted sample solution, which was adjusted to bring a final concentration of 0.59 mg protein/mL, and incubated for 5 min at 37°C. The generation of ·OH was started by the addition of 50 µL of H2O2 solution. Light emission at 430 nm was measured for 120 s immediately after initiation. 0.5 M NaCl, 15 mM sodium phosphate buffer (pH 7.5) was used as a control. The light emissions from 80 to 120 s were integrated. Among 13 samples, the residual ratio of ·OH was calculated for samples that showed a solubility more than 60% in a low ionic strength medium. Antioxidant activity was measured as the relative light units (RLU) value of a sample/RLU value of the control × 100. These values were used to evaluate RCO.
Evaluation of HORAC of GA and optimized glycated chicken Mfs The HORACs of GA and optimized glycated chicken Mfs, which indicated the smallest residual ratio of ·OH, were calculated as follows. ·OH was generated by the Fenton reaction. The RLU value containing no sample was regarded as 100%. Comparative RLUs in the presence of GA and optimized glycated chicken Mfs were calculated, and the relationship between RLU and the concentration of the chemical or optimized glycated Mfs was graphed. The amounts of GA and glycated chicken Mfs that elicited 50% RLU were determined as the half-maximal inhibitory concentration (IC50) values of GA and optimized glycated chicken Mfs, respectively. The IC50 value of an optimized glycated chicken Mfs solution (mg/mL) was converted to HORAC (GA equivalent/g of protein).
Determination of available lysine and fructosamine Assays of the available lysine and fructosamine were carried out to evaluate the progress of the Maillard reaction between the Mfs and maltose. Optimized glycated Mfs were dissolved in 0.5 M NaCl, 20 mM Tris-HCl (pH 7.5) in an Ultra-turrax T25-S1 high-speed blender (IKA-Labotechnik, Staufen, Germany) and precipitated with 7.5% trichloroacetic acid (final concentration) at room temperature for 60 min. After removing the Tris buffer, unreacted sugars, and trichloroacetic acid by decantation, the precipitated protein was redissolved in a 50 mM sodium phosphate buffer (pH 9.5) containing 2% sodium dodecyl sulfate (SDS). The available lysine content was determined by spectrophotometric analysis using o-phthalaldehyde and N-acetyl-L-cysteine (Hernandez and Alvarez-Coque, 1992).
Optimized glycated Mfs dissolved in 0.5 M NaCl, 20 mM Tris-HCl (pH 7.5) were dialyzed against the same buffer at 4°C for 2 days to remove the unreacted maltose, and fructosamine was assayed by the method of Johnson et al. (1983). Glycosylated human serum was used as a standard for determining the fructosamine content, which is regarded as the ketoamine content. Assays of available lysine and fructosamine were performed within 48 h of preparing the samples.
Formation of heat-induced gel A solution of optimized glycated chicken Mfs, which had the smallest residual ratio of ·OH among the 13 sample solutions obtained as described above, was concentrated to 15 from 7 – 9 mg protein/mL in 0.1 M NaCl, 15 mM sodium phosphate buffer (pH 7.5) using Amicon Ultra-15 Centrifugal Filter Devices (Merck Millipore Co., Darmstadt, Germany) with centrifugation at 4,500 × g and 4°C. 0.5 mL of the solution was placed in a test tube (16-mm diameter), sealed with polyvinylidene chloride film, and heated in a water bath at 90°C for 4 h. The sample was examined by SDS-polyacrylamide gel electrophoresis (PAGE) analysis. The antioxidant ability of the gel was determined by HORAC. For microscopic observation, 4 mL of the same sample in a beaker (21-mm diameter) was heated for 30 min at 90°C. An unmodified chicken Mfs sample was dissolved in 0.5 M NaCl, 15 mM sodium phosphate buffer (pH 7.5) and then heated by the same procedure.
SDS-PAGE analysis of heat-induced gels The heat-induced gels or sols were mixed with equal volumes of 125 mM Tris-HCl (pH 6.8) containing 8 M urea, 4% SDS, and 9 mM N-ethylmaleimide. The mixtures were allowed to stand overnight at room temperature. 2-Mercaptoethanol (2-ME) was added to each mixture to a final concentration of 10% (v/v), and the mixture was boiled for 3 min. Electrophoresis was carried out on a 10% acrylamide slab gel according to Laemmli's method (Laemmli, 1970), followed by staining with Coomassie Brilliant Blue R-250.
Microscopic observation The microstructures of the 30-min heat-induced gels of the optimized glycated chicken Mfs and sols of unmodified chicken Mfs were studied under a cryoscanning electron microscope (SEM; S-4100; High-Technologies Co., Tokyo, Japan) operated at 5 kV. For cryo-SEM observation, the heat-induced gels and sols were dehydrated in ethanol, frozen in liquid nitrogen, fractured using a scalpel, and transferred to the cold stage of the preparation chamber. Each sample was exposed to approximately −85°C for 10 min under a pressure of less than 7 × 10−3 Pa and with cryo-sputter coating with gold.
Protein determination The protein concentrations of dissolved and purified chicken Mfs were determined by following the methods of Kjeldahl (Kjeldahl, 1883) and Lowry (Lowry et al., 1951), with ovalbumin as a standard. The biuret method (Gornall et al., 1949), which uses bovine serum albumin as a standard, was performed in other assays.
Statistical analyses Samples for all experiments were taken from at least three different glycated Mfs lots. The results are reported as mean values of three determinations, with the error bars indicating the standard deviation. Statistical comparison between the 2 systems was made using Student's t-test, while one-way analysis of variance (ANOVA) and the Tukey method were used for multisystem comparisons. P < 0.05 or 0.01 was considered significant.
Search for the optimum preparative method of glycated chicken Mfs by RCO To determine the optimal glycated chicken Mfs with the strongest antioxidant capacity and a solubility of more than 60% in a low ionic strength medium, temperature, RH, reaction time, and the maltose mixing ratio for Mfs (w/w) were optimized using RCO (Table 1). Values for the parameters in each experiment were given by the RCO program, and then used for each preparation of glycated chicken Mfs. All data were mapped (Fig. 1). The mapping process aids in visualization of the experimental response surface, indicating the trend of the data (Nakai et al., 2009). Although RCO is usually repeated until one gets an adequate response, in this study, the approximate position of the optimal condition was clear after the first cycle of the RCO program. The best result was obtained at vertex 10 with a temperature of 57.4°C, an RH of 37.3%, a reaction time of 37.2 h, and a maltose mixing ratio for Mfs (w/w) of 5.43.
| Vertex No. | Temperature (°C) | RH (%) | Reaction Time (h) | Maltose mixing ratio for Mfs (w/w) | Evaluation (Residual ratio of ·OH)a) (%) |
|---|---|---|---|---|---|
| 1 | 46.2 | 42.4 | 47.9 | 4.77 | 100.0 |
| 2 | 57.0 | 36.6 | 28.7 | 6.79 | 66.8 |
| 3 | 68.6 | 38.0 | 38.4 | 5.16 | 100.0 |
| 4 | 49.7 | 36.7 | 44.6 | 5.63 | 68.8 |
| 5 | 46.4 | 40.0 | 38.7 | 2.62 | 100.0 |
| 6 | 40.3 | 33.3 | 32.4 | 5.66 | 100.0 |
| 7 | 58.6 | 35.0 | 44.9 | 6.95 | 68.6 |
| 8 | 63.8 | 39.2 | 31.3 | 6.25 | 100.0 |
| 9 | 64.1 | 40.7 | 30.7 | 2.36 | 100.0 |
| 10b) | 57.4 | 37.3 | 37.2 | 5.43 | 66.0 |
| 11b) | 57.3 | 36.9 | 37.4 | 6.40 | 71.1 |
| 12b) | 60.9 | 37.9 | 33.9 | 5.59 | 76.0 |
| 13b) | 58.7 | 38.3 | 33.8 | 5.26 | 76.8 |
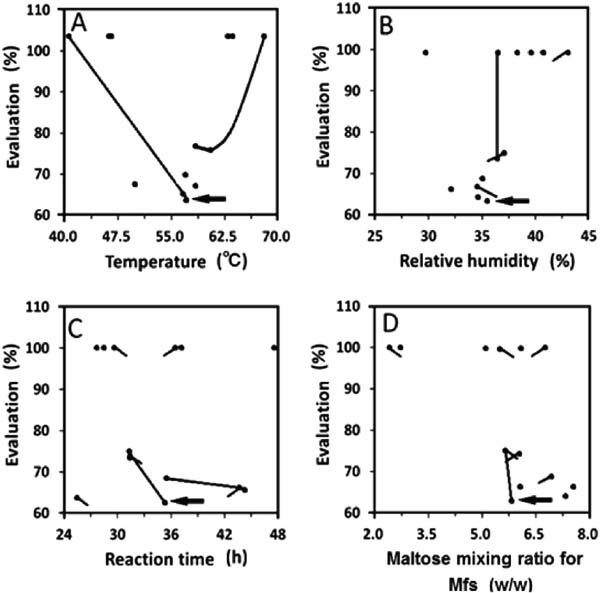
Mapping results of experiments submitted by RCO for scavenging ·OH. Evaluation: When the solubility in a low ionic strength medium of glycated Mfs did not exceeded about 60%, the evaluation of this vertex was estimated as 100%. The emitting intensity of ·OH occurred by the Fenton reaction, which was detected with fluorescence method spectroscopy, was regarded as 100%. The comparative emitting intensity of ·OH occurred by the Fenton reaction at the presence of glycated Mfs was used for the evaluation (residual ratio of ·OH) of antioxidative activity. The vertex that provided the smallest evaluation was sought. (A) temperature. (B) relative humidity. (C) reaction time. (D) maltose mixing ratio for Mfs (w/w). (●) was each vertex. Lines indicate probable trends. Arrow in each graph shows the best result.
HORAC of the optimum glycated chicken Mfs Inhibition curves for the optimized glycated and native chicken Mfs are shown in Fig. 2. Native chicken Mfs hardly demonstrated HORAC in the range of 0.35 – 1.06 mg of protein/mL, though the same phenomenon had already been observed in the evaluation of superoxide anion radical scavenging activity (Nishimura et al., 2011b; Isono et al., 2012). In contrast, optimized glycated chicken Mfs reduced the residual ratio of ·OH with increasing protein concentration, and this ratio reached 39.7 ± 0.3% (n = 3) at a concentration 1.06 mg/mL. At each concentration, there were significant differences between the values of native and optimized glycated chicken Mfs (p < 0.01).The optimized glycated chicken Mfs IC50 value was calculated from the inhibition curve in Fig. 2, giving 0.8 ± 0.1 mg/mL (n = 3).
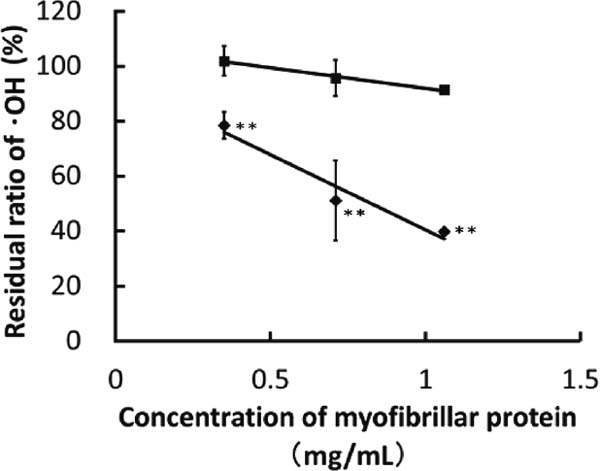
Effect of concentration of Mfs on HORAC. Antioxidative activities of native Mfs in 0.5 M NaCl solution (pH7.5) (■) and the optimum glycated chicken Mfs in 0.1 M NaCl solution (pH7.5) (◆) against ·OH were measured with fluorescence method. Values are the mean ± standard deviation (n = 3). Values with ** were significantly different from those at the same concentration of native Mfs. (p < 0.01).
HORAC of the optimized glycated chicken Mfs was converted into GA equivalent activity (µmol/mL) based on the GA IC50 value (6.4 ± 0.1 µmol/mL), a value of 7.8 ± 1.0 µmol of GA equivalent/g of protein being obtained. Boxin et al. (2002) have reported HORAC values for apple and white peach extracts of 15.0 and 23.1 µmol GA equivalent/g of extracts, respectively. These data indicate that the antioxidative activity of the optimized glycated chicken Mfs was comparable to these fruit extracts when utilized as whole material. Nishimura et al. (2011b) showed that glycated chicken Mfs with maltose obtained under conditions of a temperature of 60°C, an RH of 35%, a reaction time of 36 h, and a maltose mixing ratio for Mfs (w/w) of 4 acquired the active oxygen scavenging properties of about 200 units of superoxide dismutase (SOD)/g of protein, which is comparable to Delaware (Moriyama et al., 2002). In the present study, the optimized glycated chicken Mfs showed a value of about 300 units of SOD/g of protein (data not shown). Accordingly, the RCO program contributed to the production of glycated chicken Mfs with antioxidative abilities approximately 1.5-times greater based on their superoxide anion radical scavenging activity, while maintaining solubility of more than 60%.
Time-dependent solubility changes in optimized glycated chicken Mfs When temperature, RH, and the maltose mixing ratio for Mfs (w/w) were fixed at 57.4°C, 37.3% RH, and 5.43, respectively, time-dependent solubility changes in 0.1 and 0.5 M NaCl during the Maillard reaction were followed up to 37.2 h (Fig. 3). The solubility in 0.5 M NaCl increased from 55.1 ± 5.8% (n = 3), reached 64.7 ± 9.3% (n = 3) at 6 h, and maintained a value of more than 60% up to 24 h. The value fell below 60% after that to become 49.9 ± 3.7% (n = 3) at 37.2 h. The solubility in 0.1 M NaCl started at about 4.5% and rose with increasing reaction time to 57.5 ± 6.0% after 12 h. It subsequently increased to 61.3 ± 4.4% after 37.2 h. Overall, the solubility of optimized glycated chicken Mfs in both media was lower than the values found in previous studies (Nishimura et.al., 2011a,b). Using a temperature of 60°C, an RH of 35%, a reaction time of 36 h, and a maltose mixing ratio for Mfs (w/w) of 4, we obtained 67.3 ± 2.2% solubility in 0.1 M NaCl (n = 3) (Nishimura et.al., 2011b). Because each value of the optimized condition was similar to those in a previous study, we could not determine which condition was the rate limiting factor for solubility. However, the denaturation of Mfs and the amount of intermediate and final products that had antioxidative capacity increased as the Maillard reaction progressed. This denaturation resulted in a decrease in solubility. Because the aim of this study was to obtain glycated chicken Mfs with the strongest antioxidant ability, the RCO program provided the optimal condition, which was more severe than the previous condition used, in order to accelerate the Maillard reaction as much as possible. The denaturation gradually progressed, limiting the solubility in both media compared with those in previous studies (Nishimura et.al., 2011a,b).
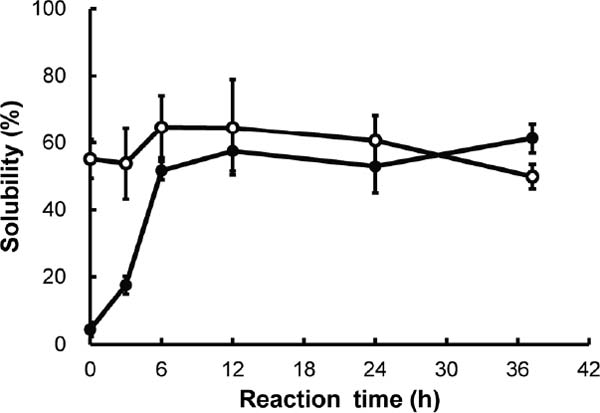
Change in the solubility of glycated chicken Mfs during the Maillard reaction.
Lyophilized chicken Mfs-maltose mixtures in weight ratio of 1:5.4(w/w) were held at 57.4°C and 37.3% RH for 37.2 h. The solubility of the optimum glycated chicken Mfs in 0.1 M (●) or 0.5 M(○) NaCl solution (pH7.5) was measured. Values are the mean ± standard deviation (n = 3).
Time-dependent amounts of available lysine and fructosamine in optimized glycated chicken Mfs The amount of available lysine decreased to 37.5 ± 3.2% (n = 3) after 24 h as the Maillard reaction progressed; however, no marked change was subsequently apparent (Fig. 4A). In contrast, the quantity of fructosamine rose by increasing the reaction time to 326 ± 32 µmol/g of protein (n = 3) in 24 h and then remained constant (Fig. 4B). The amount of available lysine for the optimized glycated chicken Mfs was less than 40%, indicating that more than 120 molecules of maltose were conjugated per myosin heavy chain, since about 200 residues of lysine are present in one molecule (Babij et al., 1991). This conjugation increases the number of hydroxyl groups, thereby improving hydration, which then leads to increased solubility of Mfs in a low ionic strength medium. Furthermore, this improved solubility would have resulted from the dissociation between myosin and actin, as well as from the loss of filament-forming ability of the myosin rods by glycation as suggested in previous studies (Nishimura et al., 2011a,b).
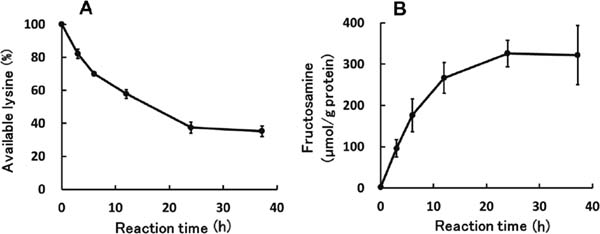
Changes of available lysine and fructosamines of the optimum glycated chicken Mfs. The amounts of available lysine (A) and fructosamine (B) were followed under the condition shown in Fig. 3. Values are mean ± standard deviation (n = 3).
The changes in the amounts of available lysine and fructosamine (Fig. 4A and B) suggest that the reaction between the lysine residues in Mfs and maltose, as well as the production of fructosamine (a product in the initial stage of the Maillard reaction), had already been terminated after the 24-h incubation. Accordingly, the optimized glycated Mfs must have had a greater content of such an intermediate product after the 37.2-h reaction than the 24-h reaction. It is known that some intermediate products of the Maillard reaction possess active oxygen scavenging properties (Hernandez et al.,1992; Pischetsrieder et al., 1998; Ando et al., 2000; Lindenmeier et al., 2002), and suppress the occurrence of ·OH. The optimal condition should provide Mfs with more than 60% solubility in a low ionic strength medium and the most active oxygen scavenging products, resulting in the strongest HORAC.
Heat-induced gel-forming abilities of optimized glycated chicken Mfs and the antioxidant abilities of the gel Optimized glycated and unmodified chicken Mfs solutions (15 mg of protein/mL) were heated at 90°C for 240 min, and their gel formation was observed (Fig. 5). The turbidity of the unmodified chicken Mfs solution (Fig. 5A) increased with heating, and no changes were observed in the color of the solution with time. Furthermore, the gel did not tighten by heating, as the gel was not fixed upon tilting the test tube. A previous study (Nishimura et al., 2015) with similar observations indicated that the absence of sugar caused denaturation of protein during incubation, leading to the loss of gel-forming ability. The optimized glycated chicken Mfs solution (Fig. 5B) became turbid upon heating, and the color of the solution changed to pale yellow after 120 min. A similar change in color occurs after 240 min with glycated chicken Mfs prepared by mixing with 1:5 maltose and incubated at 65°C and 41% RH for 36 h (Nishimura et al., 2015). The gel that formed after 30 min did not flow even when the test tube was kept in an inclined position; however, gel formation was not observed after 60 min of heating. Moreover, the obtained gel (Fig. 5B, 30 min) seemed softer than the ones created in a previous study (Nishimura et al., 2015), despite the same protein concentration (15 mg/mL). As the amounts of available lysine after 24-h incubation in both studies were similar, the binding quantities of maltose to Mfs were also considered the same. Although it is known that the gel forming ability of Mfs decreases with the presence of sugars like sucrose (Baier et al., 2001; Christ et al., 2005), this does not explain this phenomenon. Judging from the time of change in color, the Maillard reaction advanced further in the optimized glycated chicken Mfs solution compared with the previous study (Nishimura et al., 2015). Accordingly, after 60 min of heating, the formation of a gel was prevented, suggesting that some products of the Maillard reaction may inhibit the formation of a gel or destroy it. However, these phenomena were not observed in the previous study. Hence, further examination is needed.
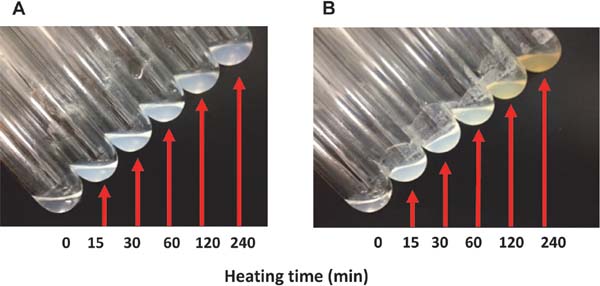
Effect of heating on both Mfs solutions. Chicken Mfs without or with maltose (a maltose mixing ratio of 5.43 for Mfs (w/w)) were incubated at 57.4°C, 37.3% RH for 37.2 h, and then unmodified Mfs(A) and the optimum glycated chicken Mfs (B) were prepared and dissolved in 0.5 M and 0.1 M NaCl solution (pH7.5), respectively. Half mL of both chicken Mfs solutions containing 15 mg of protein/mL was placed in a test tube (diameter 16 mm) and heated at 90°C in water bath for 240 min. The change of each chicken Mfs solution was followed. The status of Mfs solutions was checked by tilting the test tubes at an angle.
A previous study (Nishimura et al., 2015) suggested that heat-induced gels generated from glycated chicken Mfs retain their antioxidant abilities. Thus, HORAC of a gel prepared by heating the optimized glycated chicken Mfs at 90°C for 30 min was measured. The HORAC value was determined as 4.4 ± 1.7 µmol of GA equivalent/g of protein, about half of that before heating (7.8 ± 1.0 µmol of GA equivalent/g of protein). This result suggested that heat-induced gels of glycated chicken Mfs retain antioxidant abilities; hence, we can develop meat paste products with antioxidative abilities using glycated Mfs as a material, enabling a reduction in the amount of antioxidants added during storage.
Changes in protein subunit composition in optimized glycated and unmodified chicken Mfs during gel formation Changes in subunit composition of unmodified and optimized glycated chicken Mfs solutions during heating at 90°C were evaluated by SDS-PAGE (Fig. 6). In SDS-PAGE analysis in the absence of 2-ME (Fig. 6A), unmodified chicken Mfs mainly consisted of large molecular weight proteins stacked on the gel top: myosin heavy chain (MHC), actin, and tropomyosin (TM). On starting the heating process, the large molecular weight proteins, which could not enter the stacking gel, emerged after 15 min, and gradually the bands of MHC and actin thinned. On the other hand, with the addition of 2-ME (Fig. 6B), the amount of large molecular weight proteins decreased followed by the recovery of MHC and actin bands, suggesting that disulfide (SS) bridges between the proteins were formed by heating. However, such recovery was not observed after 240 min of heating, indicating that strong binding interactions such as covalent bonds occur among proteins. Similar changes in SDS-PAGE patterns were observed in a previous study (Nishimura et al., 2015).
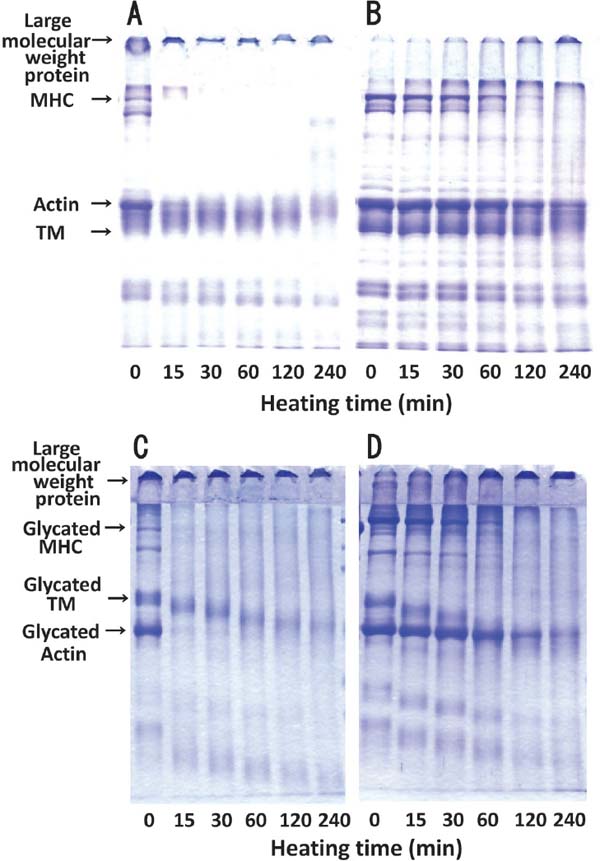
Changes in protein subunit of unmodified and optimum glycated chicken Mfs during gel formation. Unmodified (A) and optimum glycated (C) Mfs solutions obtained as described in Fig.5 were heated at 90°C for 240 min for gel formation and then subjected to SDS-PAGE analysis in the absence (A, C) or the presence (B, D) of 10% 2-ME. MHC: myosin heavy chain. TM: Tropomyosin.
In the SDS-PAGE patterns of the optimized glycated chicken Mfs solution before heating in the absence of 2-ME (Fig. 6C), glycated MHC, TM, and actin were lined up in proportion to their molecular weights. Although the molecular weight of intact TM is lower than that of actin, there are higher amounts of Lys residues in TM than in actin (Nakamura et al., 2005), so that higher molecular weight proteins are brought to TM by binding to a greater number of maltose molecules (Fig. 6C, 0 min). The densities of these bands decreased after 15 min of heating and were almost lost after 60 min. On the other hand, the addition of 2-ME (Fig. 6D) resulted in increased band widths of glycated MHC and actin and a decreased amount of large molecular weight proteins, which could not enter the stacking gel. Accordingly, the increase in glycated MHC and actin was assumed to be brought about by the depolymerization of large molecular weight proteins through SS-binding cleavage. However, glycated MHC and actin were not recovered after 240 min of heating, indicating polymerization among the protein subunits by covalent bonding such as acid-amide bonds, apart from SS bonding, followed by cross-linking. When the electrophoretic tips shown in Figs. 6C and 6D were focused, their color thickened 240 min later, indicating an increase of low molecular weight products. Heat-induced gelation of myosin in high ionic strength medium can be represented by two reactions (Ishioroshi et al., 1979; Samejima et al., 1981; Numakura et al., 1985): i) aggregation of the globular head portion of the myosin molecule that is complementary to and closely associated with the oxidation of the SH groups, and ii) the inevitable network formed by thermal unfolding of the helical tail portion. In this study, both unmodified and optimized glycated chicken Mfs solutions reached the second stage by 240 min heating at 90°C; in contrast, the formation of cross-linkages in unmodified chicken Mfs failed in a previous study (Nishimura et al., 2015). However, while the unmodified chicken Mfs solution could not form a gel, the optimized glycated chicken Mfs could form a gel only at 30 min. Despite the formation of cross-linkages in the SDS-PAGE pattern (Fig. 6D), gel formation was not observed after 60-min of heating (Fig. 5B). In order to investigate whether the gel generated at 30 min was due to the formation of microstructures, observation with a cryo-SEM was carried out.
Observation of microstructures of heat-induced gels of optimized glycated chicken Mfs The microstructures of gels of optimized glycated chicken Mfs obtained with 30-min heating, and those of unmodified chicken Mfs solution with the same heating, were observed with a cryo-SEM (×20,000) (Fig. 7). In unmodified chicken Mfs (Fig. 7A), a capricious heterogeneous microstructure with dense and coarse parts, which seemed to be caused by the aggregation of proteins, was observed. The coagulation of proteins occurred after heating so that the microstructure network could not be constructed, resulting in the failure of gel-formation. On the other hand, in the gel of the optimized glycated chicken Mfs obtained at 30 min of heating (Fig. 7B), the network structure contributing to gel formation was observed.
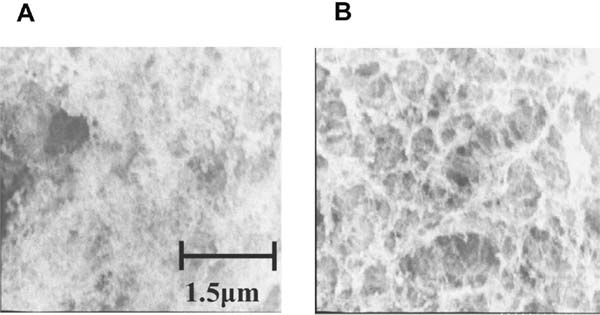
Microstructure of both Mfs after a 30 min-heating at 90°C. Unmodified (A) and optimum glycated (B) chicken Mfs solutions obtained as described in Fig.5 were heated at 90°C for 30 min. The microstructure of each Mfs was observed under cryo-SEM. Magnification, ×5,000; Scale bar = 1.5 µm.
However, the optimized glycated chicken Mfs solution could not form a gel after 60 min (Fig. 5B) despite the formation of cross-linkages among protein subunits (Fig. 6C and D). Since an increase in small molecular components was observed in this case (Fig. 6C and D), the Maillard reaction that progresses with heating seems to destroy the gel microstructure once produced. This collapse was not observed in a previous study (Nishimura et al., 2015). It was thought that a microstructure-collapsing intermediate product did not appear during the heating, since the Maillard reaction progressed slower in the glycated chicken Mfs prepared under the conditions in the previous study. Although the protein concentrations of gels formed from both glycated chicken Mfs solutions were the same, the gel from the optimized glycated chicken Mfs was more fragile. This must have been due to the presence of such intermediate products collapsing the microstructure.
In conclusion, we successfully developed glycated chicken Mfs having novel food functionalities using RCO. The new food material is soluble in a low ionic strength medium and forms heat-induced gels with antioxidant abilities. Utilization of the material may enable the development of meat paste products that do not require large amounts of salt at the time of production and excessive antioxidants as food additives during storage.
Acknowledgements The authors are grateful to assistant professor Ms. Honami Umemoto; and to students, including Ms. Kana Kuramatsu of Doshisha Women's College of Liberal Arts, for their help; and to Ms. Yuko Nambu, a technical staff member at the Graduate School of Agriculture, Kyoto University, for her skillful assistance in the microscopic observations. This study was supported in part by a Grand-Aid for Science Research (C) (No.25350107) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
gallic acid
HORAChydroxyl radical averting capacity
IC50half-maximal inhibitory concentration
Mfsmyofibrillar proteins
MHCmyosin heavy chain
·OHhydroxyl radical
PAGEpolyacrylamide gel electrophoresis
RCOrandom-centroid optimization
RHrelative humidity
RLUrelative light units
SDSsodium dodecyl sulfate
SEMscanning electron microscope
SODsuper oxide dismutase
SSdisulfide
TMtropomyosin
2-ME2-mercaptoethanol